Abstract
Background: The Leukemia related protein 16 gene (LRP16) localized on chromosome 11q12.1, is an important estrogen-responsive gene and a crucial regulator for NF-kB activation. LRP16 is frequently expressed in human cancers; however, the LRP16 gene remains unexplored in lung neuroendocrine tumors. The aim of this study was to investigate the role of LRP16 expression in primary lung neuroendocrine tumors. Methods: lung neuroendocrine tumors were analyzed for LRP16 gene expression by two-step non-biotin immunohistochemical method. Results: Fifty of ninety (55.6%) cases of neuroendocrine lung tumors tested were positive for LRP16 protein by immunohistochemistry. The expression of LRP16 was mainly located in cytoplasm and nucleus of tumor cells. LRP16 protein was corresponding to tumor type and clinical stage, as well as survival time. Conclusions: The results indicate that abnormal LRP16 expression is noted in neuroendocrine lung tumors and the expression can give insight into the pathogenesis of the disease. The LRP16 protein may serve as a potential marker in predicting prognosis of neuroendocrine lung tumors.
Keywords: Neuroendocrine lung tumors, LRP16, immunohistochemistry, prognosis
Introduction
Neuroendocrine lung tumors form a family of tumors with shared neuroendocrine differentiation features, but diverse clinical behaviors. According to the World Health Organization (WHO) classification of lung tumors [1], carcinoid tumors are low-grade neuroendocrine lung tumors consisting of typical carcinoid (TC) tumors and atypical carcinoid tumors (ATs), whereas small cell lung carcinomas (SCLCs) and large cell neuroendocrine carcinomas (LCNECs) form high-grade neuroendocrine subtype. High-grade neuroendocrine histology uniformly indicated poor prognosis regardless of its histology type [2]. Accurate discrimination of high-grade neuroendocrine carcinoma from other types of lung cancer is important for multimodal treatment [3,4]. However, histopathologic recognition of neuroendocrine differentiation, especially on small specimens, is not always reliable, as these tumors should be discriminated from undifferentiated nonsmall cell lung carcinomas, such as poorly differentiated lung adenocarcinoma or poorly differentiated squamous cell carcinoma, or other large cell carcinomas [5]. In fact, there is no immunohistochemical marker of high-grade neuroendocrine carcinoma. Thus, objective markers are needed to help in the pathologic diagnosis of those tumors [2]. It is worth searching for other factors involved in the pathogenesis of neuroendocrine lung tumors.
A growing body of evidence supports the concept that Leukemia related protein 16 gene (LRP16) has roles to play in cancer [6]. LRP16 localized on chromosome 11q12.1, is an important estrogen-responsive gene [7-11]. It has been found expressed at a high level in testicles, ovaries and mucosa of colon, at a moderate level in prostate, small intestine, spleen and thymus, and at a low level in peripheral blood leucocytes [8,9]. LRP16 is also a coactivator [12] of estrogen receptor alpha (ERα), and androgen receptor (AR), as well as being their target gene [6,11,12], and it may play an important role in ER signaling pathways. Nuclear factor kB (NF-kB)-mediated pathways have been widely implicated in cell survival, development and tumor progression. Recent evidence also indicates that LRP16 is not only a crucial regulator for NF-kB activation, but also suggest that LRP16 may be an important contributor to the aberrant activation of NF-kB in tumors [13]. LRP16 is altered in many kinds of primary or cultured carcinoma in the form of aberrant expression. Alterations of the LRP16 gene are common and have been found in primary tumors and cell lines of breast, stomach, colon and rectum, pancreas, kidney, ovary, endometrium and prostate. However, there is no research in neuroendocrine lung tumors. The role of LRP16 in neuroendocrine lung tumors has not been reported in the literature so far. In this study, we investigate the pathogenetic role of the expression of LRP16 protein in neuroendocrine lung tumors. It is hoped that this study will give information on the pathogenesis of the neuroendocrine lung tumors.
Materials and methods
Biopsy specimens
Paraffin embedded sections of ninety patients (71 men, 19 women) with histologically confirmed neuroendocrine lung tumors were obtained from the Department of Pathology, Chinese People’s Liberation Army (PLA) General Hospital (Beijing, China) from 2003 to 2013. The neuroendocrine lung tumors were classified as typical carcinoid (TC) tumors (n = 14) and atypical carcinoid tumors (ATs) (n = 20), whereas large cell neuroendocrine carcinomas (LCNECs) (n = 18) and small cell lung carcinomas (SCLCs) (n = 38) types according to the World Health Organization recommendations. Ethical approval for this study was not required by our institution as the experiments carried out did not relate to patient’s privacy, impairment or treatment.
Immunohistochemistry
A formalin-fixed, paraffin-embedded block was selected from each patient with neuroendocrine lung tumors for immunohistochemical study. Paraffin sections 4 µm thick were cut, dewaxed in xylene and rehydrated in a graded ethanol series. The sections were immersed in 3% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase activity and rinsed in running water. They were then immersed in boiling 0.01 M citrate buffer (pH 6.0) in a pressure cooker. The pressure cooker was sealed and brought to full pressure. The heating time was 2 min. After that, the pressure cooker was de-pressured and cooled under running water. The lid was then removed, and the hot buffer was flushed out with cold water from a running tap. The cooled sections were washed twice in phosphate buffered saline (PBS) before immunocytochemical staining. The primary antibodies were then added to the sections. The monoclonal rabbit antibody against human LRP16 protein was diluted 1:100 for 1 h. After exposure to primary antibody overnight, the sections were allowed to react with the polyperoxidase-anti-mouse/rabbit IgG for 20 min using the standard PV-6000 Polymer Detection System (Zymed). This is a non-biotin detection system; thus, non-specific staining due to endogenous biotin was avoided. The sections were then washed in water, counterstained with Mayer’s haematoxylin for 1 min at room temperature, dehydrated, cleaned and mounted. Paraffin blocks of human breast invasive ductal carcinoma tissues were used as the positive controls. Negative controls were sections treated the same as above but with omission of the primary antibody and replaced by 0.01M PBS.
Results
LRP16 protein was positive in 55.6% (50/90) of the neuroendocrine lung tumors by immunohistochemistry (Figures 1, 2, 3 and 4). The LRP16 protein was noted mainly in the cytoplasm and nucleus of neuroendocrine lung tumor cells (Figures 1, 2). There was a trend that LRP16 protein was mainly located in nucleus of cancer cells of high grade neuroendocrine lung tumors (Figures 3, 4). Adjacent normal or inflammatory bronchial and alveolar epithelial cells were negative for LRP16.
Figure 1.
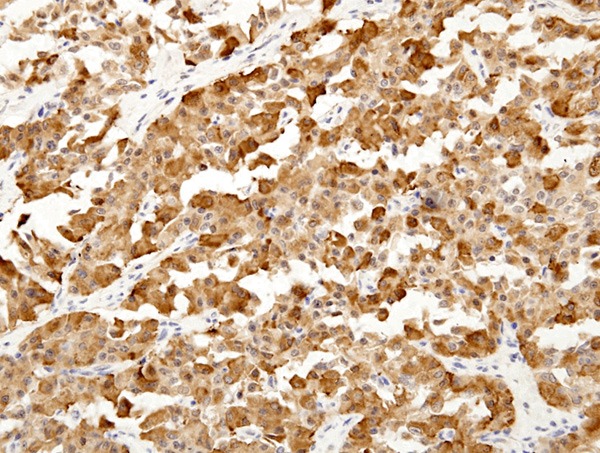
LRP16 expression in typical carcinoid tumors (TC). LRP16 protein is mainly localized in cytoplasm of tumor cells (LRP16 × 200).
Figure 2.
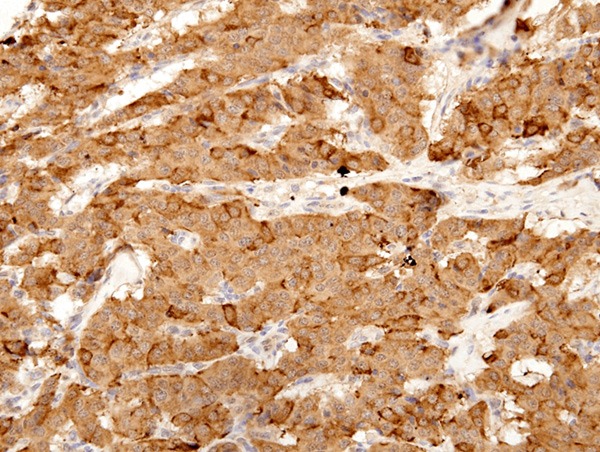
LRP16 expression in atypical carcinoid (ATs) tumors. LRP16 was stained positive in the cytoplasm of tumor cells. (LRP16 × 400).
Figure 3.
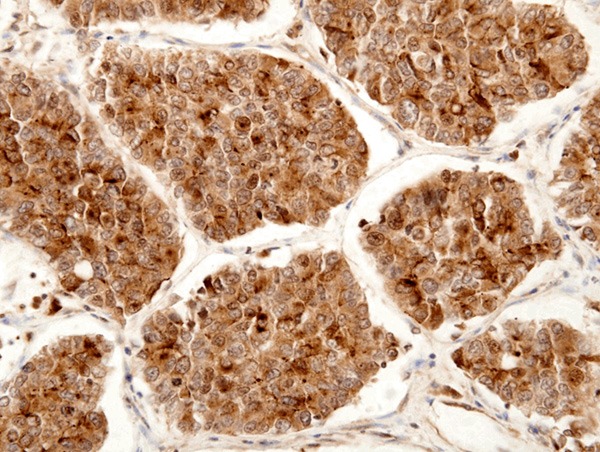
LRP16 expression in large cell neuroendocrine carcinomas (LCNECs). LRP16 was stained positive in the cytoplasm and nucleus of cancer cells. (LRP16 × 400).
Figure 4.
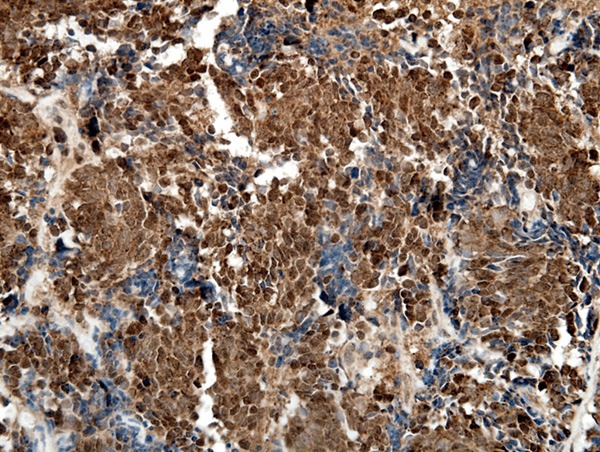
LRP16 expression in small cell lung carcinomas (SCLCs). LRP16 was stained positive mainly in the nucleus of cancer cells. (LRP16 × 400).
Relationships between LRP16 expression and histological grade, clinical stage and prognosis
LRP16 was positive in 50/90 (55.6%) cases of neuroendocrine lung tumors, in which, TC was 3 (21.4%), AC 8 (40.0%), LCNEC 12 (66.7%) and SCLC 27 (71.1%). There was a significant difference among the tumor types (P = 0.001, Table 1). In group of TNM stage, LRP16 was positive in 5/19 (26.3%) of stage I, 14/24 (58.3%) of stage II, 28/43 (65.1%) of stage III and 3/4 (75.0%) of stage IV, respectively. There was a significant difference among the TNM stages (P = 0.008, Table 1). In group of metastases, LRP16 was positive in 45/71 (63.4%) of tumors with metastasis and in 5/19 (20.8%) of those without. There was also a significant difference between tumors with and without metastasis (P = 0.004, Table 1). No significant difference in expression of LRP16 protein was found in groups of sex, age and tumor size (Table 1). Follow-up data showed that there was a significant difference in overall median survival time between the tumors with LRP16 positive expression (20.0 months) and those with negative expression (42.0 months) (Log rank = 25.799; P = 0.0001) (Figure 5). In the result of multivariate analysis by Cox Regression, LRP16 expression was an independent prognostic factor (P = 0.001).
Table 1.
The relationship between expression of LRP16 and clinicopathological features
| LRP16+ neuroendocrine tumors | LRP16- neuroendocrine tumors | P | |
|---|---|---|---|
| Age | |||
| < 60 | 24 | 20 | 0.612 |
| ≥ 60 | 26 | 20 | |
| Sex | |||
| Female | 7 | 12 | 0.666 |
| Male | 43 | 28 | |
| Tumor size (cm) | |||
| < 5 | 29 | 23 | 0.962 |
| ≥ 5 | 21 | 17 | |
| Tumor type* | |||
| TC | 3 | 11 | 0.001 |
| ATs | 8 | 12 | |
| LCNECs | 12 | 6 | |
| SCLCs | 27 | 11 | |
| Tumor stage | |||
| I | 5 | 14 | 0.008 |
| II | 14 | 10 | |
| III | 28 | 15 | |
| IV | 3 | 1 | |
| Metastases | |||
| Yes | 45 | 26 | 0.004 |
| No | 5 | 14 | |
| Survival | |||
| Median (months) | 20 | 42 | 0.0001 |
TC: typical carcinoid tumors;
ATs: atypical carcinoid tumors; LCNECs: large cell neuroendocrine carcinomas; SCLCs: small cell lung carcinomas.
Figure 5.
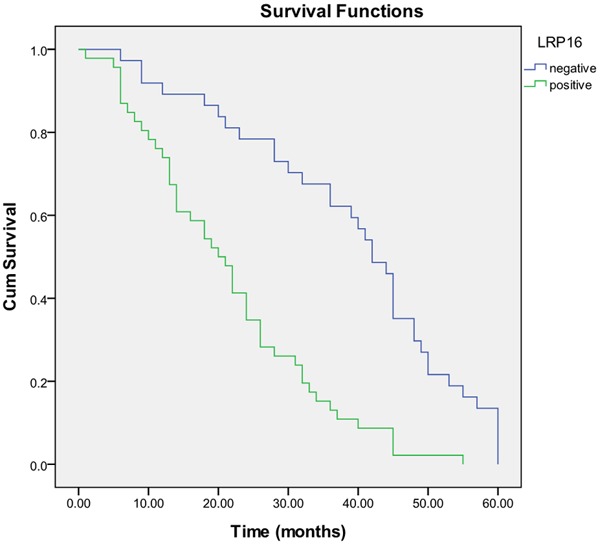
Kaplan-Meier survival analysis by LRP16 status (n = 90). The y-axis represents the percentage of patients; the x-axis, their survival in months. The green line represents LRP16-positive patients with a trend of worse survival than the blue line representing LRP16-negative patients (Log rank = 25.799; P = 0.0001. Overall median survival (OS) time was 20.0 months for the LRP16- positive group and 42.0 months for the LRP16-negative group.
Discussion
Expression of LRP16 has been found in different tissues in varying degree, including ovary, testicle, prostate, small intestine, spleen, thymus and stomach [7,8,11]. Some studies demonstrated that LRP16 gene was overexpressed as an ERa or AR coactivator and played an important role in the carcinogenesis and progression of hormone-dependent cancer in breast, endometrial and prostate [10,11]. In cancers of hormone-independent, overexpression of LRP16 was also detected in gastric and colorectal adenocarcinomas, compared with their matched normal tissues [14,15]. Recently it has been found LRP16 is as a crucial regulator for NF-kB activation indicating that LRP16 may be an important contributor to the aberrant activation of NF-kB in tumors [13]. The present study investigated the expression of LRP16 protein in neuroendocrine lung tumors by immunohistochemistry and the results showed that LRP16 was noted mainly in the cytoplasm and nucleus of tumor cells. LRP16 protein was overexpressed in 55.6% of the neuroendocrine lung tumors samples. Thus, LRP16 can be used an adjuvant marker to identify tumor cells in cases when the diagnosis of neuroendocrine lung tumors is in doubt. Although the detailed molecular mechanism involved in this phenomenon or process is unclear, the present study has potential clinical benefits. In this group of 90 neuroendocrine lung tumors, our results indicated that the LRP16 expression was corresponding to the histological type (P = 0.001), the clinical TNM stage (P = 0.008) and tumor metastasis (P = 0.004). It is suggested that LRP16 may play an important role in the evolution and development of neuroendocrine lung tumors. Follow-up data showed that there was a significant difference in overall median survival time between the tumors with LRP16 positive expression (20.0 months) and those with negative expression (42.0 months) (Log rank = 25.799; P = 0.0001) (Figure 3). In the result of multivariate analysis by Cox Regression, PHLDA1 expression was an independent prognostic factor (P = 0.001). The expression of Leukemia-related protein 16 (LRP16) could be detected by immunohistochemistry and it might be a useful molecular marker to predict the prognosis of neuroendocrine lung tumors.
Leukemia-related protein 16 (LRP16) was originally isolated from lymphocytes in order to identify a leukemia relapse-related gene, but there was no difference in patients with acute myeloid leukemia (AML) between the primarily diagnosed and the relapsed tumors [6], suggesting its function might be involved in carcinogenesis of tumors in hematopoietic and lymphoid tissues. A central role for Nuclear Factor-κB (NF-κB) in the pathogenesis of neuroendocrine lung tumors has been established and its is now clear that NF-κB controls a signaling network in neuroendocrine lung tumor cells that may stimulate tumor cell proliferation and confers resistance to apoptosis [16-20]. Our results in this study may imply LRP16 is involved in pathogenesis, or occurrence of neuroendocrine lung tumors. Although the mechanism is not clear to date, it is suggested that LRP16 may stimulate proliferation of neuroendocrine tumor cells by NF-κB pathway [13]. However, it still needs further basic research evidence.
Conclusions
Our study has suggested that expression of the LRP16 protein could be considered as a potential marker for diagnosis and prognosis of the neuroendocrine lung tumors.
Acknowledgements
The authors thank all colleagues in the Department of Molecular Biology, Chinese People’s Liberation Army General Hospital for their help and support with this study. All authors have contributed significantly, and all authors are in agreement with the content of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Travis WD. The concept of pulmonary neuroendocrine tumours. In: Travis WD, Brambilla E, Muller-Hermelink KH, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleura Thymus and Heart. Lyon: IARC; 2004. pp. 19–20. [Google Scholar]
- 2.Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, Tajima K, Nagai K. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J. Clin. Oncol. 2006;24:70–76. doi: 10.1200/JCO.2005.04.1202. [DOI] [PubMed] [Google Scholar]
- 3.Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, Bigiani N, Schirosi L, Casali C, Morandi U, Facciolongo N, Maiorana A, Bavieri M, Fabbri LM, Brambilla E. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J. Clin. Oncol. 2005;23:8774–8785. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- 4.Simon GR, Wagner H. Small cell lung cancer. Chest. 2003;123:259S–271S. doi: 10.1378/chest.123.1_suppl.259s. [DOI] [PubMed] [Google Scholar]
- 5.Sturm N, Rossi G, Lantuejoul S, Laverrière MH, Papotti M, Brichon PY, Brambilla C, Brambilla E. 34BetaE12 expression along the whole spectrum of neuroendocrine proliferations of the lung, from neuroendocrine cell hyperplasia to small cell carcinoma. Histopathology. 2003;42:156–166. doi: 10.1046/j.1365-2559.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhao YL, Han WD, Li Q, Mu YM, Lu XC, Yu L, Song HJ, Li X, Lu JM, Pan CY. Mechanism of transcriptional regulation of LRP16 gene expression by 17-beta estradiol in MCF-7 human breast cancer cells. J Mol Endocrinol. 2005;34:77–89. doi: 10.1677/jme.1.01628. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Han WD, Lou FD, Wang QS, Zhao Y, Caligiuri MA. Cloning of leukemia associated gene LRP16 in acute myeloid leukemia. Junyi Jinxiu Xueyuan Xuebao. 2000;21:81–84. [Google Scholar]
- 8.Han WD, Yu L, Lou FD, Wang QS, Zhao Y, Shi ZJ, Jiao HY, Zhou JJ. Cloning and expression characterization of the full length cDNA for a novel leukemia-associated gene LRP16. Zhongguo Shengwu Huaxue yu Fenzi Shengwu Xuebao. 2001;17:209–214. [Google Scholar]
- 9.Han WD, Lou FD, Yu L, Wang QS, Han XP, Li XJ. SAGE pattern of LRP16 gene and its expression in normal blood and leukemia cells. Junyi Jinxiu Xueyuan Xuebao. 2002;23:161–163. [Google Scholar]
- 10.Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L, Song HJ, Li M, Lu JM, Pan CY. Estrogen stimulates human breast cancer MCF-7 cell proliferation by up-regulation of LRP16 mRNA via activation of estrogen receptor-alpha. Zhonghua Neifenmi Daixie Zazhi. 2004;20:165–168. doi: 10.1677/erc.0.0100217. [DOI] [PubMed] [Google Scholar]
- 11.Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L, Song HJ, Li M, Lu JM, Zhao YL, Pan CY. Up-regulation of LRP16 mRNA by 17beta-estradiol through activation of estrogen receptor alpha (ERalpha), but not ERbeta, and promotion of human breast cancer MCF-7 cell proliferation: a preliminary report. Endocr Relat Cancer. 2003;10:217–224. doi: 10.1677/erc.0.0100217. [DOI] [PubMed] [Google Scholar]
- 12.Han WD, Zhao YL, Meng YG, Zang L, Wu ZQ, Li Q, Si YL, Huang K, Ba JM, Morinaga H, Nomura M, Mu YM. Estrogenically regulated LRP16 interacts with estrogen receptor alpha and enhances the receptor’s transcriptional activity. Endocr Relat Cancer. 2007;14:741–753. doi: 10.1677/ERC-06-0082. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Li Y, Li X, Ti D, Zhao Y, Si Y, Mei Q, Zhao P, Fu X, Han W. LRP16 integrates into NF-κB transcriptional complex and is required for its functional activation. PLoS One. 2011;6:e18157. doi: 10.1371/journal.pone.0018157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YZ, Zhao P, Han WD. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J Gastroenterol. 2009;15:4833–4837. doi: 10.3748/wjg.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi HQ, Zhao P, Han WD. Clinicopathological significance and prognostic value of LRP16 expression in colorectal carcinoma. World J Gastroenterol. 2010;16:1644–1648. doi: 10.3748/wjg.v16.i13.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haley KJ, Patidar K, Zhang F, Emanuel RL, Sunday ME. Tumor necrosis factor induces neuroendocrine differentiation in small cell lungcancer cell lines. Am J Physiol. 1998;275:L311–321. doi: 10.1152/ajplung.1998.275.2.L311. [DOI] [PubMed] [Google Scholar]
- 17.Li YD, Liu YP, Cao DM, Yan YM, Hou YN, Zhao JY, Yang R, Xia ZF, Lu J. Induction of small G protein RhoB by non-genotoxic stress inhibits apoptosis and activates NF-κB. J Cell Physiol. 2011;226:729–738. doi: 10.1002/jcp.22394. [DOI] [PubMed] [Google Scholar]
- 18.Kraus AC, Ferber I, Bachmann SO, Specht H, Wimmel A, Gross MW, Schlegel J, Suske G, Schuermann M. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–8695. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhu W, Chen Z, Luo L, Huang J, Zhang F, Li M, Guo Y, Guo L. Fibroblast growth factor-inducible 14 regulates cell growth and multidrug resistance of small-cell lung cancer through the nuclear factor-κB pathway. Anticancer Drugs. 2014;25:1152–1164. doi: 10.1097/CAD.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Guo Y, Jiang H, Zhang T, Jin C, Young CY, Yuan H. Differential regulation of MMPs by E2F1, Sp1 and NF-kappa B controls the small cell lung cancer invasive phenotype. BMC Cancer. 2014;14:276. doi: 10.1186/1471-2407-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]


