Abstract
Recent data strongly suggest the important role of miRNAs in various cancer-related processes. Osteosarcoma is the most common type of primary malignant bone tumor and is characterized by complex genetic changes and resistance to conventional treatments. In this study, the role of miRNA-15a (miR-15a) in the progression and metastasis of osteosarcoma was investigated. The result demonstrated that the expression of miR-15a was down-regulated in osteosarcoma tissues and cell lines as compared with that in adjacent non-neoplastic bone tissues and the osteoblastic cell line. In functional assays, miR-15a inhibited cell proliferation, migration and invasion in U2OS and MG-63 cells. Meanwhile, bioinformatic analysis combined with experimental confirmation demonstrated that tumor necrosis factor; α-induced protein 1 (TNFAIP1) gene is a potential target of miR-15a and can be directly regulated by miR-15a. Down-regulation of TNFAIP1 induced effects on osteosarcoma cell lines similar to those induced by miR-15a. Taken together, these data suggest that miR-15a may act as a tumor suppressor, which is commonly down-regulated in both osteosarcoma tissues and cells. TNFAIP1 plays an important role in mediating miR-15a dependent biological functions in osteosarcoma. Reintroduction of miR-15a may be a novel therapeutic strategy by down-regulating TNFAIP1 expression.
Keywords: miRNA-15a (miR-15a), osteosarcoma, proliferation, invasion, migration, tumor necrosis factor, α-induced protein 1 (TNFAIP1)
Introduction
Osteosarcoma is the most common type of human primary sarcoma of the bone and a leading cause of cancer death in adolescents due to its rapid proliferation [1]. It is an aggressive malignant neoplasm arising from primitive transformed cells of mesenchymal origin that exhibit osteoblastic differentiation and produce malignant osteoid. The mechanisms of formation and development of osteosarcoma have been studied for a long time. Recently, more and more evidence showed that miRNAs play important roles in regulating tumor growth.
MicroRNAs (miRNAs) are a class endogenous small non-coding RNAs that regulate gene expression by the inhibition of the translation and/or decreasing of the stability of target mRNAs [2]. MiRNAs are differentially expressed in various tissues and cells, suggesting their potential applications as biomarkers and therapeutic targets [3]. MiRNAs are deregulated in several diseases including cancers, where they play important roles by regulating the expression of various tumor oncogenes and suppressors [4,5]. MiRNAs also can act as oncogenes or tumor suppressors and involve in numerous cellular processes, playing roles in tumorigenesi by regulating cell differentiation, cell proliferation and cell cycle [6-9]. To date, down-regulated miRNAs and their roles in osteosarcoma development have attracted much attention. Some of them, including miR-21, miR-31, miR-34a, miR-20a, miR-125b and miR-143, have been reported to participate in the initiation and progression of osteosarcoma and modulate the biological properties of cancer cells [10-14]. However, the role of miR-15a in osteosarcoma tumor development and metastasis has only recently been investigated and remains largely unknown.
In the present study, we investigated the role of miR-15a in osteosarcoma. Our results showed that the expression of miR-15a was down-regulated in osteosarcoma cells and tissues compared to paired adjacent non-tumor bone tissues. Moreover, in vitro experiments proved that miR-15a inhibited cell proliferation, migration and invasion in the osteosarcoma cells. In addition, TNFAIP1 was identified as a novel direct target gene of miR-15a. Our findings suggested that miR-15a has a tumor suppressive effect in osteosarcoma by inhibiting cell proliferation and invasion.
Materials and methods
Cell culture
Human osteosarcoma cell lines (MG63 and U2OS cells) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (GIBCO, NY, USA) and streptomycin (100 mg/ml), penicillin (100 U/ml). Cultures were incubated at 37°C with 5% CO2 in a humidified incubator.
Cell transfection
Cells were grown in the appointed medium 12-16 h before transfection. The cells were transfected with 20 nmol/L of miR-15a mimics, inhibitor and the scramble mimics using lipofectamine 2000 (Invitrogen) according to the protocol of the manufacturer. The miRNA mimics, inhibitors, and the scramble, which are non-homologous to the human genome, were from GenePharma (Shanghai, China). TNFAIP1 siRNAs were purchased from Ribobo (Guangzhou, China).
RNA extraction and quantitative real-time RT-PCR analysis
Total RNA was extracted by TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. For miRNA detection, 2 μg of small RNA was reverse transcribed to cDNA using miRNA First-Strand cDNA Synthesis kit (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) analysis for miR-15a was performed in triplicate with the SYBR Green PCR Master Mix (Perkin-Elmer, Applied Biosystems) according to the manufacturer’s instructions. U6 was used to normalize expression. To detect the target genes, 2 μg of total RNA was reverse transcribed to cDNA using oligo (dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega). β-actin levels were used to normalize expression. Data analysis was performed using the 2-ΔΔCt method.
Cell proliferation assay
Cell proliferation was analyzed by using cell counting assay Kit-8 (CCK-8) (DOJINDO, Kumamoto, Japan) according to the manufacture’s protocol. Cells were cultured in 96-well flat bottomed microplate and were incubated in 10% CCK-8 (Dojindo; Kumamoto, Japan) diluted in normal cultured medium and then were incubated for 1 h at 37°C. Proliferation rates were determined at 1, 2, 3, 4 and 5 days after transfection. The absorbance of each well was measured with a microplate reader set at 450 nm. The experiment was repeated three times.
Cell migration and invasion assay
MG-63 cells were grown to confluence in 12-well plastic dishes and were treated with miR-15a mimics, inhibitors or scrambled. Then, 24 h after transfection, linear scratch wounds (intriplicate) were created on the confluent cell monolayers using a 200 mL pipette tip. To remove cells from the cell cycle prior to wounding, cells were maintained in serum-free media. To visualize migrated cells and wound healing, images were taken at 0, 24 and 48 h. A total of ten areas were selected randomly from each well, and the cells in three wells from each group were quantified.
For the invasion assays, 24 h after transfection, 1×105 cells in serum-free media were seeded in transwell migration chambers (8 mm pore size; Millipore, Zurich, Switzerland). The upper chamber of these transwell inserts was coated with Matrigel (Sigma-Aldrich, St. Louis, MO, USA). Medium containing 20% FBS was added to the lower chamber. After 24 h, the cells that did not invade through the pores were carefully wiped out with cotton wool. Then cells located on the lower surface of the chamber were stained with 0.1% crystal violet (Sigma) and counted using a microscope (Olympus, Tokyo, Japan). These experiments were repeated three times.
Western blotting analysis
Proteins were separated on 10% SDS-PAGE gel, and then transferred to a PVDF membrane (Amersham, Buckinghamshire, UK). After blocked with 5% non-fat dried milk, the membrane was incubated with anti-tumor necrosis factor, α-induced protein 1 (TNFAIP1) antibody (Abcam, England) at 1:1000 dilution; anti-GAPDH antibody (Proteintech, Chicago, USA) at 1:50,000 dilution. After washing with TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.1% Tween 20), the membranes were incubated for 2 h with goat anti-rabbit antibody at 1:5000 dilution and 1:50000 dilution. Proteins bands were visualized using ECL reagents (Pierce, Rockford, IL, USA).
Luciferase reporter assay
Primers were designed in accordance with the Genbank TNFAIP1 gene mRNA sequence. A fragment of the 3’-UTR of TNFAIP1 was amplified from cells by PCR using the forward primer 5’-CGAGCTCGTGCTGCCTGGGTCTCTGC-3’ and the reverse primer 5’-GCTCTAGAGCAGCTGCTCTGTCGGATGTTT-3’. Following digestion of the PCR product by SacI and XbaI, the FASN 3’-UTR was cloned into pmirGLO (Promega, Madison, WI, USA) at the SacI and XbaI sites. All PCR products were verified by DNA sequencing. U2OS cells were co-transfected with the pmirGLO vectors containing the 3’-UTR variants and miR-15a or the negative control miRNA. Twenty-four hours after transfection, cells were analyzed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega). Normalized firefly luciferase activity (firefly luciferase activity/Renilla luciferase activity) for each construct was compared with that of the pmirGLO Vector no insert (NO) control. For each transfection, luciferase activity was averaged from three replicates.
The 3’-UTRs of TNFAIP1 containing these predicted binding sites of miR-15a were amplified using PCR from human cDNA using primers, and inserted into the pMIR-REPORT luciferase reporter vectors (Ambion, Austin, TX, USA) to get the constructs containing the wild-type TNFAIP1 3’-UTR (TNFAIP1-WT). TNFAIP1-Mut contained the sequences with mutations in the first putative binding site of TNFAIP1 3’-UTR. Mutations of these predicted seed regions in these mRNA sequences were created using the primers including the mutated sequences. The recombination constructs, pRL-TK (Promega, WI, USA) and miR-15a or control mimic were co-transfected into cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The plasmid of pRL-TK containing Renilla luciferase was used as the internal control. Firefly and Renilla luciferase activity were measured using Dual Luciferase Assay (Promega, WI, USA) according to the manufacturer’s instructions at 24 h after transfection.
Statistical analysis
Data were presented as the means ± standard deviation of at least three experiments. Statistical analysis was performed using SPSS 15.0. A one-way analysis of variance (ANOVA) test, least significant difference (LSD) test, Chisquare test and Student’s t test were used for statistical analysis.
Results
Expression of miR-15a is down-regulated in osteosarcoma tissues and cell lines
We examined the expression of miR-15a in two human osteosarcoma cells lines (MG-63 and U2OS) and in hFOB using real-time RT-PCR. As shown in Figure 1A, expression of miR-15a was much lower in two osteosarcoma cell lines than that in human osteoblast cell line hFOB. To confirm miR-15a expression was different between tumor and nontumor tissues, we examined 45 pairs of human osteosarcoma samples. The result showed that the miR-15a expression was down-regulated in osteosarcoma tissues compared with the corresponding adjacent normal tissues (Figure 1B).
Figure 1.
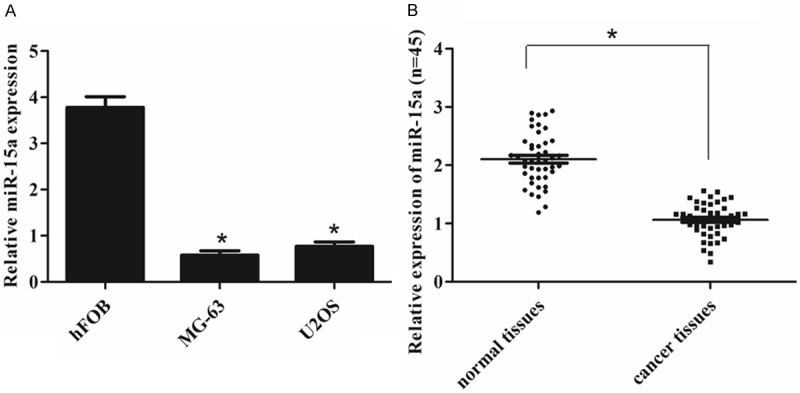
miR-15a is down-regulated in osteosarcoma. A. The relative expression levels were determined by qRT-PCR in human osteoblast cell line (hFOB) and osteosarcoma cells (MG-63 and U2OS). *P < 0.05 vs. hFOB. B. qRT-PCR analysis of miR-15a expression in 45 pairs osteosarcoma tissues and their corresponding adjacent normal bone tissues. The expression of miR-15a was normalized to U6. *P < 0.05 vs. normal tissues.
miR-15a inhibits osteosarcoma cell proliferation
Cells were firstly transfected with miR-15a mimics, inhibitors or negative control, which showed high transfection efficiency (Figure 2A and 2B). The results of CCK-8 proliferation assay showed that cell proliferation capacity was reduced in miR-15a mimics-transfected the MG-63 and U2OS cells compared with either the scrambled miRNA-transfected cells or the untreated cells (Figure 2C, 2D). Conversely, miR-15a inhibitor significantly promoted the cell proliferation of the MG-63 and U2OS cells (Figure 2C, 2D). These results indicated that the miR-15a significantly suppressed the cell proliferation of the osteosarcoma cells.
Figure 2.
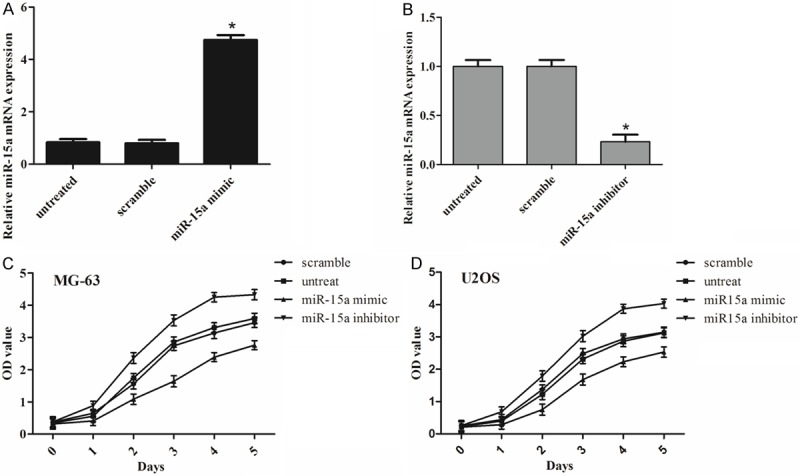
miR-15a inhibits osteosarcoma cell proliferation. (A) qRT-PCR analysis of miR-15a expression after the transfection of miR-15a mimics or scramble or no transfection. (B) qRT-PCR analysis of miR-15a expression after the transfection of miR-15a inhibitors or scramble or no transfection. Proliferation curves of MG-63 (C) and U2OS (D) cells, respectively. The effect of miR-15a on cell proliferation was evaluated by CCK-8 assay after miR-15a transfection of MG-63 and U2OS. *P < 0.05 vs. untreated or scramble.
miR-15a suppress osteosarcoma cell migration and invasion
To further investigate the effect of miR-15a on MG-63 and U2OS cells migration and invasion, we transfected the cells with miR-15a mimics, inhibitors or negative control and then evaluated migration and invasion ability of osteosarcoma cells. As the Figure 3A shown, the cells that were treated with the miR-15a mimic were distinctively less migratory than the scrambled control or untreated cells. By contrast, the miR-15a inhibitor significantly increased the cell migration of the osteosarcoma cells. Furthermore, we conducted cell invasion Matrigel assays and then stained the invaded cells to measure the directional invasion ability of the cells after ectopically expressing miR-15a in cells. The invasiveness of the cells that were transfected with the miR-15a mimic was dramatically decreased compared with the scrambled control and untreated cells. Conversely, the miR-15a inhibitor significantly increased the invasiveness of the osteosarcoma cells (Figure 3B). These observations suggest that miR-15a had an important role in reducing the migration and invasion of osteosarcoma in vitro.
Figure 3.
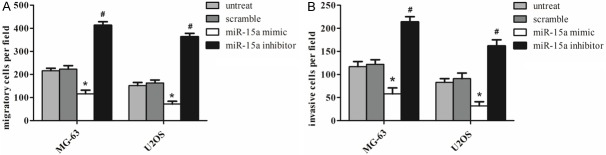
miR-15a inhibits osteosarcoma cell migration and invasion. A. Migration assays of the MG-63 and U2OS cells after treatment with miR-15a mimics, inhibitors or scramble or no transfection. B. Invasion analysis of the MG-63 and U2OS cells after treatment with miR-15a mimics, inhibitors or scramble or no transfection. Each bar represents the mean ± SD. The results were reproduced in three independent experiments. *P < 0.05 vs. untreated or scramble, #P < 0.05 vs. untreated or scramble.
TNFAIP1 is a direct target of miR-15a
As is well known, miRNAs exert their function through suppressing the expression of their target genes. To better understand the molecular mechanisms of miR-15a, we performed bioinformatics analyses the using the miRNA target analysis tools PicTar, TargetScan, and microRNA.org in order to predict putative miRNAs binding to the TNFAIP1 3’-UTR. According to the analysis, all three programs predicted that a binding sequence in the 3’-UTR of TNFAIP1 was a very good match for the miR-15a seed (Figure 4A). The bioinformatics analysis thus indicated a potential functional link between TNFAIP1 and miR-15a.
Figure 4.
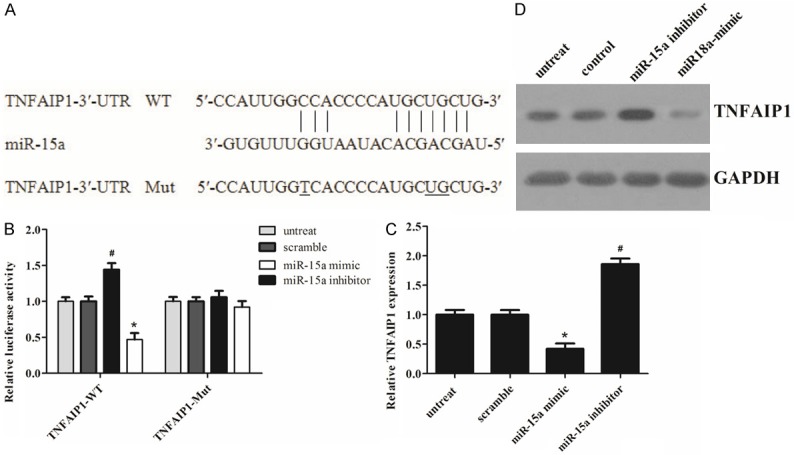
miR-15a directly targets TNFAIP1 by binding to its 3’-UTR. A. The predicted miR-15a binding site within the TNFAIP1 3’-UTR and a mutated version generated by site directed mutagenesis are shown. B. Luciferase reporter assay illustrating direct binding of miR-15a to the WT, but not Mut sequences within the 3’-UTR of TNFAIP1. C. mRNA levels of TNFAIP1 were determined by real-time PCR in transfected MG-63 cells. D. Western blots were used to confirm the expression of TNFAIP1 in MG-63 cells after transfection; GAPDH was used as a control. The statistical analysis was based on three independent experiments. Error bars represent mean ± SD. *P < 0.05 vs. untreated or scramble, #P < 0.05 vs. untreated or scramble.
Furthermore, we performed a luciferase reporter assay to further confirm whether miR-15a can directly target the 3’-UTR region of TNFAIP1 in osteosarcoma cells. Both the TNFAIP1 wild-type 3’-UTR containing the miR-15a-binding site and a mutated TNFAIP1 3’-UTR sequence were cloned into modified pGL-3 luciferase reporter vectors, which were co-transfected into MG-63 cells together with NC, the miR-15a mimic, or miR-15a inhibitor. As shown in Figure 4B, miR-15a over-expression significantly reduced the luciferase reporter activity by the TNFAIP1 3’-UTR in a consistent manner, and inhibition of miR-15a had the opposite effect. However, TNFAIP1 3’-UTR luciferase reporter activity was unaffected by point mutations in the miR-15a-binding seed region. Collectively, these data suggest that miR-15a may inhibit TNFAIP1 expression by targeting its 3’-UTR. As predicted, a western blot showed that, at 48 h after transfection, the enhanced miR-15a in MG-63 cells significantly repressed TNFAIP1 protein expression compared to cells transfected with a scrambled control. By comparison, down-regulation of miR-15a by inhibitors in MG-63 cells led to a moderate increase in the TNFAIP1 mRNA level (Figure 4C). Meanwhile, apparent alterations in TNFAIP1 protein expression were also observed (Figure 4D). Together, these data provide strong evidence that TNFAIP1 is a specific target of miR-15a in osteosarcoma cells.
TNFAIP1 is involved in miR-15a-inhibited proliferation, migration and invasion in MG-63 cells
Based on the findings above, we hypothesized that miR-15a might inhibit osteosarcoma cell proliferation, migration and invasion by repressing TNFAIP1 expression. First we transiently transfected TNFAIP1 siRNA into MG-63 cells, and qRT-PCR and western blotting confirmed the down-regulation of TNFAIP1 (Figure 5A). As shown in Figure 5B and 5C, TNFAIP1 silencing significantly suppressed cell proliferation, migration and invasion of MG-63 cells, which was similar to the phenotype induced by miR-15a.
Figure 5.
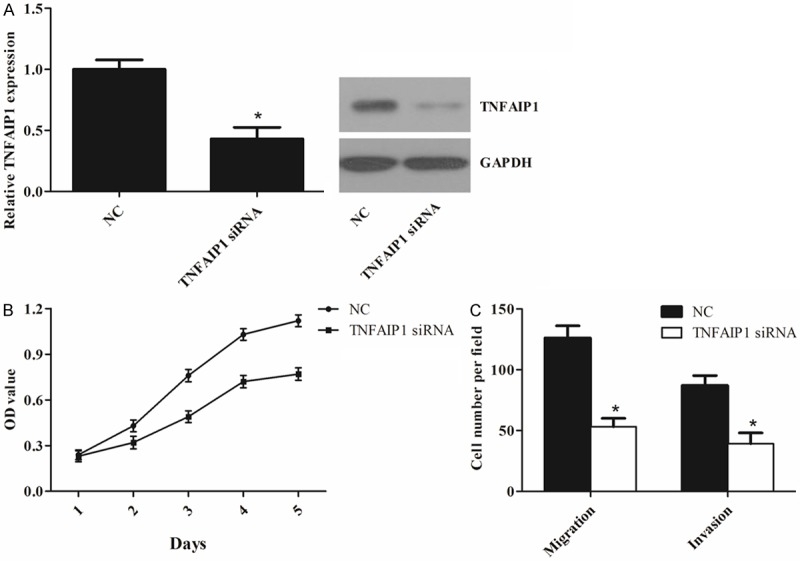
TNFAIP1 is involved in miR-15a-inhibited growth, migration and invasion in MG-63 cells. A. qRT-PCR and western blotting analysis of TNFAIP1 expression in MG-63 cells transfected with TNFAIP1 siRNA or negative control (NC). B and C. Down-regulation of TNFAIP1 suppressed cell proliferation, migration and invasion of MG-63 cells. The values represent the mean ± SD of three replicates. *P < 0.05 vs. NC.
Discussion
Accumulating evidence has shown that combined miRNA and mRNA profiling may be involved in multiple processes in cancer development and progression. Recent years, a series of studies have report that miRNA remarked in osteosarcoma development [15,16]. However, the expression and function of miR-15a in osteosarcoma has not been reported. In the current study, we found miR-15a was down-regulated in osteosarcoma cells. In addition, we analyzed the expression of miR-15a in 45 osteosarcoma patients and found that the expression of miR-15a was much lower in osteosarcoma tissues in comparison with paired adjacent non-tumor bone tissues. All of these evidences indicated that miR-15a might play a significant part in the development of osteosarcoma.
MiR-15a regulates the apoptosis and proliferation of cells by functioning in the regulation of multiple intracellular signaling pathways. It has been reported that over-expression of miR-15a inhibited cellular growth, suppressed migration and arrested cells at the G1 phase, but did not promote cellular apoptosis [16]. It has specifically been shown that miR-15a down-regulated CCND1 and induced apoptosis and cell cycle arrest in osteosarcoma [17]. To determine the biological relevance of miR-15a in osteosarcoma, we performed functional assays. Ectopic expression of miR-15a significantly inhibited osteosarcoma cell proliferation, migration and invasion. These findings suggested that miR-15a might act as a tumor suppressor gene whose down-regulation may contribute to the progression and metastasis of osteosarcoma.
Identification of cancer-specific miRNAs and their targets is pivotal for understanding their roles in tumorigenesis [18,19]. TNFAIP1 is an immediate-early response gene of endothelium induced by TNFα and IL-6 [20]. It may play roles in DNA synthesis, DNA repair, cell apoptosis and human diseases including cancer, and may be involved in tumor progression and metastases [21,22]. In this study, we identified TNFAIP1 as a direct target of miR-15a in the MG-63 cells. Complementary sequence of miR-15a was identified in the 3’-UTR of TNFAIP1 mRNA.
Recent study has demonstrated that TNFAIP1 was highly expressed in normal cell lines while it was lowly expressed in cancer cell lines [23]. Moreover, the over-expression of TNFAIP1 could accelerate the apoptosis of HeLa cells [24]. It has been report that silencing of TNFAIP1 was able to inhibit the growth and invasion, and induces apoptosis in osteosarcoma cells [25]. In the current study, further investigation was conducted to explore the molecular mechanism by which miR-15a suppressed osteosarcoma cell growth, migration and invasion. We identified TNFAIP1 as a functional target of miR-15a, and verified the positive effects of TNFAIP1 on osteosarcoma cell proliferation, migration and invasion using RNA interference. These results indicated that miR-15a might function as a tumor suppressor partly mediated by repressing TNFAIP1 expression in osteosarcoma.
In conclusion, the present study provided an novel evidence that miR-15a function as a tumor suppressor miRNA in osteosarcoma through repressing TNFAIP1 expression. Consequently, our findings provided a molecular basis for the role of miR-15a/TNFAIP1 in the progression of human osteosarcoma and suggested that this miRNA could be a potential target for the treatment of osteosarcoma in future.
Disclosure of conflict of interest
None.
References
- 1.Jiang L, Tao C, He A, He X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J Surg Oncol. 2014;12:383. doi: 10.1186/1477-7819-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Wynsberghe PM, Chan SP, Slack FJ, Pasquinelli AE. Analysis of microRNA expression and function. Methods Cell Biol. 2011;106:219–252. doi: 10.1016/B978-0-12-544172-8.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W, Gao B, Fu P, Xu S, Qian Y, Fu Q. The miRNAs in the pathgenesis of osteosarcoma. Front Biosci. 2012;18:788–794. doi: 10.2741/4142. [DOI] [PubMed] [Google Scholar]
- 4.Tang JT, Fang JY. MicroRNA regulatory network in human colorectal cancer. Mini Rev Med Chem. 2009;9:921–926. doi: 10.2174/138955709788681672. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Maruyama R, Yamamoto E, Kai M. DNA methylation and microRNA dysregulation in cancer. Mol Oncol. 2012;6:567–578. doi: 10.1016/j.molonc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schotte D, Pieters R, Den Boer M. MicroRNAs in acute leukemia: from biological players to clinical contributors. Leukemia. 2012;26:1–12. doi: 10.1038/leu.2011.151. [DOI] [PubMed] [Google Scholar]
- 7.Sandoval J, Esteller M. cancer epigenomics. beyond genomics. Curr Opin Genet Dev. 2012;22:50–55. doi: 10.1016/j.gde.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Rottiers V, Najafi-Shoushtari SH, Kristo F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N, Mostoslavsky R. In Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press; 2011. MicroRNAs in Metabolism and Metabolic Diseases; pp. 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song B, Ju J. Impact of miRNAs in gastrointestinal cancer diagnosis and prognosis. Expert Rev Mol Med. 2010;12:e33. doi: 10.1017/S1462399410001663. [DOI] [PubMed] [Google Scholar]
- 10.Ziyan W, Shuhua Y, Xiufang W, Xiaoyun L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol. 2011;28:1469–1474. doi: 10.1007/s12032-010-9563-7. [DOI] [PubMed] [Google Scholar]
- 11.Creighton CJ, Fountain MD, Yu Z, Nagaraja AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–1915. doi: 10.1158/0008-5472.CAN-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, Ma B. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One. 2012;7:e33778. doi: 10.1371/journal.pone.0033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LH, Li H, Li JP, Zhong H, Zhang HC, Chen J, Xiao T. miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem Biophys Res Commun. 2011;416:31–38. doi: 10.1016/j.bbrc.2011.10.117. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Cai X, Wang Y, Tang H, Tong D, Ji F. microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep. 2010;24:1363–1369. doi: 10.3892/or_00000994. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi E, Hornicek FJ, Duan Z. MicroRNA involvement in osteosarcoma. Sarcoma. 2012;2012:359739. doi: 10.1155/2012/359739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Q, Li X, Li J, Kong X, Zhang J, Chen L, Huang Y, Fang L. MiR-15a is underexpressed and inhibits the cell cycle by targeting CCNE1 in breast cancer. Int J Oncol. 2013;43:1212–1218. doi: 10.3892/ijo.2013.2034. [DOI] [PubMed] [Google Scholar]
- 17.Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma YL, Ji ZW, Li XX, Han K, Gao J. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol Rep. 2012;28:1764–1770. doi: 10.3892/or.2012.1995. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Yang J, Duan P, Xu J, Luo X, Luo F, Zhang Z, Hou T, Liu B, Zhou Q. MicroRNA-24 inhibits osteosarcoma cell proliferation both in vitro and in vivo by targeting LPAATβ. Arch Biochem Biophys. 2013;535:128–135. doi: 10.1016/j.abb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Zhong D, Gao Q, Zhai W, Ding Z, Wu J. MicroRNA-34a inhibits human osteosarcoma proliferation by downregulating ether a go-go 1 expression. Int J Med Sci. 2013;10:676. doi: 10.7150/ijms.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf F, Marks R, Sarma V, Byers M, Katz R, Shows T, Dixit V. Characterization of a novel tumor necrosis factor-alpha-induced endothelial primary response gene. J Biol Chem. 1992;267:1317–1326. [PubMed] [Google Scholar]
- 21.Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, Wood DE, Sahagan BG. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging. 2003;24:397–413. doi: 10.1016/s0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Liu N, Hu X, Zhang W, Wang T, Li H, Zhang B, Xiang S, Zhou J, Zhang J. CK2 phosphorylates TNFAIP1 to affect its subcellular localization and interaction with PCNA. Mol Biol Rep. 2010;37:2967–2973. doi: 10.1007/s11033-009-9863-1. [DOI] [PubMed] [Google Scholar]
- 23.Yang LP, Zhou AD, Li H, Zhang WF, Wu YY, Zhang J, Han M. [Expression profile in the cell lines of human TNFAIP1 gene] . Yi Chuan. 2006;28:918–922. [PubMed] [Google Scholar]
- 24.Kim DM, Chung KS, Choi SJ, Jung YJ, Park SK, Han GH, Ha JS, Song KB, Choi NS, Kim HM. RhoB induces apoptosis via direct interaction with TNFAIP1 in HeLa cells. Int J Cancer. 2009;125:2520–2527. doi: 10.1002/ijc.24617. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CL, Wang C, Yan WJ, Gao R, Li YH, Zhou XH. Knockdown of TNFAIP1 inhibits growth and induces apoptosis in osteosarcoma cells through inhibition of the nuclear factor-κB pathway. Oncol Rep. 2014;32:1149–1155. doi: 10.3892/or.2014.3291. [DOI] [PubMed] [Google Scholar]


