Abstract
In this study, we try to testify the relationship between the programmed cell death receptor-1 (PD-1)/programmed cell death ligand 1 (PD-L1) passway and Treg cells in maternal-fetal immune regulation through PD-1 blockade on lymphocytes of normal early pregnancy in vitro and investigation of the PD-1 and PD-L1 changes in early recurrent miscarriage patients. CD4+ CD25+ Treg cells and PD-1 (CD279) positive cell were detected in deciduas in early recurrent miscarriage patients by flow cytometry. And the normal early pregnant women were as controls. Meanwhile the mRNA level of PD-1 and molecular expression of PD-L1 in deciduas of early recurrent miscarriage patients were detected by real time RT-PCR test and Immunohistochemical staining respectively. Also through antibody blocking assay to block PD-1 on lymphocytes of normal early pregnancy in vitro further testify the relationship between PD-1/PD-L1 and Treg cells, the results were analyzed by flow cytometry. CD4+ CD25+ Treg cells decreased both in deciduas in RM (P < 0.05), and for all almost 100% Treg cells (CD4+ CD25+) expressed PD-1, but there was no difference between the PD-1 positive cells in decidual lymphocytes in RM and that in normal pregnancy women (P > 0.05). PD-L1 mRNA in deciduas decreased in RM (P < 0.001), but PD-1 mRNA no difference (P > 0.1). After PD-1 blockade there was no change in CD4+ CD25+ Treg cells percentage, while the CD4+ T cell percentage increased (P < 0.01), as well as the level of IFN-gamma in cells supernatant (P < 0.01). PD-1 blockade has a little influence on the number of Treg cells, and may lead to impaired Treg cells function, the decrease of PD-L1 may closely relates to the occurrence of early recurrent miscarriage and implies that Treg cells may through PD-1/PD-L1 pathway play a role of immunosuppression regulation, and the impairment of Treg cells function in recurrent early abortion cases may be due to PD-L1 decrease in deciduas or trophoblast cells rather than PD-1 change.
Keywords: Early pregnancy, miscarriage, programmed cell death receptor-1, regulatory T cells, immunoregulation
Introduction
Recurrent miscarriage (RM) refers to the consecutive spontaneous miscarriage, which happens 3 or more than 3 times prior to 20 gestational weeks with about 1-3% incidence [1]. RM happening prior to 12 weeks (including 12 weeks) gestation is termed early recurrent miscarriage.
T regulate cells (Treg cells) are identified by the surface marker CD4+ CD25+. Its control gene is transcription FoxP3. They can inhibit CD4+ and CD8+ T cell proliferation and cytokine production as well as the production of B cells, the cytotoxic activity of natural killer cells (NK cells) and dendritic cells (DLs) mutations. They effect in the immune suppression and may be one of the important cells to induce and maintain peripheral immune tolerance and play an important role in pregnancy immune tolerance and maintaining a successful pregnancy [2,3].
Studies found that quite a number of Treg cells existed in uterine deciduas of normal pregnant women [4]. These cells significantly inhibit autologous T cells proliferation through the anti-CD3 stimulation, and doses dependently reduce autologous CD4+ CD25-T cells proliferation. According to previous reports, the increase of Treg cells in the endometrium parallels that in peripheral blood [5], it can down regulate Th1 cells emerging in endometrium after fertilization, which can damage fetus. And the CD4+ CD25+ cells (Treg cells) were found reduce in deciduas both in spontaneous abortion women and women with RM [4,6]. In the RM cases, the inhibition function of CD4+ CD25+ CD127low Treg cells to effect T cells weakens mainly due to reducing the secretion of IL-10 and TGF-β [7], and the signaling conduction of IL-6 inhibits Treg cells growth, while induces Th17 cell differentiation [8]. It implies that type Th1 immune enhancement may be caused by the impaired Treg cells function.
Impaired Treg cells have been described in several organ-specific autoimmune diseases and system autoimmune diseases, including SLE, multiple sclerosis and type I diabetes [9-11]. At present, a lot of experimental evidence suggests that women with even tiny autoimmune defects may suffer from an increasing frequency of RM, which may be associated with the reduction of Treg cells function [12]. The declining of Treg cells function weakens their down-regulation role for Th17 and Th1 cells, so that lead to relative increased activity of Th17 and Th1 cells, which is toxic to early pregnancy embryo. Nevertheless the mechanism of Treg cells regulating in maternal-fetal immunity is still unclear. Immune inhibitory receptor PD-1 (programmed cell death receptor-1) and its ligand PD-L1 (programmed cell death ligand 1) may participate in the immune regulatory mechanism of Treg cells.
PD-1/PD-L1 pathway inhibits autoreactivity T and B cells and effector T cells, which induces immune tolerance and regulates local inflammation. So it maybe plays a critical role in the delicate balance between effective immunity and self-tolerance, setting up and maintaining peripheral immune tolerance [13]. Studies have shown that Treg cells high express PD-1 and PD-L1 [14,15]. Moreover in the same allogeneic pregnancy, lacking of PD-Ll decreases the regulating function of Treg cells, which leads to embryo absorption increase and fetal survival rate drop [16].
In this study, we try to testify the relationship between PD-1/PD-L1 and Treg cells in maternal-fetal immune regulation through PD-1 blockade on lymphocytes of normal early pregnancy in vitro and investigation of the PD-1 changes in early recurrent miscarriage patients.
Materials and methods
Patients and tissue samples source
Decidual samples were taken from 19 cases early recurrent miscarriage patients who had at least 3 times history of early spontaneous abortion before and diagnosed embryo still by ultrasonic examination this time again, for flow cytometry analysis, real-time semi-quantitative RT-PCR and immunohistochemical staining. And the normal decidual samples as control were from 22 healthy pregnancy women who requested for a termination during the first trimester. Pregnancy diagnosis was made by blood β-HCG determination and ultrasonic examination.
Decidual tissues were collected in uterus curettage with sterile negative pressure suction device. The decidual tissues for flow cytometry analysis were processed as following right now. The decidual tissues for real-time semi-quantitative RT-PCR were put in sterile freezing tubes and stored in -80°C icebox, and that for immunohistochemistry were put in formalin.
There were no differences in the age and pregnancy duration between test groups and control groups, as shown in Table 1. All subjects were ruled out systemic disease and genital infection. And the study had obtained informed consent and permission from the provincial hospital ethics committee of Shandong University.
Table 1.
Characteristics of RM and control groups
| Groups | n | Age (years) | P value | Pregnancy Days | P value |
|---|---|---|---|---|---|
| N | 22 | 28.56 ± 3.01 | 0.06 | 56.13 ± 7.86 | 0.07 |
| RM | 19 | 30.12 ± 3.24 | 61.44 ± 9.62 |
Age: RM vs. N, P = 0.06; Pregnancy days: RM vs. N, P = 0.07.
Cells preparation and flow cytometry analysis
The decidual tissues got with above method were instantly rinsed off blood with sterile saline, and quickly transferred to the laboratory in sterile containers in ice boxes. Then, the tissues were collected into RPMI 1640 medium. After further removal of blood clot, they were chopped into small pieces with sterile ophthalmic scissors and strained through a 100 um sterile cell strainer. The cells that passed through the strainer were collected and incubated with red blood cell lysing buffer for 5 min and then centrifuged at 300 g for 5 min. The cell pellet washed in PBS then, after a further centrifugation at 300 g, was re-suspended in cold fluorescence-activated cell sorting(FACS) buffer to get deciduas monocytes (DMC) suspension. The total number of single cells was counted by a haemocytometer. Living cells were distinguished by 0.2% trypan blue dyeing test. After treated by the detection antibodies, the DMCs were analyzed on a FACS ARIA-III (BD) to detect expressions of PD-1, CD25 and CD4. Detection antibodies were respectively marked with three different colors fluorescent pigment, namely PE anti-human CD279 (PD-1) (Bio Legend EH12.2 H7), APC anti-human CD25 (Bio Legend BC96), PerCP anti-human CD4 (Bio Legend OKT4). Each tag was set a blank control and an isotype control respectively. FCM analysis data were processed with FLOW-Jo software.
Cell culture conditions and PD-1 blockade method
The cells for culture were DMCs prepared from deciduas of 12 normal pregnancy women with above method. The culture cells concentration is 1 × 106 cells/ml. First, each DMC sample was separated into 2 equal parts. One part for blockade test group to be blocked by LEAFTM purified anti-human CD279 (PD-1) (BioLegend EH12.2 H7) monoclonal antibody (5 µg/ml), and the other without antibody as the blank controls. Next, two groups of cell samples were incubated in refrigerator at 4-8°C for 30 minutes at the same time. Then, after washing by buffer, the cells were added in lymphocyte culture (Lonza 04-418 q), containing 100 U/ml penicillin, 100 mg/ml streptomycin and 10% heat-fetal bovine serum (FBS). After culturing in 37°C CO2 incubator for 72 hours, cells were collected for analysis with flow cytometry. Finally, the percentage of CD4+ CD25+ cells, CD4+ cells, and CD126 (IL-6Rα) + CD4+ CD25+ cells was detected by flow cytometry. At the same time IFN-γ and IL-10 levels were determined by ELISA technique in the cells supernatant.
Real time RT-PCR and immunohistochemistry
The real time RT-PCR test was taken used Takara PCR Thermal Cycler Dice Real Time system (Takara TP800), and all reagents were provided by TAKARA. In the test, Beta actin was chosen as the reference gene. The primer sequences of reference gene and purpose genes were as follows: PD-1 F: 5-CTCAGGGTGACAGAGAGAAG-3; PD-1 R: 5-GACACCAACCACCAGGGTTT-3; PDL-1 F: 5-TCA ATGCCCCATACA ACAAA-3; PDL-1 R: 5-GCTTGTCCAGATGACTTCG-3; β-actin F: 5-TGGATCAGCAAGCAGGAGTATG-3; β-actin R: 5-GCATTTGCGGTGGACGAT-3. Each gene was set five gradient standards and negative controls.
Paraffin sections of deciduas were stained with streptavidin-perosidase (SP) method. The detection antibody was purified mouse anti-human CD274 (B7-H1, PD-L1), (BioLegend 29 e. 2 a3). The second antibody was goat anti-mouse IgG (Zmyed). Developing was by DAB. The Results were analyzed by using positive cells counting score method. At least 5-10 HPF were observed and percentage of positive cells was counted. The final total < 1.0 was regarded as weakly positive, 1.0-1.5 as positive, > 1.5 as strong positive.
Statistical analysis
Student t test was used for comparison of means between experimental groups, the non-parametric Wilcoxon rank sum test for paired samples, and the Mann-Whitney U test for non-paired samples. Dates were showed with mean ± SD. Values of P < 0.05 were considered significant.
Results
Flow cytometry analysis of PD-1 positive cells and Treg cells change in the peripheral blood and deciduas in women with recurrent early miscarriage
As shown in Figures 1 and 2, The CD4+ CD25+ Treg cells percentages reduced in deciduas of pregnant early recurrent miscarriage patients (n = 19) compared to normal pregnant women (n = 22). The result was 22.8 ± 12.1% versus 36.6 ± 11.4%, P < 0.05. PD-1 (CD279) positive cells percentage in deciduas of the recurrent miscarriage group declined slightly. But there was no statistical differences compared to normal pregnancy group (61.4 ± 10.3% versus 69.4 ± 10.7%, P > 0.05). And for all almost 100% CD4+ CD25+ cells expressed PD-1. In addition, the ratio of CD4+ CD25+ cells and CD4+ cells decreased in RM group compared to normal control (0.457 ± 0.0211 versus 0.589 ± 0.0242, P < 0.01).
Figure 1.
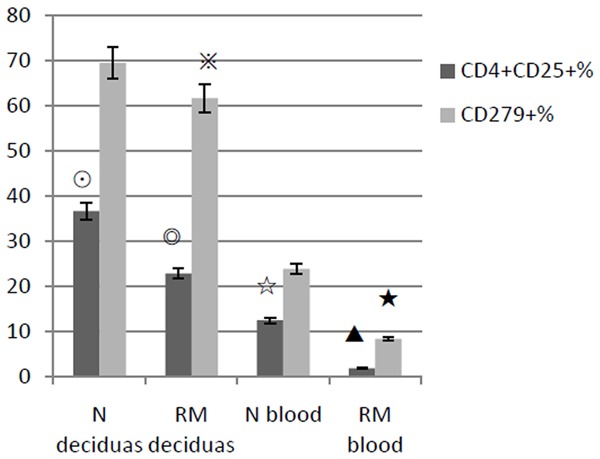
This shows the CD4+ CD25+ % and CD279+ % of lymphocytes in deciduas and peripheral blood of early recurrent miscarriage women (RM) and normal control (N). CD4+ CD25+ % in deciduas and blood of RM are both less than that of N (◎▲both P < 0.01). CD279+ % in deciduas slightly decreases in RM compared to N, but there is no significant difference between two groups (※P > 0.05), while in peripheral blood it decreases in RM (★P < 0.01).
Figure 2.
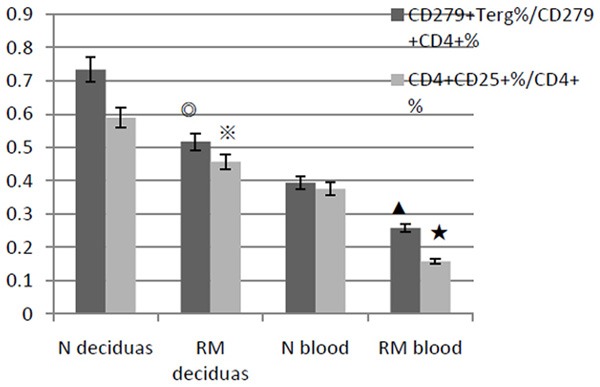
The ratio of Treg cells (CD4+ CD25+) and CD4+ cells are lower in RM than that in normal control (N), both in deciduas (※P < 0.05) and in peripheral blood (★P < 0.01). And it is similar to the ratio of CD279 (PD-1)+ Treg cells and CD279+ CD4+ cells (◎▲both P < 0.05).
PD-1 blockade experimental results show that PD-1 blockade may impair Treg cells and up-regulate the level of CD4+ cells
As shown in Figure 3, the culture cells suspension of PD-1 blocked group and the blank control group were tested using flow cytometry after 72 hours incubation. The results showed that there was no difference in CD4+ CD25+ cells percentage between the two groups (0.102 ± 0.0063% versus 0.116 ± 0.0069%, P > 0.05), as well as the percentage of CD4+ CD25+ cells with high CD126 (IL-6 R alpha) expression between two groups (70.3 ± 8.92% versus 78.7 ± 11.3%, P > 0.05). But the percentage of CD4+ cells increased in PD-1 blocked group (55.6 ± 6.78% versus 31.4 ± 5.32%, P < 0.01). As shown in Figure 4, ELISA experiment showed that the IFN-gamma concentration in cells supernatant fluid in PD-1 blocked group was higher than that in control (975.98 ± 66.35 pg/ml versus 781.57 ± 78.16 pg/ml, P < 0.01). And there was no obvious change in IL-10 concentration (260.66 ± 22.14 ng/L versus 199.73 ± 22.85 ng/L, P > 0.05).
Figure 3.
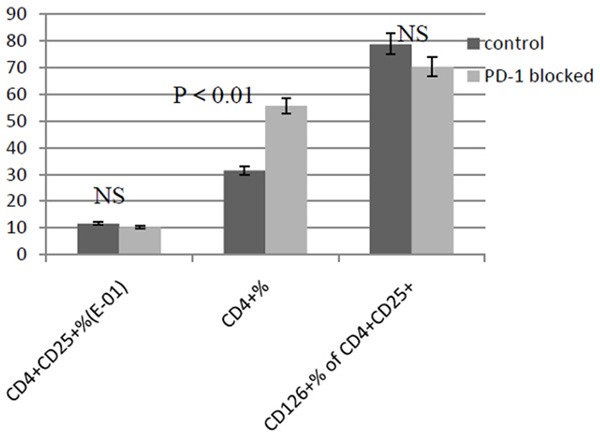
The percentage change of CD4+ cells and CD25+ cells between PD-1 blocked and control group is detected with FCM. CD4+ CD25+ % and CD126 (IL-6Rα)+ % of CD4+ CD25+ cells do not have significant changes (both P > 0.05) between two groups, while CD4 % is significantly higher in PD-1 blocked group (P < 0.01).
Figure 4.
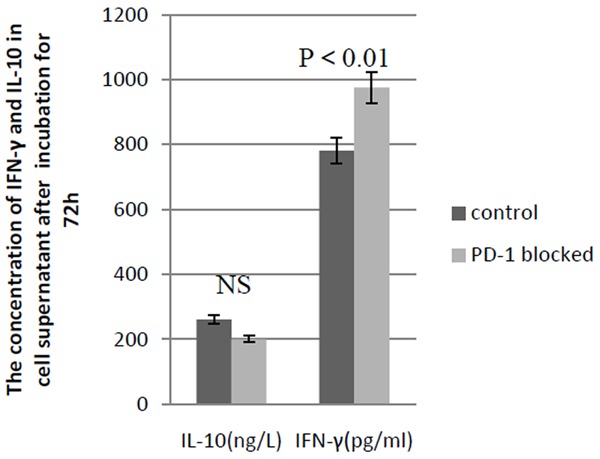
The concentration of IFN-γ and IL-10 in cell supernatant in PD-1 blocked and control group after 72 h incubation is detected with ELASE. The concentration of IFN-γ increases in PD-1 blocked group compared with control group (P < 0.01) and there is no change in IL-10 concentration (P > 0.05).
Immunohistochemistry and PCR experiment results showed that the decidual PD-L1 and its mRNA expression decreased in RM women
The results of Immunohistochemical staining were analyzed with the method of positive cells counting score analysis. The decidual PD-L1 expression in early RM group (n = 19) was less than that in normal control (n = 22) (0.436 ± 0.002 versus 1.15 ± 0.037, P < 0.01), namely the PD-L1 in deciduas of early RM women was weakly positive expression, and in normal early pregnancy was positive expression. Real time RT-PCR relative quantitative analysis results showed that the PD-L1mRNA content in the deciduas in RM group (n = 19) decreased, comparing to normal control (n = 22) (relative value 1.17 ± 0.37 versus 2.67 ± 1.23, P < 0.001), but there was no difference in the PD-1 mRNA content between two groups (relative value 1.43 ± 0.64 versus 1.53 ± 0.71, P > 0.1). As shown in Table 2 and Figure 5.
Table 2.
PD-1 and PD-L1 content in deciduas of RM and normal control group by real time RT-PCR
| Group | n | PD-1 | PD-L1 | β-actin | Relative value of PD-1 | Relative value of PD-L1 |
|---|---|---|---|---|---|---|
| RM | 19 | 2.58 ± 0.59 | 26.8 ± 7.75 | 25.9 ± 2.75 | 1.43 ± 0.64 | 1.17 ± 0.37 |
| N | 22 | 2.74 ± 0.81 | 55.6 ± 20.7 | 26.3 ± 3.60 | 1.53 ± 0.71 | 2.67 ± 1.23 |
Note: Relative value have been correction, choosing the lowest normal control value in each target gene group as a calibration standard for ratio correction.
Figure 5.
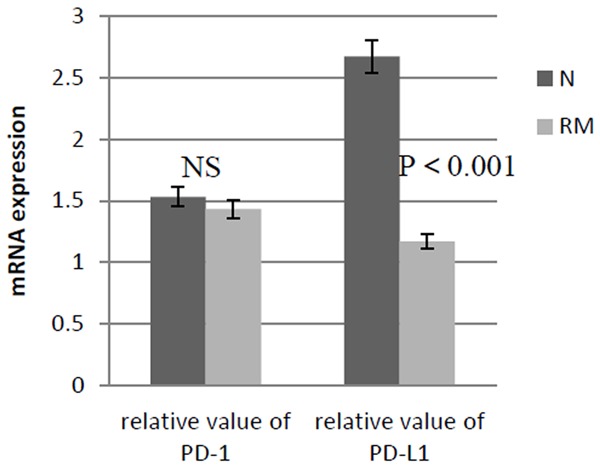
The mRNA expression of PD-1 and PD-L1 in deciduas of early RM women and normal early pregnancy women is detected with real time RT-PCR. Between two groups the expression of PD-1 mRNA has no significant difference (P > 0.1), but the expression of PD-L1 mRNA differs significantly, decreasing in early RM women (P < 0.001). Relative value of mRNA expression is got through the relative transcript level correction by the lowest value in each target gene group.
Discussion
In recent years, the relationship between regulatory T cells (Treg cells) and recurrent miscarriage has been proposed gradually. Most scholars believe that Treg cells play an important role in inducing and maintaining maternal-fetal immune tolerance and the decrease or impairment of Tregs may lead to fetal loss [2-6]. However, seldom study on Treg cells and PD-1 simultaneously in recurrent early miscarriage women. More importantly, the immune-regulation mechanism of Treg cells is still unclear. Animal experiments have shown that the PD-1/PD-L1 immunosuppression pathway is closely related to pregnancy loss. Blocking the PD-L1 can down-regulate the ratio of Treg cells and effector T cells in mice spleen and lymph nodes, while raise Th17 and Th1 cytokine levels [17]. So it is assumed that Treg cells may play immunosuppression role through the PD-1/PD-L1 pathway.
Our experimental results showed that the percentage of CD4+ CD25+ Treg cells was lower in deciduas (P < 0.05) in early recurrent miscarriage patients comparing with normal early pregnancy women. And the ratio of CD4+ CD25+ cells and CD4+ cells in deciduas in the cases of early recurrent miscarriage also decreased, which implied that the level of CD4+ cells is higher relatively. It suggested that Treg cells were necessary for early pregnancy. Flow cytometry analysis also showed high expression of PD-1 in CD4+ cell group, and almost 100% Treg cells (CD4+ CD25+) expressed PD-1. But there was no significant difference between the proportions of PD-1 positive cells in decidual lymphocytes in early recurrent miscarriage and in normal pregnancy women. It indicates that PD-1 content may be not associated with the fetal loss.
Moreover, we took decidual lymphocytes from normal pregnancy women, and blocked PD-1 on cells using monoclonal antibody through antigen-antibody reaction. CD4+ cells and CD4+ CD25+ cells were determined after 72 hours incubation. Results showed that the percentage of CD4+ CD25+ Treg cells had no significant change compared with blank control, but the CD4+ T cell percentage increased. This matched the increase of CD4+ T cells in early recurrent miscarriage patients. At the same time, the detection of the expression of IL-6Rα on CD4+ CD25+ cells showed no obvious change. But the level of IFN-gamma in cells supernatant increased. According document the signaling conduction of IL-6 inhibits Treg cells growth [8]. It suggested that PD-1 blockade had a little influence on the number of Treg cells, and might lead to impaired Treg cells function.
In addition, studies have shown that PD-L1 expression is rich in the placenta [5]; our experiment indicated that PD-L1 was also expressed rich in the uterine deciduas in early pregnancy women. The immunohistochemical staining showed PD-L1 expression in deciduas most located in glandular epithelial cell membrane and cytoplasm, implying that PD-L1 might relate to glandular secretion. And the PD-L1 molecules and mRNA levels in deciduas both decreased in the recurrent early abortion women compared with normal pregnancy women. But there was no difference in the PD-1 expression between the two groups. It indicated that down-regulating the PD-L1 expression was closely related to recurrent early pregnancy loss, which could be considered to be one of the symptoms of an autoimmune disorder. It implied that Treg cells might play a role of immunosuppression through PD-1/PD-L1 way, thus participated in maternal-fetal immune tolerance. And the impairment of Treg cells function in recurrent early abortion cases might be caused by PD-L1 decrease in deciduas or trophoblast cells rather than PD-1 change. D’addio’s PD-L1 blockade model studies have shown that PD-L1 blockade can lead to embryo size decrease and embryo loss in model mice, while raising Th17 and Th1 cells in their peripheral lymphoid tissues [17]. It also illustrated that the absence or reduce of PD-L1 might cause spontaneous abortion pathogenesis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NO. 81170590).
Disclosure of conflict of interest
None.
References
- 1.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci (Landmark Ed) 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 5.Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, Scherjon SA. Differential distribution of CD4+ CD25bright and CD8+CD28- T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–S53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Bao SH, Wang XP, Lin QD, Wang WJ, Yin GJ, Qiu LH. Decidual CD4+ CD25+ CD127dim_regulatory T cells in patients with unexplained recurrent spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol. 2011;155:94–98. doi: 10.1016/j.ejogrb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Arruvito L, Billordo A, Capucchio M, Prada ME, Fainboim L. IL-6 trans-signaling and the frequency of CD4+FOXP3+ cells in women with reproductive failure. J Reprod Immunol. 2009;82:158–165. doi: 10.1016/j.jri.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn A, Beissert S, Krammer PH. CD4+CD25+ regulatory T cells in human lupus erythematosus. Arch Dermatol Res. 2009;301:71–81. doi: 10.1007/s00403-008-0891-9. [DOI] [PubMed] [Google Scholar]
- 11.Crispín JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–325. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009;123:977–983. doi: 10.1016/j.jaci.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, Yagita H, Azuma M, Turka LA, Sayegh MH. Role of the programmed death-1 pathway in regulation of alloimmune response in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 14.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR. Program death-1 (PD-1) signaling and regulatory T cells (Tregs) collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes (CTLs) in advanced acute myeloid leukemia (AML) Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 17.D’Addio F, Riella LV, Mfarrej BJ, Chabtini L, Adams LT, Yeung M, Yagita H, Azuma M, Sayegh MH, Guleria I. The Link between the PDL1 Costimulatory Pathway and Th17 in Feto- maternal Tolerance. J Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]


