Abstract
Purpose: We aimed to observe the effects and mechanism of rhBNP treatment on myocardial fibrosis (MF) after myocardial infarction (MI). Methods: SPF rats were separated into 3 groups: normal, MI (ligation of left coronary artery), and MI + rhBNP (recombinant human brain natriuretic peptide). Rats in MI + rhBNP group were given 30 μg/kg for 2 days before modeling and for 4 weeks after modeling. mRNA levels and the expression levels of TGF-β1 (transforming growth factor) and CTGF (connective tissue growth factor) in 3 groups were analyzed using the RT-qPCR and western blotting analysis, respectively. Furthermore, myocardial volume fraction (CVF) was analyzed using the Sirius Red F3B (SR) while the percentage of type I and III collagen in 3 groups were analyzed using the immunohistochemical staining. Results: Compared with the normal group, the levels of TGF-β1, CTGF, CVF, type I and III collagen were higher in MI group. However, mRNA levels of TGF-β1 and CTGF were significantly decreased in MI + rhBNP compared to MI groups. Expression of TGF-β1 was lower while that of CTGF was higher in MI + rhBNP group than that in MI group. Besides, CVF, and type I and III collagen were lower in MI + rhBNP group compared with MI group. Conclusion: rhBNP could significantly decrease the TGF-β1 and CTGF levels in post-MI so as to inhibit the type I and III collagen deposition in MF of post-MI. rhBNP will be benefit for the improvement of MF.
Keywords: Recombinant human brain natriuretic peptide (rhBNP), TGF-β1, connective tissue growth factor (CTGF), myocardial infarction, myocardial fibrosis
Introduction
Myocardial infarction (MI) is one of the main diseases that leading to death or disability worldwide [1]. The main cause for MI is the sustained and serious myocardial ischemia resulted by myocardial necrosis due to the imbalance of myocardial blood supply and demand [2]. Myocardial fibrosis (MF), a subsequent development of MI, is characterized by the abnormal level and excess deposition of collagen in myocardial matrix that leading to the dysfunction of ventricular and heart failure [3]. Although many studies have devoted to the exploration of MI control and treatment methods, the mechanisms of MI still remain largely unknown. Therefore, it will be of great significance to investigate the mechanism of MI and dig several biomarkers or useful drugs for MI treatment.
Previous studies referred that inflammatory factors participated in MF progression of post-MI. For instance, tumor necrosis factor (TNF-α) contributes to the MF in post-MI [4] and interleukin-1β (IL-1β) levels is strongly associated with the myocardial remodeling in post-MI [5]. Also, IL-17A promotes the ventricular remodeling in post-MI [6]. On the other hand, the newly reported connective tissue growth factor (CTGF) is another factor that functions in myocardial remodeling and MF. The activated CTGF is related to the left ventricular remodeling after MI [7]. In addition, the TGF-β-stimulated cardiac fibroblast collagen synthesis and CTGF expression are decreased by the soluble expoxide hydrolase in post-MI [8].
Recombinant human brain natriuretic peptide (rhBNP) is a heart failure treatment drug that approved by America Food and Drug Administration (FDA) in 2001 [9]. The role of rhBNP on MF in post-MI has been discussed in previous studies. For example, BNP is an important prognostic drug for heart failure [10] and rhBNP improves the sympotoms in patients with acute decompensated heart failure [11]. Also, the combined rhBNP therapy and bone mesenchymal stem cell transplantation is benefit for the heart failure treatment [12]. In spite of many explorations of rhBNP in improving MF. However, few studies demonstrated the mechanisms of rhBNP in the TGF-β1/CTGF-stimulated MF of post-MI.
In this study, we constructed a rat model of MI and treated the MI rat with rhBNP. Comprehensive experimental methods were used to detect the mRNA level and protein levels of TGF-β1 and CTGF in MI model and MI + rhBNP models compared to the normal groups. Further experiments were conducted to detect the CVF, type I and III collagen of myocardial cells. We aimed to explore the intervention effect and possible mechanism of rhBNP on MF in post-MI. This study might provide basis for the future research of rhBNP on MF in post-MI.
Materials and methods
Modeling of rat MI
All experimental procedures in this study were approved by the Institute of Health Services Research at Harbin Medical University on the protection of animal used for scientific purpose. The manuscript was prepared according to the guidelines of the declaration of Helsinki for biomedical research. The SPF C57BL/6J mice (Shanghai laboratory animal center, Chinese Academy of Sciences) weighting 18-20 g were individually housed in a rodents feeding room with the adjustable temperature, humidity, light, and pressure. The environment was maintained under a 12/12 hour light/dark cycle (lights on at 150-200 LX), with the relative humidity at 50-70%, the temperature set at 22 ± 1°C, and noise < 50 dB. A total of 12 mice were separated into 3 treatment groups (each group containing 4 repeats): sham-operated, MI, and MI + rhBNP. Rats in MI + rhBNP group were intravenous injected with 30 μg/kg rhBNP for 2 days before modeling.
Anesthesia was conducted using the anesthesia apparatus and maintained with 2% isoflurane. Endotracheal intubation was completed via oral, and then rats were connected with HSE-HA MiniVent (USA). Chest at the fourth rib was open to exposure the heart, the left coronary artery was ligated (with the 8.0 nylon suture) about 1 mm from their origin. The immediate color change of the heart surface (pale appearance about 1 min) and the slower movement of ventricular wall were the signs of successful coronary ligation (Figure 1). The diaphragm was sutured with an absorbable 5.0 suture starting at the muscular segment towards its tendinous part, and final suture was tightened after the air was expelled from thoracic cavity. Rats in MI + rhBNP group were given 30 μg/kg rhBNP for 4 weeks after modeling, while rats in the normal and MI groups were given the same dose of water.
Figure 1.
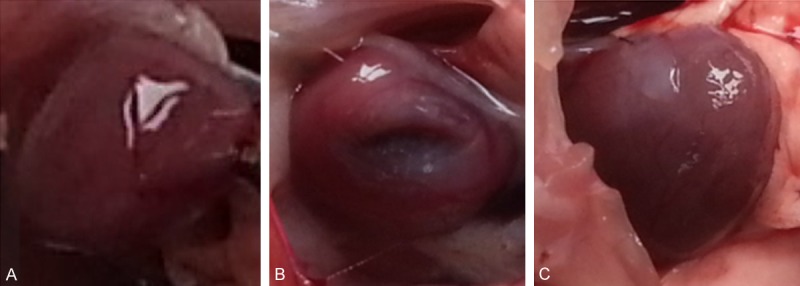
Rat heart. A: Normal heart of rat (Normal), B: MI model heart of rat (Model), C: MI model heart given with the rhBNP (Model + rhBNP).
Quantitative RT-PCR
The fresh heart tissues of rats collected at week 4 were grinded in the liquid nitrogen and then washed with the PBS buffer (PH 7.4). The total RNA was extracted from heart tissues using the Trizol reagent [13] (Invitrogen life technologies, Carlsbad, CA) and treated with RNse-free Dnase I (Promega Biotech). The concentration and purity of extracted RNA was detected using SMA4000 UV-VIS (Merinton, Shanghai, China). The purified RNA that adjusted to 0.5 μg/μL with nuclease-free water was used for cDNA synthesis using the PrimerScript 1st Strand cDNA Synthesis Kit (Takara, China). Primers for the targets amplification were shown in Table 1. Then RT-PCR was carried out in an Eppendorf Mastercycler (Brinkman Instruments, Westbury, NY) using the SYBR ExScript RT-qPCR Kit (Takara, China). The reaction system of 20 μL volume containing 1 μL cDNA from the above PCR, 10 μL SYBR Premix EX Taq, 1 μL each of the primers (10 μM), and 7 μL dd H2O. The PCR program was as follows: denaturation at 95°C for 2 min; followed by 45 cycles of 95°C for 10 s, 59°C for 20 s, and 72°C for 30 s. Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one product was amplified and detected.
Table 1.
Primers used for the targets amplification in this study
| Target | Primer | Sequence (5’-3’) | Length |
|---|---|---|---|
| TGF-β1 | sense | 5’-GGCGGTGCTCGCTTTGTA-3’ | 18 |
| anti-sense | 5’-GTTGCGGTCCACCATTAGC-3’ | 19 | |
| CTGF | sense | 5’-TCTTCGGTGGGTCGGTGTA-3’ | 19 |
| anti-sense | 5’-GGCAGCTTGACCCTTCTCG-3’ | 19 | |
| β-actin | sense | 5’-CCACAGCTGAGAGGGAAATC-3’ | 20 |
| anti-sense | 5’-AAGGAAGGCTGGAAAAGAGC-3’ | 20 |
Western blotting analysis
Tissues harvested at 4 weeks post-MI were lapped in RIPA (radioimmunoprecipitation assay, Sangon Biotech) lysate containing PMSF (phenylmethanesufonyl fluoride) and then were centrifuged at 12,000 rpm/min for 10 min at 4°C. The supernatant was collected to determine the concentration of protein using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rochford, IL).
A total of 30 μg protein per cell lysates was subjected to a 10% SDS-PAGE (sodium dodecylsulfate-polyacrylamide gel electrophoresis) and then transferred onto a Polyvinylidencefluoride (PVDF) membrane (Mippore). The membrane was blocked in TBST (Tris Buffered Saline Tween) with 5% non-fat milk for 1 h, and subsequently incubated with rabbit anti-human TGF-β1 or CTGF monoclonal antibodies (1:100 dolution) overnight at 4°C, followed by incubation with horseradish peroxidase labeled goat anti-rat secondary antibody (1:100 dilution) at room temperature for 1 h. The PVDF was washed using the 1 × TBST buffer for 10 min with 3 times. Detection was performed using the development of X-ray after chromogenic substrate with an enhanced CEL (chemiluminescence) method. In addition, β-actin (Sigma-Aldrich, USA) served as the internal control.
Sirius Red F3B (SR) for myocardial volume fraction (CVF)
One third part of the fresh heart tissues harvested at 4 weeks post-MI was removed with a scalpel along the heart transverse section, and were washed with the precooling PBS, then fixed in the 10% formalin (100 mL formalin + 900 mL PBS) for 24 h. Briefly, paraffin was sliced and then dewaxed to water. Tissues were put into the Sirius Red F3B liquid for 1 h, and then washed with distilled water for 5 min. Then the nuclear was treated with hematoxylin (Mayer) for 5 min, followed by washed with distilled water for 10 min. Xylene was used to transparent the staining tissues and neutral gum was used to seal the tissues. Sections were observed in at least five areas at × 200. The percentage of type I/III collagen (the ratio of red area and yellow area in a field vision) was analyzed using the Image-Pro plus 6.0 software.
Immunohistochemical staining
Immunohistochemical staining was conducted to detect the expressions of type I and III collagen in MI heart tissues. The fresh rat heart tissues harvested at 4 weeks were fixed in 4% formaldehyde, and then dehydrated with an automatic dehydration device (70% alcohol for 30 min, 80% alcohol for 2 h, 95% alcohol for 2 h with 2 cycles, 100% alcohol for 2 h with 2 cycles). The dehydrated tissues were transparented in xylene for 30 min and embedded in paraffin (melting point 52°C) for 1 h with 3 cycles, and then 6 μm sections were cut series. Sections were treated with xylene 3 times for 15 min and then treated with 99% ethanol 3 times for 10 min. The sections were immersed in 3% H2O2 solution and incubated at room temperature for 10 min to deactivate internal peroxidase and then washed 3 times for 5 min using the PBS buffer (PH 7.4).
Non-specific binding was blocked by incubation in 1.5% normal goat serum for 1 h at room temperature. The sections were incubated overnight at 4°C with the following primary antibodies: rabbit anti-human type I collagen monoclonal antibody (BOSTER, China, 1:100 dilution); Rabbit anti-human type III collagen (BOSTER, China, 1:1000). Sections were incubated at 37°C for 1 h with the horseradish peroxidase (HRP) labeled goat anti-rabbit (Jacksong, 1:100 dilution). At lase, 3’-diaminobenzidine (DAB) was used for color development and hematoxylin for counterstaining. The sections were mounted, examined and photographed. In negative control slides, phosphate buffer solution (PBS) was used instead of primary antibody.
After treated with the above steps, pictures from sections were observed in at least five areas at × 200 using the Olympus BX53 digital microscope (Olympus BX53, Tokyo, Japan).
Statistical analysis
The data were expressed as mean ± standard error of mean (SEM). The independent sample t-test method was used to calculate the difference among groups (normal vs. MI, MI vs. MI + rhBNP) with the Prism 5.0 software (GraphPad Prism, San Diego, CA). The P < 0.05 was defined as statistically significant.
Results and discussion
mRNA expression levels of TGF-β1 and CTGF
We used RT-qPCR to analyze the mRNA levels of TGF-β1 and CTGF in MI rats (Figure 2). The RT-qPCR products were analyzed using the agarose gel electrophoresis (Figure 2A). The results showed us that mRNA levels of TGF-β1 and CTGF significantly increased in MI group compared with the normal group (P < 0.05). Besides, the mRNA levels of TGF-β1 and CTGF were lower in MI + rhBNP groups than that in MI groups (Figure 2B and 2C).
Figure 2.
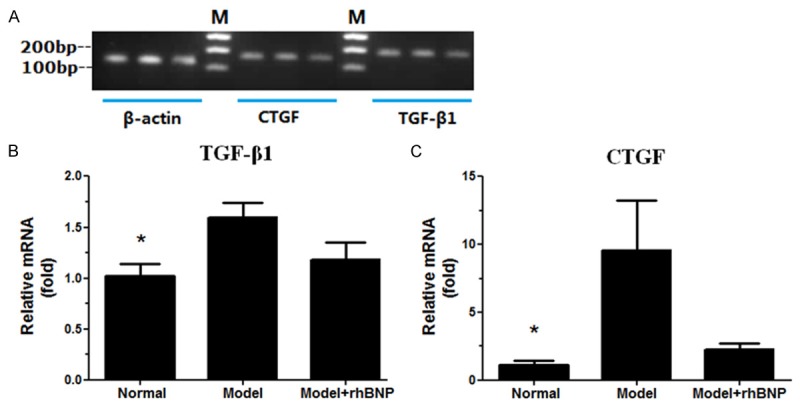
RT-qPCR analyses of TGF-β1 and CTGF mRNA levels. A: The agarose gel electrophoresis of the RT-PCR products, M: DL 2,000 marker; B: The relative mRNA expression level of TGF-β1 in 3 groups, C: The relative mRNA expression level of CTGF in 3 groups. β-actin served as the internal control. *: the significance between Normal and Model group (P < 0.05). Red color stands for the myocardial collagen fiber, while yellow color stands for the myocardial cells.
Western blotting for the expressions of TGF-β1 and CTGF protein
To identify the association of rhBNP on TGF-β1 and CTGF in heart tissues of post-MI, we conducted the western blotting analysis to further detect their expression (Figure 3A). The relative expression levels of TGF-β1 and CTGF in MI group were lower than that in normal group (Figure 3B and 3C). However, TGF-β1 level in MI group was higher than that in MI + rhBNP group (Figure 3B), while the CTGF level in MI group was lower than that in MI + rhBNP group (Figure 3C).
Figure 3.
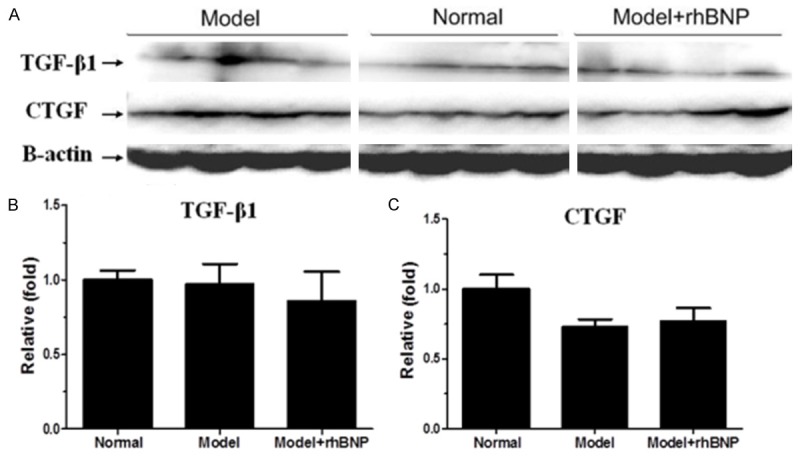
Western blotting analysis of TGF-β1 and CTGF protein expression levels. A: Expression levels of TGF-β1 and CTGF protein in 3 groups, B: The relative fold of TGF-β1 in 3 groups, C: The relative fold of CTGF in 3 groups. β-actin served as the internal control.
Sirius Red F3B (SR) for myocardial volume fraction (CVF)
The CVF of heart in each kind group was analyzed using the Sirius Red F3B (SR) method (Figure 4A). The results displayed that myocardial collagen fibers were stained with red, while myocardial cells were stained with yellow. Also, the percentage of CVF was calculated using the Image-Pro plus 6.0 software (Figure 4B). Compared to the normal group, CVF was significantly increased in MI group (P < 0.05), while CVF significantly decrease in MI + rhBNP group compared with the MI group (P < 0.05).
Figure 4.
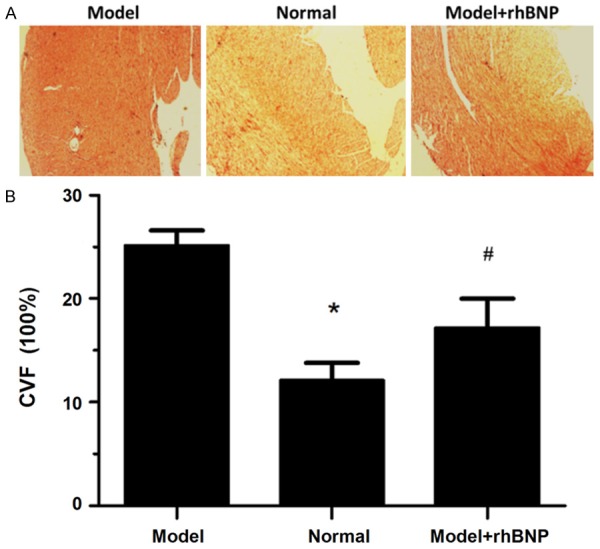
Sirius Red F3B (SR) for CVF. A: The Sirius red F3R for CVF in 3 groups, B: The area percentage CVF in 3 groups. *: the significance between Normal and Model group (P < 0.05). #: the significance between Model group and Model + rhBNP group (P < 0.05).
Immunohistochemical staining of type I and III collagen in post-MI tissues
Immunohistochemical analysis on paraffin sections displayed that post-MI cells expressed strong reactivity for type I and III collagen in post-MI tissues (Figure 5A and 5B). Compared with the normal groups, expressions of type I and type III collagen were significantly higher in MI group (P < 0.05) (Figure 5C and 5D). Besides, both the type I and III collagen levels in MI groups were higher than that in MI + rhBNP groups. No statistical significance was observed of type I and III collagen between MI group and MI + rhBNP group (P > 0.05).
Figure 5.
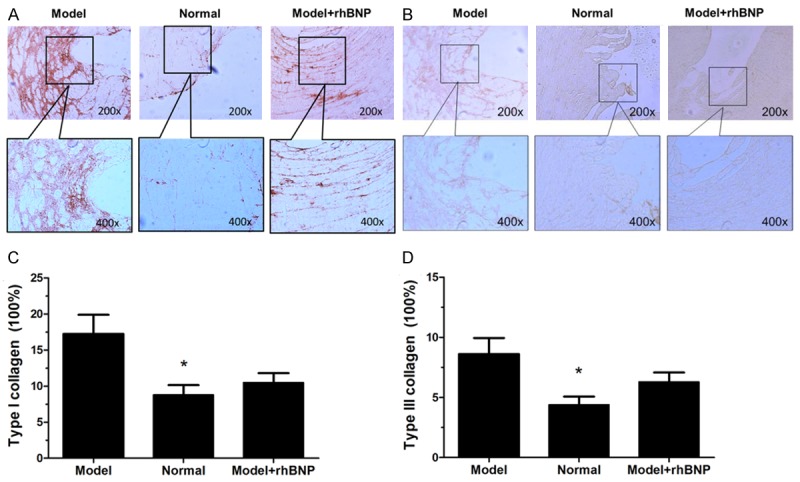
Immunohistochemical staining of type I and III collagen in post-MI tissues. A: Immunohistochemical staining of type I collagen in 3 tissues, C: The area percentage of type I collagen in 3 groups, B: Immunohistochemical staining of type III collagen in 3 tissues, D: The area percentage of type III collagen in 3 groups. *: the significance between Normal and Model group (P < 0.05).
MF in post-MI is a disease that associated with the heart dysfunction and characterized by the excess deposition or abnormal higher level of collagen in normal heart tissues [1,3]. Studies have devoted to the mechanisms of some useful drugs on cytokines and other factors in the MF of post-MI, such as aminomethylbenzoic acid and angelica sinensis [14,15]. In this study, we built a rat MI model and treated the MI rat with rhBNP to explore the mechanism of rhBNP on MF in post-MI. The mRNA level and protein levels of TGF-β1 and CTGF in MI were significantly higher than that in normal groups. rhBNP could significantly decrease the TGF-β1 and CTGF levels in MI. Moreover, the deposition of CVF, type I and III collagen were inhibited by rhBNP in MI + rhBNP group compared with that in MI group.
The complement in immune system was activated in post-MI, characterized by the leukocytes gathered together to the damage heart. TGF-β1 is a member of the TGF-β family proteins that involved in embryogenesis, cell differentiation, and inflammatory reaction and damage repairers in the early stage of MI [16]. Baxter et al. proved that TGF-β1 alleviated the areas of myocardial apoptosis and infraction via activating the p42/p44 MAPK in MI [17]. Also, TGF-β1 prevented the apoptosis of cardiac fibroblast through the Smad3 signaling pathways [18]. Besides, TGF-β1 level could be decreased with the long-term treatment of rhBNP in heart failure [19] and rhBNP prevented the serum TGF-β1 level and induced its fibrosis in heart failure [20]. Hence, rhBNP might play roles in lowering the TGF-β1 level in MI. On the other hand, CTGF (also named CCN2) is a mitogen which is secreted by vascular endothelial cells and plays a role in the osteogenesis, tissue repair, and fibrosis [21]. CTGF was correlated with the cardiac diastolic dysfunction in heart failure [22]. A high activity of CTGF in post-MI was related to attenuate the remodeling of left ventricular via inhibiting the cell apoptosis and some inflammatory responses [23]. Therefore, CTGF may be a contributor for MI. It has been reported that CTGF might cooperate with TGF-β1 during the myocardial fibrosis of heart via mapping their mRNA levels in the infarct zone [24]. Also, Dean et al. said that TGF-β1 was involved in the initial at the acute stage of inflammation and repair after MI, while CTGF was involved in the ongoing fibrosis of heart [25]. Thus, TGF-β1 might induce the CTGF expression in the cardiac fibrosis of post-MI. Ritchie et al. said that rhBNP had the potential role to protect the ischemic of myocardial [26]. Also, the continuous application of exogenous rhBNP reduced the infarction areas of heart and alleviated the ventricular remodeling in post-MI [27]. Our data showed that both the mRNA levels and the protein levels of TGF-β1 and CTGF in MI group were high while these levels were low in MI + rhBNP group, indicating that rhBNP may be an inhibitor of MI in early stage via suppressing the expression of TGF-β1 and CTGF. Thus, rhBNP may be the suppressor drug for MI treatment in early stage.
Meanwhile, CVF and the deposition of collagen were the most important factors that affecting the MF formation. Deposition of type I collagen might be associated with the MF in myocardium [28]. The level of collagen type III was high in rats of cardiac fibroblast [29]. Myocardial collagen metabolism was significantly enhanced in patients underwent surgical ventricular restoration [30]. rhBNP is a potential marker for the prognosis of patients with acute CAD [31] and clinical diagnosis of heart failure can be detected by the rhBNP [32]. Kapoun et al. proved that rhBNP could prevent cardiac remodeling in pathological conditions of heart failure by inhibiting TGF-β-induced effects on cardiac fibroblasts [33]. Hypertension et al. proved that TGF-β1 activated the synthesis of myofibroblasts and increased the collagen production in rats cardiac fibroblasts [34] and the synthesis of collagen induced by TGF-β was mediated via the down-regulate cAMP [35]. Thus, type I and III collagen deposition might be induced by TGF-β1 and CTGF. The association between rhBNP and type I and III collagen in MI has not been fully discussed. However, rhBNP could suppress the serum TGF-β1 and CTGF levels in MI. Also, Magga et al. said that rhBNP was the antifibrotic factor in heart and degradation of type I collagen was regulated by rhBNP and increased after acute MI rhBNP [36]. Based on our results, type I and III collagen level were high in MI rats while that was low in MI + rhBNP group, we speculated that rhBNP might prevent the MI progression by decreasing the expression of TGF-β1 and CTGF to inhibit the expression of type I and III collagen. Therefore, rhBNP may be a therapeutic drug for the improvement of MF in MI.
In conclusion, our findings suggest that the levels of TGF-β1, CTGF, CVF, and type I and III collagen are high in the MI rats. Treatment with rhBNP can better suppress the expression levels of TGF-β1, CTGF, as well as can improve the deposition of CVF, and type I and III collagen in MI, suggesting that rhBNP may be associated with the inhibition of MF in post-MI, which may be one of the mechanisms of rhBNP inhibition role in the MI therapeutics.
Disclosure of conflict of interest
None.
References
- 1.Jneid H, Alam M, Virani SS, Bozkurt B. Redefining myocardial infarction: what is new in the ESC/ACCF/AHA/WHF third universal definition of myocardial infarction? Methodist Debakey Cardiovasc J. 2013;9:169. doi: 10.14797/mdcj-9-3-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano FG, Guido MC, Barbeiro HV, Caldini EG, Lorigados CB, Nogueira AC. Endotoxemic myocardial dysfunction: subendocardial collagen deposition related to coronary driving pressure. Shock. 2014;42:472–9. doi: 10.1097/SHK.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AW. Do TNF inhibitors reduce the risk of myocardial infarction in psoriasis patients? JAMA. 2013;309:2043–2044. doi: 10.1001/jama.2013.4695. [DOI] [PubMed] [Google Scholar]
- 5.Orn S, Ueland T, Manhenke C, Sandanger O, Godang K, Yndestad A, Mollnes TE, Dickstein K, Aukrust P. Increased interleukin-1beta levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med. 2012;272:267–276. doi: 10.1111/j.1365-2796.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou SF, Yuan J, Liao MY, Xia N, Tang TT, Li JJ, Jiao J, Dong WY, Nie SF, Zhu ZF, Zhang WC, Lv BJ, Xiao H, Wang Q, Tu X, Liao YH, Shi GP, Cheng X. IL-17A promotes ventricular remodeling after myocardial infarction. J Mol Med. 2014;27:27. doi: 10.1007/s00109-014-1176-8. [DOI] [PubMed] [Google Scholar]
- 7.Gravning J, Orn S, Kaasboll OJ, Martinov VN, Manhenke C, Dickstein K, Edvardsen T, Attramadal H, Ahmed MS. Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One. 2012;7:20. doi: 10.1371/journal.pone.0052120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kompa AR, Wang BH, Xu G, Zhang Y, Ho PY, Eisennagel S, Thalji RK, Marino JP Jr, Kelly DJ, Behm DJ, Krum H. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol. 2013;167:210–219. doi: 10.1016/j.ijcard.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 9.Strain W. The use of recombinant human B-type natriuretic peptide (nesiritide) in the management of acute decompensated heart failure. Int J Clin Pract. 2004;58:1081–1087. doi: 10.1111/j.1368-5031.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 10.Brenyo A, Barsheshet A, Rao M, Huang DT, Zareba W, McNitt S, Hall WJ, Peterson DR, Solomon SD, Moss AJ, Goldenberg I. Brain natriuretic peptide and cardiac resynchronization therapy in patients with mildly symptomatic heart failure. Circ Heart Fail. 2013;6:998–1004. doi: 10.1161/CIRCHEARTFAILURE.112.000174. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Qu X, Qu Y, Yu Y, Feng W. The effect of B-type brain natriuretic peptide on patients with acute decompensated heart failure coexisting with lung cancer: a randomized controlled clinical trial. Pharmazie. 2014;69:212–216. [PubMed] [Google Scholar]
- 12.Fujii H, Itoh Y, Ohnishi N, Sakamoto M, Ohkawara T, Sawa Y, Nishida K, Ohkawara Y, Yamaguchi K, Minami M, Okanoue T. Factors associated with the overall survival of elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18:1926–1932. doi: 10.3748/wjg.v18.i16.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rio DC, Ares M, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5439. pdb. prot5439. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Shen Z, Yu Y. Application of P-aminomethylbenzoic acid in heart valve replacement surgery under cardiopulmonary bypass. Jiangsu Medical Journal. 2012;3:025. [Google Scholar]
- 15.Zhang S, He B, Ge J, Li H, Luo X, Zhang H, Li Y, Zhai C, Liu P, Liu X. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int J Biol Macromol. 2010;47:546–550. doi: 10.1016/j.ijbiomac.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Zhang JQ, Zhang J, Lamparter S. Cardiac remodeling by fibrous tissue after infarction in rats. J Lab Clin Med. 2000;135:316–323. doi: 10.1067/mlc.2000.105971. [DOI] [PubMed] [Google Scholar]
- 17.Baxter GF, Mocanu MM, Brar BK, Latchman DS, Yellon DM. Cardioprotective effects of transforming growth factor-β1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol. 2001;38:930–939. doi: 10.1097/00005344-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Vivar R, Humeres C, Ayala P, Olmedo I, Catalán M, García L, Lavandero S, Díaz-Araya G. TGF-β1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013;1832:754–762. doi: 10.1016/j.bbadis.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Lian X, Yang G, Yang L, Chen Z, Kang H, Ye Z, Wei Z, Qin P, Li G, Li D. Effects of recombinant human brain natriuretic peptide on plasma TGF-β1 and PDCD5 levels in heart failure. Life Sci J. 2013:10. [Google Scholar]
- 20.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Wu CK, Wang YC, Lee JK, Chang SN, Su MY, Yeh HM, Su MJ, Chen JJ, Chiang FT, Hwang JJ. Connective tissue growth factor and cardiac diastolic dysfunction: human data from the Taiwan Diastolic Heart Failure Registry and molecular basis by cellular and animal models. Eur J Heart Fail. 2014;16:163–172. doi: 10.1002/ejhf.33. [DOI] [PubMed] [Google Scholar]
- 23.Gravning J, Ørn S, Kaasbøll OJ, Martinov VN, Manhenke C, Dickstein K, Edvardsen T, Attramadal H, Ahmed MS. Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One. 2012;7:e52120. doi: 10.1371/journal.pone.0052120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuva De Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, Ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- 25.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie RH, Rosenkranz AC, Kaye DM. B-type natriuretic peptide: endogenous regulator of myocardial structure, biomarker and therapeutic target. Curr Mol Med. 2009;9:814–825. doi: 10.2174/156652409789105499. [DOI] [PubMed] [Google Scholar]
- 27.Palazzuoli A, Silverberg DS, Iovine F, Calabrò A, Campagna MS, Gallotta M, Nuti R. Effects of β-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007;154:645.e649–645.e615. doi: 10.1016/j.ahj.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Gu D, Jiao W, Huang Y, Liu P, Zhao L, Yu H. [Effects of spironolactone on type I, III collagen concentration in myocardium of spontaneous hypertension rats] . Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:81–85. doi: 10.3785/j.issn.1008-9292.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Bao J, Jin M, Yang Y, Gao X, Shu L, Xing H, Jia L. [In vitro effect of total flavones of Fructus Chorspondiatis on expression of collagen type I and type III mRNA and protein of cultured rat cardiac fibroblasts] . Yao Xue Xue Bao. 2014;49:136–141. [PubMed] [Google Scholar]
- 30.ten Brinke EA, Witkowski TG, Delgado V, Klein P, Klok M, Marsan NA, Klautz RJ, van der Wall EE, Bax JJ, van der Laarse A, Steendijk P. Myocardial collagen turnover after surgical ventricular restoration in heart failure patients. Eur J Heart Fail. 2011;13:1202–1210. doi: 10.1093/eurjhf/hfr097. [DOI] [PubMed] [Google Scholar]
- 31.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 32.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 33.Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-β in primary human cardiac fibroblasts fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 34.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-β1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 35.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor β-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 36.Magga J, Puhakka M, Hietakorpi S, Punnonen K, Uusimaa P, Risteli J, Vuolteenaho O, Ruskoaho H, Peuhkurinen K. Atrial natriuretic peptide, B-type natriuretic peptide, and serum collagen markers after acute myocardial infarction. J Appl Physiol. 2004;96:1306–1311. doi: 10.1152/japplphysiol.00557.2003. [DOI] [PubMed] [Google Scholar]


