Abstract
The surface marker PROM1 is considered one of the most important marker of tumor-initiating cells, and its high expression is believed to be an adverse prognostic factor in gliomas, medulloblastoma and in other malignancies. The aims of our research were to explore the expression profile of the PROM1 in non-small cell lung cancer (NSCLC) and to assess its possible role as a prognostic factor. The protein expression profiles were determined via immunohistochemical staining assay. The clinical prognostic values of protein expression were investigated with univariate and multivariate survival analysis. The quantitative variable PROM1 expression was dichotomized according to the best cutoff value obtained by the receiver operating characteristics (ROC) analysis. The protein level of PROM1 of NSCLC was higher compared with normal tissues, and the survival analysis demonstrated the positive membrane expression and combination of membrane/cytoplasm groups of PROM1 had worse prognosis than those negative expression groups. Also, multivariate Cox regression analysis showed membrane expression of PROM1 and lymph node invasion were the independent prognostic factors. The expression of PROM1 was significantly higher than normal tissue, and high levels of PROM1 membrane expression and combination of membrane/cytoplasm expression were associated with adverse prognosis.
Keywords: PROM1, CD133, non-small cell lung cancer, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide and the incidence increased year by year. Non-small cell lung cancer (NSCLC) is the main type of lung cancer. CD133, a member of prominent family, formerly known as PROML-1 or AC133, was first discovered as a pentaspan transmembrane glycoprotein of murine neuroepithelial stem cells located in plasma membrane protrusions [1]. CD133 is found in embryonic stem cells, normal tissue stem cells, stem cell niches, and circulating endothelial progenitors as well as cancer stem cells. Its antigen has been identified as a putative stem cell marker in normal and malignant brain tissues. According to the cancer stem cell hypothesis, PROM1 (CD133) - positive cells determine long-term tumor growth and, therefore, are suspected to influence clinical outcome [2]. Although most researchers had studied PROM1 expression in correlation with prognosis and clinicopathological variables, that indicated high PROM1 expression is associated with decreased survival in a variety of human tumors, including brain, liver, stomach, endometrium, ovary, colorectum, gliomas [2] and medulloblastoma [3], to date, the relationship between PROM1 and NSCLC lack of in-depth study. Thus, in this study, we aimed to evaluate the expression profile of the PROM1 in NSCLC with use of immunohistochemical staining assay and to determine its possible prognostic significance.
Material and methods
Tissue collection
One hundred and eighty-three surgically resected primary NSCLC cases, during the period from 2007 to 2008, were obtained from the archives of the Pathology Department of West China Hospital, Sichuan University, 175 cases of NSCLC patients included in survival analysis at last. A total of 96 normal control tissue samples were randomly taken from the normal tissues that adjacent to the tumor tissues according to the surgically resected. Data on stage were according to the International Union Against Cancer’s tumor-node-metastasis system, and differentiation and histological type were according to the World Health Organization classification for NSCLC [4,5]. The tissue specimens consisted of 91 adenocarcinomas (ADC), 74 squamous cell carcinomas (SCC), and 10 other types. All the tissues were fixed in 10% formalin immediately and embedded with paraffin within 12 to 24 hours post resection. All patients were adjuvant therapy-free before surgical resection and underwent standard therapeutic procedure after surgical resection according to the Clinical Oncology Information Network guidelines for nonsurgical management of lung cancer [6]. Institutional review board approval for this research was obtained from West China Hospital. All the participants provide their verbal informed consent to participate in this study. Because all the patients came from different parts of China, it is really difficult to let them written consent, but we have their contact information, so we ask them if they agree to participate in our research by telephone. If “yes”, we included these patients’ tissues. The ethics committees in our hospital (Medical Ethics Committee of Sichuan University) approve this consent procedure.
Antibody preparation
All the cases of lung cancer tissue samples underwent Envision two-step immunohistochemical staining. Primary antibodies used as follows: Prominin-1 mouse monoclonal antibody (17A6.1, 1:100 dilution, #MAB4399, Millipore). Secondary antibodies of Dako EnVision were purchased from Dako Corporation. All paraffin tissues were made of 4 μm slices. Envision method according to kit instructions, and antigen retrieval was done by heating Tris/ethylenediaminetetraacetic acid retrieval solution (pH 6.0) at 95°C for 45 minutes in water bath. The method of immunohistochemical staining according to the literature [7].
Immunohistochemical scoring
We used dual-rate semi-quantitative method according to the literature [7], and the scores composed of stained area and staining intensity of the tumor cells. Evaluation of sections was carried out by 2 pathologist (Drs. DN Liang and XS Qiu) without the knowledge of clinical information as previous described. The fraction score was defined as the average of 10 randomly selected fields by light microscope: 0, no tumor cell stained; 1, <20% of cells stained; 2, 20% to 50% of cells stained; and 3, >50% of cells stained. The intensity score was defined as follows: 0, no appreciable staining in the tumor cells; 1, barely detectable staining in the tumor cells; 2, readily appreciable brown staining; and 3, dark brown staining in tumor cells. The total score was calculated by multiplying the fraction score and the intensity score, producing a total range from 0 to 9. Then the quantitative variable PROM1 expression was dichotomized according to the best cutoff value obtained by the receiver operating characteristics (ROC) analysis (Supplementary 1: ROC analysis).
Statistical analysis
The association of clinical characteristics with status of protein expression was determined by Pearson chi-square test. The Kaplan-Meier method was used to estimate univariate survival. The log-rank test and univariate Cox regression analysis were used to compare survival distributions between positive and negative staining groups. Independent prognostic factors of survival were identified with a multivariate Cox regression analysis. “PROM1 expression” was dichotomized by the ROC. P<0.05 (2-side) was considered to be statistically significant. Data analysis and summarization were conducted using SPSS 17.0 for Windows (SPSS Inc., Chicago, III) [7] and Graphpad. prism. 6. x. C.
Results
Comparison of expression levels of proteins in non-small cell lung cancers versus normal control tissue
The positive expression of PROM1 could be detected in the cell membrane, cytoplasm, nuclear membrane (only a few cases) and nucleus. Comparison of PROM1 expression in NSCLC with normal controls is shown in Table 1. Protein level of PROM1 was significantly increased (all P<0.001) in NSCLC compared with normal controls. Representative examples of PROM1 expression at different locations are shown in Figure 1.
Table 1.
Expression of PROM1 protein in NSCLC and normal control group
| Location | Total (No.) | Positive expression (No. %) | Negative expression (No. %) | P value |
|---|---|---|---|---|
| Normal tissue | 96 | 14 (14.6%) | 82 (85.4%) | Ref. |
| Tumor membrane | 175 | 81 (46.3%) | 94 (53.7%) | <0.001a |
| Tumor cytoplasm | 183 | 107 (58.5%) | 76 (41.5%) | <0.001a |
| Tumor nucleus | 28 | 14 (50%) | 14 (50%) | <0.001a |
Statistically significant.
Figure 1.
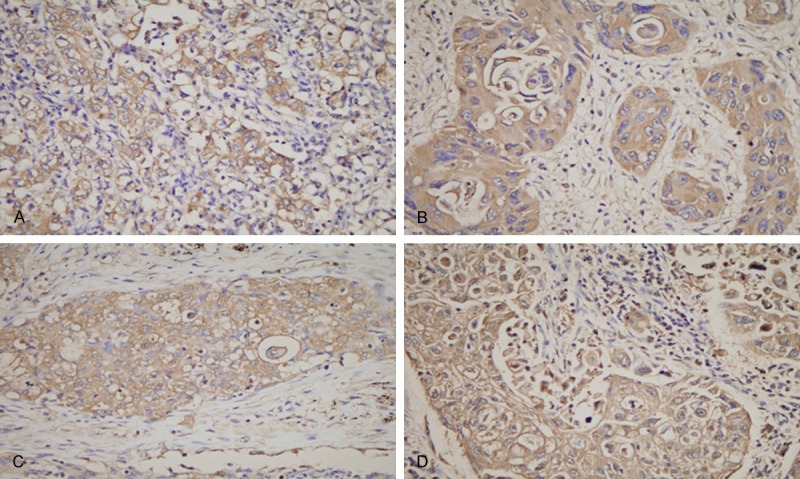
Representative examples of PROM1/CD133 expression at different locations. A. Positive expression of tumor cell membrane; B. Positive expression of tumor cell cytoplasm; C. Positive expression of tumor cell nuclear membrane; D. Positive expression of tumor cell nuclear. Red arrows shown. Original magnification, × 400.
Relationship between protein expression profiles and clinical characteristics of NSCLC
The relationship between protein phenotypes and clinical variables was estimated by the univariate analysis (Table 2). The membrane expression of PROM1 was associated with gender (P = 0.018). As it shown, positive membrane expression of PROM1 was much more in men than in women.
Table 2.
Association between clinical variables and PROM1 (CD133) expression
| Variable | Membrane expression | P value | Cytoplasm expression | P value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Positive | Negative | Positive | Negative | |||
| Gender | ||||||
| Male | 67 | 63 | 0.018a | 76 | 54 | 0.391 |
| Female | 14 | 31 | 23 | 22 | ||
| Age | ||||||
| ≤60 | 36 | 55 | 0.063 | 46 | 45 | 0.094 |
| >60 | 45 | 39 | 53 | 31 | ||
| Histological type | ||||||
| SCC | 34 | 40 | 0.665 | 45 | 29 | 0.128 |
| ADC | 41 | 50 | 46 | 45 | ||
| Others | 6 | 4 | 8 | 2 | ||
| Differentiation | ||||||
| Poor | 26 | 28 | 0.168 | 32 | 22 | 0.850 |
| Moderate | 27 | 21 | 27 | 21 | ||
| Well | 28 | 44 | 39 | 33 | ||
| Tumor size | ||||||
| T1 | 13 | 18 | 0.992 | 16 | 15 | 0.213 |
| T2 | 40 | 43 | 47 | 36 | ||
| T3 | 14 | 15 | 21 | 8 | ||
| T4 | 14 | 18 | 15 | 17 | ||
| Lymph node invasion | ||||||
| N0 | 43 | 53 | 0.500 | 53 | 43 | 0.190 |
| N1 | 21 | 16 | 25 | 12 | ||
| N2 | 16 | 24 | 19 | 21 | ||
| N3 | 1 | 1 | 2 | 0 | ||
| Distant metastasis | ||||||
| M0 | 77 | 90 | 0.829 | 103 | 72 | 0.619 |
| M1 | 4 | 4 | 4 | 4 | ||
| Clinical stage | ||||||
| I | 29 | 35 | 0.587 | 37 | 27 | 0.319 |
| II | 22 | 18 | 27 | 13 | ||
| III | 26 | 37 | 31 | 32 | ||
| IV | 4 | 4 | 4 | 4 | ||
ADC, Adenocarcinoma; SCC, Squamous cell carcinoma;
Statistically significant.
Association of clinical characteristics with survival
Clinical pathology data was missing in a small fraction of the cases that were excluded from the survival analysis. The relationship between the survival of patients and clinical pathological features for evaluable cases is shown in Table 3. It was indicated that the lymph node invasion and clinical stage were associated with survival (all P<0.05).
Table 3.
Relation between clinical variables and survival
| Variable | Category | Available No. for Survival (Died/Total) | Median survival (Mean/mo.) | Log-rank P |
|---|---|---|---|---|
| Gender | Male | 71/130 | 45/43.856 ± 2.384 | 0.064 |
| Female | 17/45 | -/51.978 ± 3.844 | ||
| Age | <60 | 40/84 | 46/44.253 ± 2.859 | 0.451 |
| ≥60 | 48/91 | -/47.786 ± 2.915 | ||
| Histological type | ADC | 48/91 | 46/45.253 ± 2.769 | 0.832 |
| SCC | 34/74 | -/46.459 ± 3.287 | ||
| Others | 6/10 | 46/47.300 ± 6.989 | ||
| Differentiation | Poor-Moderate | 59/121 | -/46.121 ± 2.500 | 0.727 |
| Well | 29/54 | 49/45.556 ± 3.545 | ||
| Tumor size | T1 | 16/31 | 47/44.548 ± 4.917 | 0.967 |
| T2 | 40/83 | -/46.910 ± 2.978 | ||
| T3 | 15/29 | 54/46.000 ± 4.921 | ||
| T4 | 17/32 | 46/44.281 ± 4.693 | ||
| Lymph node invasion | N0 | 36/96 | -/52.271 ± 2.629 | <0.001a |
| N1 | 24/37 | 36/40.378 ± 4.190 | ||
| N2 | 26/40 | 35/37.650 ± 4.354 | ||
| Distant metastasis | M0 | 82/167 | -/46.608 ± 2.089 | 0.094 |
| M1 | 6/8 | 15/30.375 ± 7.756 | ||
| Clinical stage | I | 25/64 | -/51.672 ± 3.229 | 0.034 a |
| II | 19/40 | -/48.025 ± 4.172 | ||
| III | 38/63 | 37/40.571 ± 3.447 | ||
| IV | 6/8 | 15/30.375 ± 7.756 |
ADC, Adenocarcinoma; SCC, Squamous cell carcinoma; No, number; mo, month;
Statistically significant.
Comparison of survival among different status of single and combined proteins expression
In the total 175 cases of NSCLC patients, the 5-year survival rates of PROM1 with membrane positive and negative expression were 24.7% and 71.3% respectively, the median survival time were (35.012 ± 2.699) months and (55.367 ± 2.657) months, the difference was statistically significant (P<0.001). The 5-year survival rates of PROM1 with cytoplasm positive and negative expression were 43.4% and 57.9% respectively, the median survival time were (43.081 ± 2.713) months and (49.681 ± 3.063) months, there has no statistical difference (P = 0.1170). Additionally, The 5-year survival rates of PROM1 with nuclear positive and negative expression were 61.5% and 40.0% respectively, the median survival time were (50.231 ± 7.414) months and (37.067 ± 7.321) months, there has no statistical difference (P = 0.1288). Compared the 5-year survival rate between the expression was positive with both membrane and cytoplasm and the expression was negative with both membrane and cytoplasm, the rates were 32.2% and 76.8% respectively, with the median survival time were (39.119 ± 3.313) months and (59.032 ± 3.096) months, the difference was statistically significant (P<0.001) (Figure 2). Furthermore, we assessed the prognosis value of expression of proteins (Table 4).
Figure 2.
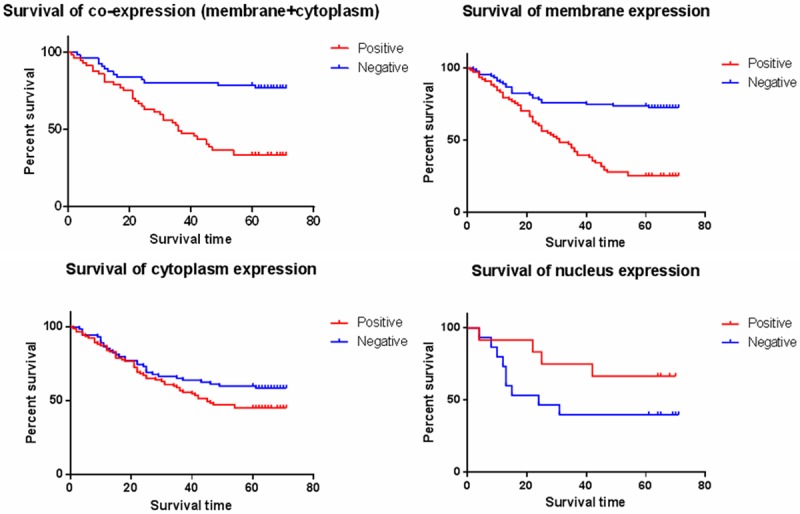
Relation between protein expression and survival are shown. Kaplan-Meier curves of proteins compare positive (red lines) with negative phenotype (blue lines).
Table 4.
Relation between protein expression and survival (Univariate analysis)
| Protein | P/N | HR | 95% CI | P value |
|---|---|---|---|---|
| Membrane expression | P vs. N | 3.521 | 2.232-5.556 | <0.001a |
| Cytoplasm expression | P vs. N | 1.475 | 0.955-2.278 | 0.080 |
| Nuclear expression | P vs. N | 0.526 | 0.175-1.580 | 0.252 |
| Co-expression of membrane and cytoplasm | P vs. N | 3.922 | 2.092-7.407 | <0.001a |
P/N indicates positive/negative; HR, hazard ratio; CI, confidence interval;
Statistically significant.
Multivariate Cox regression analysis for prognosis
According to the results above, by using multivariate Cox regression model, we evaluated whether membrane expression of PROM1, lymph node invasion and clinical stage could have prognosis value in the assessment of NSCLC (Table 5). The analysis revealed that membrane expression of PROM1 and lymph node invasion were independent prognosis factors for NSCLC development (log-rank, all P<0.05) (Supplementary 2: Multivariate Survival Analysis (Cox Regression Model)).
Table 5.
Multivariate survival analysis (Cox regression model)
| Variants | HR | 95% CI | P value |
|---|---|---|---|
| Lymph Node Invasion | 9.709 | 1.805-52.632 | 0.008a |
| Clinical Stage | 1.217 | 0.399-3.704 | 0.730 |
| Membrane expression | 3.774 | 2.374-6.061 | <0.001a |
HR, hazard ratio; CI, confidence interval;
Statistically significant.
Discussion
Our research suggests that there is a significant relationship between high membrane protein levels of PROM1 and the prognosis of NSCLC, finding that PROM1 expression is a potential predictor of survival independent to clinical variables.
During the past years, great advances have been made in developing therapeutic approaches for NSCLC, but the best method to stratify patients with NSCLC into prognostic risk groups and stratify by the optimal treatment is unknown. Cancer stem cell (CSC), also called tumor-initiating cells (TICs), have self-renewal capacity and can produce heterogeneity of tumor cells, it may cause tumors aggressive too [8,9]. The expression of the PROM1 (CD133) gene, which encoding a 5 transmembrane domain protein, has the feature that identifies brain TICs [10,11], it also has been used as a marker for purifying CSC in many other solid tumors, including liver, colon, pancreas, prostate and melanoma [12-16]. In addition, high PROM1 expression is associated with poor survival in various human solid tumors, including colon, prostate, etc. However, these studies are limited by relatively small sample size and retrospective study design and thus limit definitive conclusions. Thus, targeting and monitoring PROM1 may lead to significant advances in outcome prediction and cancer therapy.
This study via immunohistochemical analysis found that expression level of PROM1 was significantly increased in NSCLC compared with normal controls. The membrane expression of PROM1 was related to the gender, and the positive expression in men was significantly higher than women (all P<0.05). To define a cutoff value that could dichotomize the range of quantitative variables of PROM1 expression, a ROC curve was calculated. In this research, combined with the results of immunohistochemical staining and the 5-year median survival time, found that the 5-year median survival time of membrane positive group of PROM1 and combination of membrane/cytoplasm positive were obviously shorter than the negative groups (P< 0.05). In addition, based on the results before, we analyzed whether the membrane expression of PROM1, lymph node invasion and clinical stage were as the independent prognostic factors in NSCLS. The results indicated that only PROM1 membrane expression and lymph node invasion indeed as the independent prognostic factors in NSCLS (log-rank, all P<0.05).
As we all known that the mechanisms regulating tumorigenesis and progression is multifactorial, so it’s hard and insufficiently to use only one biomarker to optimally stratify patients with NSCLC. But, we consider that PROM1 may play a crucial role in determining the patient’s outcomes and may be helpful in making treatment strategies.
In summary, the expression of PROM1 was significantly higher in tissues of patients with NSCLC than in normal tissues, and high levels of PROM1 membrane expression and combination of membrane/cytoplasm expression were associated with adverse prognosis. However, in order to incorporate PROM1 expression into risk classification system to be used in the clinical setting, the results of our study need to be confirmed in larger prospective researches.
Acknowledgements
We thank the patients and their families for participating in this study with patience and cooperation, and technicians in the Department of Pathology, West China Hospital, Sichuan University, who assisted us in collecting the tissue samples. This work was supported by the National Natural Science Foundation of China (No. 81372504); the Science and Technology Support Program of Science and Technology Department of Sichuan Province (2014SZ0148); the International Cooperation Program of Science and Technology Department of Sichuan Province (2014AA022202-2).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Fargeas CA, Corbeil D, Huttner WB. AC133 antigen, CD133, prominin-1, prominin-2, etc. : prominin family gene products in needof a rational nomenclature. Stem Cells. 2003;21:506–508. doi: 10.1634/stemcells.21-4-506. [DOI] [PubMed] [Google Scholar]
- 2.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 3.Raso A, Mascelli S, Biassoni R, Nozza P, Kool M, Pistorio A, Ugolotti E, Milanaccio C, Pignatelli S, Ferraro M, Pavanello M, Ravegnani M, Cama A, Garrè ML, Capra V. High levels of PROM1 (CD133) transcript are a potential predictor of poor prognosis in medulloblastoma. Neuro Oncol. 2011;13:500–508. doi: 10.1093/neuonc/nor022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology & Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. [Google Scholar]
- 5.International Union Against Cancer. TNM Classification of Malignant Tumors. 6th edition. New York, NY: Wiley & Sons; 2002. [Google Scholar]
- 6.The Royal College of Radiologists Clinical Oncology Information Network. Guidelines on the non-surgical management of lung cancer. Clin Oncol (R Coll Radiol) 1999;11:S1–S53. [PubMed] [Google Scholar]
- 7.Liu D, Huang Y, Chen B, Zeng J, Guo N, Zhang S, Liu L, Xu H, Mo X, Li W. Activation of mammalian target of rapamycin pathway confers adverse outcome in nonsmall cell lung carcinoma. Cancer. 2011;117:3763–3773. doi: 10.1002/cncr.25959. [DOI] [PubMed] [Google Scholar]
- 8.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Biophys Res Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- 10.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 11.Corbeil D, Röper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 12.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 14.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 15.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 16.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


