Abstract
Endometrial carcinoma is the most common gynecological malignancy among women worldwide. Although treatment for EC has improved with the introduction of Paclitaxel (Tax) chemotherapy, the majority of patients will develop resistance to the treatment, leading to poor prognosis. One of the causes of chemoresistance is the increased ability to undergo autophagy. In this study, we identified that miR-218 was significantly down-regulated in Tax-resistant EC cells compared to the non-drug resistant cell lines, and overexpression of miR-218 sensitized paclitaxel resistant EC cells to paclitaxel. Moreover, we demonstrated that miR-218 directly binds to the 3’-UTR of HMGB1 gene. HMGB1 was upregulated in paclitaxel resistant EC cells, it mediated autophagy and contributed to chemotherapy resistance in endometrial carcinoma in vitro. HMGB1-mediated autophagy could be suppressed by miR-218 overexpression in Tax resistant EC cells. In summary, we determined the targeting role of miR-218 to HMGB1 and the regulation of miR-218 on the HMGB1-mediated cell autophagy during chemotherapy resistance in endometrial carcinoma cells. These results reveal novel potential role of miR-218 against chemotherapy resistance during the treatment of endometrial carcinoma.
Keywords: miR-218, endometrial carcinoma, chemoresistance, autophagy, HMGB1
Introduction
Endometrial carcinoma is one of the most common female malignancies worldwide [1]. Presently, surgery combined with adjuvant or postsurgical chemotherapy is the predominant treatment strategy for endometrial cancer. Paclitaxel, one of the most promising antitumor agents for women with endometrial cancer, makes a great contribution to the improvement of the life quality and overall survival of patients bearing endometrial cancers [2]. Unfortunately, some patients observed the paclitaxel resistance and suffered cancer recurrence or metastasis. Therefore, it is crucial to understand the mechanism of paclitaxel-induced resistance to obtain the better clinical application of paclitaxel.
Drug resistance can be intrinsic or acquired and arises from a variety of factors, including genetic differences in somatic cell tumors and individual patient-specific variations [3]. In culture, drug-resistant of cancer cells have been shown to associate with different mechanisms, such as autophagy, apoptosis etc. [3]. Recently, an increasing number of studies showed that phosphorylated cofilin 1 [4], Akt isoforms [5], Mdm2 [6] and cyclophilin A [2] associated with drug resistance in endometrial cancer cell line and placental leucine aminopeptidase (P-LAP) [7] as a determinant of chemoresistance in endometrial carcinoma cells.
MicroRNAs (miRNAs) are an abundant group of endogenous non-coding RNAs (about 22 nt) that bind to target mRNAs mainly at their 3’-UTR [8]. Recent progress in cancer biology has shown that microRNAs as important gene regulators in human genomes have been found to dysregulate proliferation in types of human cancer [9,10], including endometrial carcinoma [11,12]. Recently, the association between miRNA expression and the resistance to anticancer drug in cancer is closely noticed, which is regarded for predicting chemosensitivity in cancers. miR-375 was up-regulated in acquired Tax resistance in cervical cancer [13] and overexpression of miR-17-5p sensitized paclitaxel resistant lung cancer cells to paclitaxel induced apoptotic cell death [14].
MiR-218, as a candidate tumor suppressor, greatly down-regulated in several types of cancer, such as cervical cancer [15], colon cancer [16], lung adenocarcinoma [17], prostate cancer [18] etc. In this study, we were interested to examine the role of miR-218 in the development of Tax resistance in EC. Our data demonstrated that HMGB1, one of the most important regulators of cellular autophagy, was a direct target of miR-218 in EC cells. MiR-218 played a critical role in the development of Tax resistance by regulating cellular autophagy.
Material and methods
Cell culture
Human endometrial carcinoma cell lines RL95-2 and Ishikawa were used in this work. RL95-2 cells (Shanghai Cell Bank, China) were routinely cultivated in complete growth medium comprising Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Invitrogen, Carlsbad, CA, USA), 1% penicillin-streptomycin (Invitrogen), and 0.005 mg/mL insulin (Sigma-Aldrich, St. Louis, MO, USA). Ishikawa (ISK) cells were stored in our laboratory and maintained in RPMI-1640 (HyClone) containing 1% penicillin- streptomycin (Invitrogen) with 10% FBS (Gibco Invitrogen). The cells were incubated at 37°C, with 5% CO2. miR-218 mimics, miR-218 inhibitor and their negative control were transiently transfected into endometrial carcinoma cells using Lipofectamine 2000 reagents (Invitrogen), following the manufacturer’s instructions. Cells were harvested after transfection for 48 h.
Tax treatment of cancer cell in vitro
Cells were seeded and allowed to attach overnight. On the second day, freshly prepared Tax was added. The concentration of Tax was 10 µmol/L. The chemotherapeutical-treated cells were further cultured for cell proliferation assay at indicated time points or for western blot assay for 72 h.
Cell viability assay
Cells were seeded at 1000 per well in 96-well plates. Cell viability was evaluated using a Cell Counting Kit (CCK-8) according to the manufacturer’s instruction at indicated time. All results were from three separate experiments with six replicates.
mRNA and miRNA extraction and analysis
The total RNA (including miRNA) was extracted by Trizol (Invitrogen) according to the manufacturer’s instructions. For miRNA reverse transcription (RT), special miR-218 RT forward primer (5’-AGAAGTGCTCAGAGAGGTGGA-3’) and reverse primer (5’-CCTTTGGGAGGGATATAGGTT-3’) were used. RNU6B (U6 small nuclear B non-coding RNA) was used as an internal control. The forward primer of U6 was: 5’-AGGGGCCGGACTCGTCATACT-3’) and reverse primer (5’-GGCGGCACCACCATGTACCCT-3’). For the RT of total RNA, oligo (dT) was used as a common primer and β-actin was used as an internal control. The real-time polymerase chain reaction (PCR) was performed by the SYBR Green PCR master Mix (Applied Biosystems, Foster City, USA) according to the following conditions: 95°C for 5 min followed by 35 cycles of amplification at 95°C for 10 s, 58°C for 30 s, and 72°C for 30 s.
Western blot analysis
Whole cell extracts were prepared with a cell lysis reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manual, and then, the protein was quantified by a BCA assay (Pierce, Rockford, IL, USA). Then, the protein samples were separated by SDS-PAGE (10%) and detected by Western blot using polyclonal (rabbit) anti-LC-3II, HMGB1 and anti-Beclin1 antibody (Santa Cruz Bio-technology, Santa Cruz, CA, USA). Goat anti-rabbit IgG (Pierce, Rockford, IL, USA) secondary antibody conjugated to horseradish peroxidase and ECL detection systems (SuperSignal West Femto, Pierce) were used for detection.
Luciferase activity assay
According to the expression of miR-218 with the HMGB1 3’-UTR, 3’-UTR sequence or three copies of the mutated 3’-UTR sequence of HMGB1 were amplified by PCR from human genomic DNA and inserted into psiCHECK-2 vector immediately downstream from the stop codon of luciferase as described previously [19]. Endometrial carcinoma cell RL95-2 and ISK in six-well plates were transfected with 1 μg of the firefly luciferase report vector, 1 h post-transfection with 25 nM of miR-218 mimics, miR-218 inhibitor or miR control. At 24 h post-transfection, firefly luciferase activities were measured consecutively using luciferase assays (Promega, Madison, WI, USA).
Plasmid construction and dual-luciferase reporter assay
For miR-218 precursor transfection, RL95-2 and Ishikawa cells were cotransfected with miR-218 and HMGB1/mut-HMGB1 dual-luciferase miRNA target expression vectors using Lipofectamine 2000 (Invitrogen). Luciferase activity was assessed using the Dual-Luciferase Reporter Assay System (Promega, 48 hours after transfection).
TALEN design and construction
The exon and its immediate flanking regions of HMGB1 (high mobility group box-1 protein) were scanned for putative TALEN binding pairs using the TAL Effector Nucleotide Targeter 2.0 [20]. The site: TCACAGCCATTGCAGTACA was chose. Performing as previously, the binding pairs HMGB1-L and HMGB1-R were respectively assembled into the pTALEN-v2-L and pTALEN-v2-R backbones, yielding pTALEN-HMGB1-L and pTALEN-HMGB-R. RL95-2 and Ishikawa cells were transfected with a mixture of pTALEN-HMGB1-L, pTALEN-HMGB1-R (untreated cells were used as control). Cells were trypsinized and resuspended after transfection for 4 days. The transfected cells were expanded. To confirm HMGB1 disruption, western blot and PCR were performed. Whole cell extracts were analyzed by western blot, and the targeted exon was PCR-amplified from genomic DNA isolated from individual clones. The following day, medium were refreshed and grown an additional 24 h prior to harvesting for further analysis.
Statistical analysis
Data in the present study were represented as means ± SD of at least three independent experiments except as indicated. Student’s unpaired t test was used for comparison between two groups. Values were considered significantly different when P < 0.05.
Results
MiR-218 is down-regulated in Tax-resistant endometrial carcinoma cells
We prepared Tax-resistant endometrial carcinoma cell (RL95-2 and Ishikawa) from Tax sensitive cells by exposing cells to Tax for 72 hours [13]. We further validated the array data for miR-218 by qRT-PCR. The qRT-PCR assay revealed that miR-218 is down-regulated in Tax-resistant cells compared to Tax sensitive cells. Figure 1A showed a ~71.6% down-regulation in the relative miR-218 expression level in RL95-2 cells. Ishikawa-Tax cells exhibited almost ~66.5% down-regulation of relative miR-218 expression compared to that of Tax sensitive Ishikawa cells, indicating association between miR-218 and Tax resistance was not cell line specific.
Figure 1.
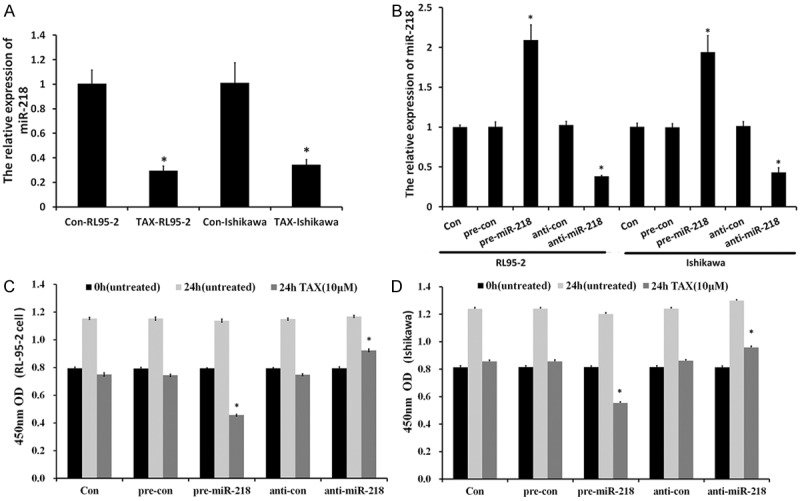
miR-218 affected proliferation of anticancer drug-treated endometrial carcinomas. (A) miR-218 is down-regulated in Tax-treated endometrial carcinoma cells. The expression of miR-218 was measured by qRT-PCR. (B) RL95-2 and Ishikawa cells with overexpressed/silenced miR-218. miR-218 overexpression decreased RL95-2 (C) and Ishikawa (D) cell growth. Cell viability was measured using a CCK-8 kit at indicated time point. Data were means ± SD from three independent experiments performed in sextuple.
Overexpressed miR-218 sensitized EC cells to paclitaxel
To explore the biological roles of miR-218 in Tax-treated endometrial carcinoma cells, we stably overexpressed miR-218 in RL95-2 and Ishikawa cells by transfecting miR-218 mimics and miR-218 inhibitor and then selected by puromycin. Also, we established mimics control (pre-con), inhibitor control (anti-con) and untransfected (con) groups as control. The overexpression/silencing of miR-218 in endometrial carcinoma cells were confirmed by RT-PCR (Figure 1B). The stable cells were seeded in 96-well plates and measured by CCK-8 kit for cell growth at indicated time points. Overexpression of miR-218 exhibited much lower cell viability (Figure 1). As shown in Figure 1C, overexpressed-miR-218 decreased Tax-treated RL95-2 cells viability (0.626 ± 0.007) compared with pre-con group (0.744 ± 0.005), while miR-218 silencing increased Tax-treated RL95-2 cells viability (0.824 ± 0.014) compared with anti-con group (0.748 ± 0.003). Similar results were obtained in Tax-treated Ishikawa cells (Figure 1D). In conclusion, our results demonstrated that overexpression miR-218 inhibited the proliferative capability of EC cell post-anticancer drug treatment, thus sensitizing the cancer cells to those drugs.
MiR-218 targets 3’ UTR of HMGB1 and suppresses the expression of HMGB1
High mobility group box 1 (HMGB1), a chromatin-binding nuclear protein, plays a role in facilitating autophagy following cytotoxic insults including starvation [21-23]. Recent studies showed that HMGB1 was direct target of miR-218 which was validated in cancer cells before [19]. As shown in Figure 2A and 2B, co-transfection of miR-218 suppressed the luciferase activity of the reporter containing wild-type HMGB1 3’ UTR sequence, but failed to inhibit that of mutated HMGB1 by dual-luciferase reporter assay. These data suggested that miR-218 could directly target the 3’-UTR sequences of HMGB1. As shown in Figure 2C, HMGB1 protein is up-regulated in Tax-treated endometrial carcinoma cells, and the up-regulation was also demonstrated in HMGB1 mRNA level (Figure 2E). Figure 2F revealed that HMGB1 was downregulated by miR-218 overexpression and upregulated by miR-218 silencing in protein level. These results agree with the fact that miR-22 regulates HMGB1 by targeting the 3’-UTR of it and suppressing its translation.
Figure 2.
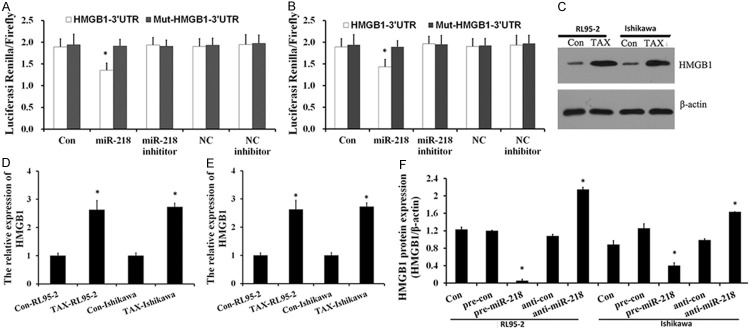
miR-218 targets 3’ UTR of HMGB1 and suppresses the expression of HMGB1. (A, B) Luciferase reporter assay with co-transfection of wild-type or mutant HMGB1 and miR-218 mimics or miR-218 inhibitor or mimics-control or inhibitor-control or blank control in RL95-2 (A) and ISK (B) cells. Error bars represent ± S.E. and *, P < 0.05 versus control. (C, D) Western blot analysis was performed to examine the effects of Tax-treat on expression of HMGB1. HMGB1 is up-regulated in Tax-treated endometrial carcinoma cells. (E) qRT-PCR analyses were performed to determine the mRNA levels of HMGB1. (F) The decreased expression of HMGB1 in miR-218 over expressing cells.
HMGB1 regulates autophagy during chemotherapy in EC cells
Tax induces programmed cell death by autophagic induction in cancer cells [24-26]. Tumor cells use this cytoprotective autophagy as a defense from apoptotic cell death which in turn contributes to development of Tax resistance. Previous study showed that HMGB1 interacted with Beclin1 [27,28] and LC3B, thereby promoting autophagy [28]. Beclin1 and LC3B are autophagy-related markers and are critical for regulating autophagy. Microtubule-associated LC3 tends to monitor levels of autophagy. When autophagy is upregulated, LC3 is cleaved (LC3-I) and then conjugated to phosphatidylethanolamine (LC3-II), which is recruited to the autophagophore. In this study, the autophagy stimulation by the anticancer drug treatment in EC cells was also confirmed by the assay of autophagy-related biomarker expressions using western blot analysis. As shown in Figure 3, accumulation of LC3 puncta in EC cells was significantly higher in Tax treatment groups (Figure 3A) and significantly high levels of LC3-I to LC3-II conversion and Beclin1 expression were also observed in the EC cells which were treated with Tax. Then, we determined the correlation of the high autophagy level following Tax treatment with the upregulated HMGB1 in the EC cells, we knockout HMGB1 in RL95-2 cells and Ishikawa cells via the method as previously described and then treated with Tax for 72 h [29]. The talen-knockout treat significantly decreased HMGB1 expression by Western blotting (Figure 3D). As expected, HMGB1 knockdown in Tax-treated cells decreased levels of autophagy as shown by decreased the expression of Beclin1, but had no further effects on LC3B-II and LC3B punctae (Figure 3F). These results confirmed the HMGB1-mediated autophagy during chemotherapy in EC cells.
Figure 3.
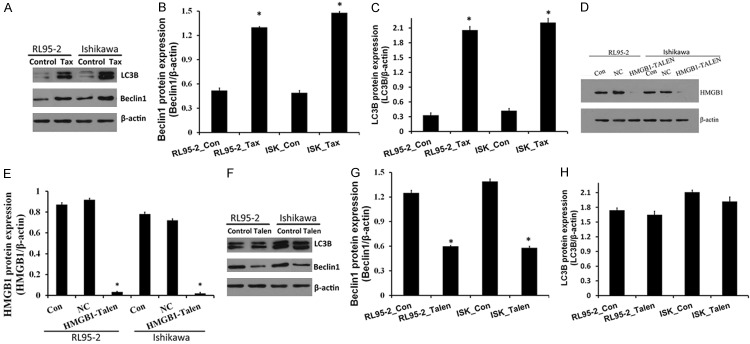
HMGB1 regulates autophagy during chemotherapy in osteosarcoma cells. A-C. Tax treatment promoted the conversion of LC3B-I to LC3B-II and the Beclin1 expression and assayed by Western blot. β-action was detected as the loading control, bars represent mean ± S.E. from three independent experiments (**P < 0.05 vs. control, where n = 3). D and E. HMGB1 gene knocked out in RL95-2 cells and Ishikawa cells. Western blot results showed the expression of HMGB1 protein in endometrial carcinoma cells. F-H. HMGB1 silencing affected the expression of LC3B and the Beclin1 in RL95-2 cells and Ishikawa cells. In control group, RL95-2 cells and Ishikawa cells were treated with Tax. In Talen group, RL95-2 cells and Ishikawa cells were HMGB1 gene knock-out cells and treated with Tax.
Overexpressed miR-218 inhibits the anticancer agent-induced autophagy
To determine the possible contribution of miR-218 to the autophagy in the drug-treated EC cells, we assayed the autophagy in RL95-2 cells and Ishikawa cells post Tax treatment and the miR-218 mimics or miR-Con transfection. Results showed that miR-218 overexpression significantly decreased Beclin1 expression, while miR-218 silencing increased Beclin1 protein level (Figure 4). And a significantly high level of LC3-I to LC3-II conversion and decreased accumulation of LC3B punctae were also confirmed by WB assay, in both RL95-2 cells and Ishikawa cells transfected with miR-218 mimics. These results revealed that overexpressed miR-218 inhibited the anticancer drug-induced autophagy in EC cells.
Figure 4.
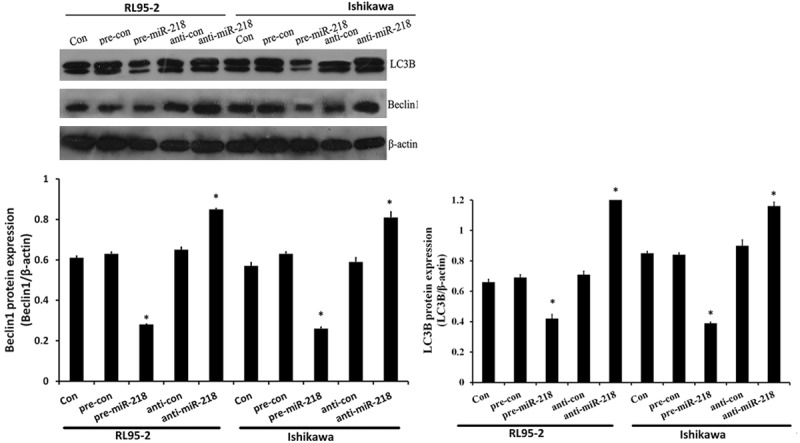
miR-218 suppressed HMGB1-mediated autophagy in endometrial carcinoma cells. Cells were not transfected (con), or transfected either with miR-218 mimics (pre-miR-218), mimics control (pre-con), miR-218 inhibitor (anti-miR-218) or inhibitor control (anti-con). After 24 h, cell lysates were prepared for Western blotting with antibody against Beclin1 and LC3B, and β-action was used as loading control. Relative Beclin1 protein and LC3B punctae expression levels were quantified by WB analysis in Tax-treated RL95-2 cells and Ishikawa cells, bars represent mean ± S.E. from three independent experiments (**P < 0.05 vs. control, where n = 3).
Discussion
Despite recent advances in understanding the cancer signaling network and in developing new therapeutic strategies, endometrial carcinoma shows poor prognosis and high incidence. Drug resistance is major obstacle in the treatment of endometrial carcinoma by limiting the efficacy both of conventional chemotherapy and novel biological agents. Therefore, new and more innovative approaches are required for treatment of this cancer. In the past decade, a large body of researches has demonstrated that microRNAs were involved in the regulation of cellular chemosensitivity [30-32]. For example, miR-449a was reported to regulate proliferation and chemosensitivity to cisplatin by targeting cyclin D1 and BCL2 in SGC7901 cells [30] and miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1 [33]. MiR-218 is a tumor suppressor microRNA, and previous studies have confirmed that miR-218 inhibited cancer cell proliferation and invasion through targeting oncogenic genes in certain cancers [18,34,35]. A recent study has shown that miR-218 could inhibit the growth of cervical cancer and promote its sensitivity to drug-based chemotherapy [36-38]. In this study, we also confirmed that miR-218 was significantly down-regulated in Tax-resistant endometrial carcinoma cells, and overexpression of miR-218 increased Tax-induced chemosensitivity both in RL95-2 cells and Ishikawa cells. MiR-218 overexpression inhibited the proliferative capability of EC cell post-anticancer drug treatment, thus sensitizing the cancer cells to Tax.
Autophagy is a tightly regulated lysosomal degradation pathway, helping to maintain a balance among the synthesis, degradation, and subsequent recycling of cellular products [39]. To date, an increasing number of studies have revealed a critical role for autophagy in cancer development and therapy. High-mobility group box 1 (HMGB1), known primarily regulator of autophagy, is expressed in many normal cells and has also been confirmed to involve in cancer development by interfering with several signaling pathways [40-42]. Recently, studies suggested that HMGB1 bounded to the autophagy regulator Beclin1 and regulated the formation of the Beclin1-PI3KC3 complex that facilitates autophagic progression [21]. Induction of autophagy by HMGB1 is important for tumor development and a novel target for cancer therapy [21,43]. Present study clearly reconfirmed that the expression of HMGB1 and Beclin1 were upregulated decreased by anticancer agents, Tax, in EC cells in vitro, which were reversed by overexpression of miR-218. Moreover, HMGB1 knockout also suppressed Beclin1 expression in Tax treated EC cells. In conclusion, our results revealed that HMGB1 was directly targeted by miR-218, and overexpressed miR-218 could suppress the HMGB1 level, and block the HMGB1-mediated autophagy in drug-treated EC cells.
In summary, our study shows that miR-218 expression was downregulated, following the HMGB1 upregulation during the anticancer drug treatment in EC cells in vitro. And the miR-218 targeted HMGB1 directly and overexpressed miR-218 inhibited the HMGB1-mediated autophagy in endometrial carcinoma cells. These findings identified the role of miR-218 in HMGB1-mediated autophagic chemotherapy resistance during the treatment of endometrial carcinoma, suggesting that miR-218 may be a novel target for endometrial carcinoma therapy.
Acknowledgements
This study was supported by the Research foundation of Health Department Hunan Province (no. c2014-33).
Disclosure of conflict of interest
None.
References
- 1.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, Zhang DD. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Min W, Gou J. Knockdown of cyclophilin A reverses paclitaxel resistance in human endometrial cancer cells via suppression of MAPK kinase pathways. Cancer Chemother Pharmacol. 2013;72:1001–1011. doi: 10.1007/s00280-013-2285-8. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Yin J, Mao N, Pan L. Upregulation of phosphorylated cofilin 1 correlates with taxol resistance in human ovarian cancer in vitro and in vivo. Oncol Rep. 2013;29:58–66. doi: 10.3892/or.2012.2078. [DOI] [PubMed] [Google Scholar]
- 5.Girouard J, Lafleur MJ, Parent S, Leblanc V, Asselin E. Involvement of Akt isoforms in chemoresistance of endometrial carcinoma cells. Gynecol Oncol. 2013;128:335–343. doi: 10.1016/j.ygyno.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Mir R, Tortosa A, Martinez-Soler F, Vidal A, Condom E, Perez-Perarnau A, Ruiz-Larroya T, Gil J, Gimenez-Bonafe P. Mdm2 antagonists induce apoptosis and synergize with cisplatin overcoming chemoresistance in TP53 wild-type ovarian cancer cells. Int J Cancer. 2013;132:1525–1536. doi: 10.1002/ijc.27832. [DOI] [PubMed] [Google Scholar]
- 7.Kondo C, Shibata K, Terauchi M, Kajiyama H, Ino K, Nomura S, Nawa A, Mizutani S, Kikkawa F. A novel role for placental leucine aminopeptidase (P-LAP) as a determinant of chemoresistance in endometrial carcinoma cells. Int J Cancer. 2006;118:1390–1394. doi: 10.1002/ijc.21509. [DOI] [PubMed] [Google Scholar]
- 8.Yanokura M, Banno K, Kobayashi Y, Kisu I, Ueki A, Ono A, Masuda K, Nomura H, Hirasawa A, Susumu N, Aoki D. MicroRNA and endometrial cancer: Roles of small RNAs in human tumors and clinical applications (Review) Oncol Lett. 2010;1:935–940. doi: 10.3892/ol.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang YW, Chen LA. microRNAs as tumor inhibitors, oncogenes, biomarkers for drug efficacy and outcome predictors in lung cancer (review) Mol Med Rep. 2012;5:890–894. doi: 10.3892/mmr.2012.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su N, Qiu H, Chen Y, Yang T, Yan Q, Wan X. miR-205 promotes tumor proliferation and invasion through targeting ESRRG in endometrial carcinoma. Oncol Rep. 2013;29:2297–2302. doi: 10.3892/or.2013.2400. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang Z, Liu X, Huang T, He W, Shen Y, Liu X, Hong K, Cao Q. miR-124 functions as a tumor suppressor in the endometrial carcinoma cell line HEC-1B partly by suppressing STAT3. Mol Cell Biochem. 2014;388:219–231. doi: 10.1007/s11010-013-1913-2. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X, Cheng X, Lu W, Xie X. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br J Cancer. 2013;109:92–99. doi: 10.1038/bjc.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee A, Chattopadhyay D, Chakrabarti G. miR-17-5p Downregulation Contributes to Paclitaxel Resistance of Lung Cancer Cells through Altering Beclin1 Expression. PLoS One. 2014;9:e95716. doi: 10.1371/journal.pone.0095716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Wang Y, Dong R, Huang X, Ding S, Qiu H. Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J Cancer Res Clin Oncol. 2012;138:671–674. doi: 10.1007/s00432-012-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan FK, Sung JJ, Yu J. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol Med. 2012;18:1491–1498. doi: 10.2119/molmed.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sher YP, Wang LJ, Chuang LL, Tsai MH, Kuo TT, Huang CC, Chuang EY, Lai LC. ADAM9 up-regulates N-cadherin via miR-218 suppression in lung adenocarcinoma cells. PLoS One. 2014;9:e94065. doi: 10.1371/journal.pone.0094065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa R, Goto Y, Sakamoto S, Chiyomaru T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya Y, Ichikawa T, Seki N. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014;105:802–11. doi: 10.1111/cas.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Ge S, Hu C, Yang N, Zhang J. MiRNA-218, a new regulator of HMGB1, suppresses cell migration and invasion in non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2013;45:1055–1061. doi: 10.1093/abbs/gmt109. [DOI] [PubMed] [Google Scholar]
- 20.Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012;72:230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D, Ni J. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy. 2012;8:275–277. doi: 10.4161/auto.8.2.18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang R, Livesey KM, Zeh HJ 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256–1258. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 24.Ajabnoor GM, Crook T, Coley HM. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012;3:e260. doi: 10.1038/cddis.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, Yuan H. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011;307:141–148. doi: 10.1016/j.canlet.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Chi EY, Viriyapak B, Kwack HS, Lee YK, Kim SI, Lee KH, Park TC. Regulation of paclitaxel-induced programmed cell death by autophagic induction: A model for cervical cancer. Obstet Gynecol Sci. 2013;56:84–92. doi: 10.5468/OGS.2013.56.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Fang Y, Cao Y, Qin R, Chen Q. miR-449a Regulates proliferation and chemosensitivity to cisplatin by targeting cyclin D1 and BCL2 in SGC7901 cells. Dig Dis Sci. 2014;59:336–345. doi: 10.1007/s10620-013-2923-3. [DOI] [PubMed] [Google Scholar]
- 31.Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W. MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther. 2008;7:1–9. doi: 10.1158/1535-7163.MCT-07-0573. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Lu Y, Cui D, Li E, Zhu Y, Zhao Y, Zhao F, Xia S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol Rep. 2014;31:910–918. doi: 10.3892/or.2013.2897. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L. miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol. 2014;35:6293–302. doi: 10.1007/s13277-014-1821-4. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita T, Hanazawa T, Nohata N, Kikkawa N, Enokida H, Yoshino H, Yamasaki T, Hidaka H, Nakagawa M, Okamoto Y, Seki N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin- 332 in head and neck squamous cell carcinoma. Oncotarget. 2012;3:1386–1400. doi: 10.18632/oncotarget.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin J, Cai L, Liu ZM, Zhou XS. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev. 2013;14:3681–3684. doi: 10.7314/apjcp.2013.14.6.3681. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Ping Z, Ning H. MiR-218 Impairs Tumor Growth and Increases Chemo-Sensitivity to Cisplatin in Cervical Cancer. Int J Mol Sci. 2012;13:16053–16064. doi: 10.3390/ijms131216053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan W, Xiaoyun H, Haifeng Q, Jing L, Weixu H, Ruofan D, Jinjin Y, Zongji S. MicroRNA-218 Enhances the Radiosensitivity of Human Cervical Cancer via Promoting Radiation Induced Apoptosis. Int J Med Sci. 2014;11:691–696. doi: 10.7150/ijms.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan R, Zhong J, Zheng S, Wang Z, Xu Y, Li S, Zhou J, Yuan F. microRNA-218 increase the sensitivity of gastrointestinal stromal tumor to imatinib through PI3K/AKT pathway. Clin Exp Med. 2015;15:137–44. doi: 10.1007/s10238-014-0280-y. [DOI] [PubMed] [Google Scholar]
- 39.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–183. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song JX, Lu JH, Liu LF, Chen LL, Durairajan SS, Yue Z, Zhang HQ, Li M. HMGB1 is involved in autophagy inhibition caused by SNCA/alpha- synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy. 2014;10:144–154. doi: 10.4161/auto.26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 2013;19:4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567–577. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, Tang D, Cao L. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]


