Abstract
The main purpose of this study was to investigate the infiltration of tumor-associated macrophages (TAMs) in normal and malignant breast tissue and the draining lymph nodes, and to explore its effect on breast cancer invasion and metastasis. The infiltration densities of TAMs was observed using immunohistochemical staining of CD68 in 100 cases of breast cancer specimens and its paired adjacent non-cancer breast tissues and draining lymph modes, and then to evaluate the relation of TAMs to various clinicopathological features including patients prognosis in breast carcinoma. We observed the infiltration densities of TAMs were significantly higher in breast carcinoma tissue than in adjacent normal tissue and significantly higher in much larger size and higher stage cases. Furthermore, infiltration densities of TAMs have negative correlation with the 5-year survival rates of breast cancer patients. But in matched lymph-nodes, the infiltration densities of TAMs were significantly lower in cancerous metastatic lymph-node samples than in non-metastatic one. Therefore, our data suggests that TAMs infiltration in primary tumor promote invasion and lymphatic metastasis of breast cancer and have negative correlation with patients prognosis in breast cancer, but in lymph-node TAMs may play another role and need further study in the future.
Keywords: Breast cancer, tumor-associated macrophages, invasion and metastasis
Introduction
Breast cancer is the most prevalent malignant disease which seriously affects the physical and mental health of women, and it is one of the leading causes of death among women [1,2]. The prevalence of breast cancer tended to increase. As the considerable progress in treatment (comprehensive as well as radiation, hormonal and targeted therapies) and early detection, breast cancer mortality decreased for few years [3]. However, not all patients have benefited from those advances, there are still a part of patients eventually failed to obtain the effective treatment, and invasion and metastasis is still a great challenge in the treatment [4].
As technologies advance, we know more and more about breast cancer. Many studies have focused on the ability of tumor cell’s invasion and metastasis which due to the change of the cell’s genetic and epigenetic. In recently years, the tumor microenvironment has been suggested to play an important role in tumor initiation and promoting the invasion and metastasis of malignant tumors including breast cancer, however, rare data is available in metastatic lymph nodes. Scholars had made some research work to find that the number of T cells [5], dendritic cells [6] and angiogenesis [7] in metastatic lymph nodes’ microenvironment effect tumor prognosis.
The tumor microenvironment of malignant tumors is populated by many cells including immune cells, stromal cells and the secretion of the active medium with tumor cells [8], most of which participate as abettors or double-edged swords in tumor carcinogenesis. The tumor-associated macrophages (TAMs) are the predominant components of the cells in tumor microenvironment, and it is associated with poor prognosis of solid tumors. CD68 is the best marker protein TAMs, and the pan-macrophage marker CD68 is now generally utilized in prognosis prediction in many tumors, like in thyroid, pancreatic, lung and hepatocellular cancer [9].
Breast cancer metastasis primarily happens through lymphatic system, where the extent of lymph node metastasis is the major factor influencing staging, prognosis and therapeutic decision of the disease. Therefore, it’s meaningful to study the influence of the main cells (TAMs) in the microenvironment of lymph node, and recent studies have showed that the increase of TAMs within breast tumors of breast cancer patients correlates with poor prognosis [10]. We speculate that TAMs in primary tumor of breast cancer and corresponding lymph nodes may be associated with breast cancer invasion and lymphatic metastasis. Consequently, in this paper, we examine the infiltration densities of TAMs in primary cancer tissue and its paired adjacent non-cancer breast tissue and corresponding lymph node metastases, and investigate a novel metastasis mechanism involving tumor-associated macrophages. We found that TAMs infiltration in primary tumor was significantly associated with TNM stages and tumor sizes, and TAMs infiltration in non-metastatic lymph-node samples was significantly more than the cancerous metastatic lymph-node samples in breast carcinomas. We observed the 5 years survival rate of patients is significantly poorer in tumor primary tissue’s TAMs high-infiltration density group than TAMs low-infiltration ones.
Materials and methods
Tissue samples
All of the tissue sample and clinical data collection was approved by the Hospital Research Ethics Committee, and consent was obtained from all patients prior to conducting the research. Normal and malignant breast tissue and the corresponding lymph nodes were obtained from the Department of Pathology at the Affiliated the Fifth People’s Hospital of Shanghai of Fudan University from January 1, 2005 to December 31, 2013. There were 100 patients with invasive breast carcinoma involved in this study, 48 cases with lymph node metastasis, 52 cases without lymph node me-tastasis. The median age of these patients was 55 years with a range of 28-80 years. The tumor breast tissues were examined by pathologists. The formalin-fixed, paraffin-embedded tissues were sectioned with a microtome into 4 μm sections. The diagnosis and certified pathologist were according the World Health Organization tumor classification criteria.
Immunohistochemistry and antibodies
Immunohistochemical staining was performed using the streptavidin-perosidase (SP) method. Paraffin-embedded sections were processed according to previously published standard laboratory protocol, deparaffinized, dehydrated and immersed in sodium citrate buffer (PH 6.0), blocked with 3% hydrogen peroxide for 10 min at room temperature, rinsed with phosphate-buffered saline (PBS) for 10 min, pretreated in a microwave oven for 10 min, then the slides were incubated at 4°C overnight with monoclonal mouse anti-CD68 (DAKO, Carpinteria, CA, diluted 1:100 in 10 mM PBS). After the slides were stained with the 2-step plus Poly-HRP anti-Rabbit IgG Detection System, the slides were visualized the reaction with the DAB chromogen, counterstained with haematoxylin and covered with a glyceringel. PBS was used to substitute the primary antibody for negative controls.
Evaluation of immunohistochemical staining
CD68+ TAMs was estimated by counting the number of CD68+ TAMs by light microscope in 400 times in intensive areas, selected 5 regions from each slice, and the mean of 5 counts was taken. The median value was used to cut the groups of all immunohistochemical variables in our data. The patients were then divided into TAMs high-infiltration group and low-infiltration group.
5-year follow-up
Pathological and clinical records of all patients on this study were reviewed periodically. Patients were followed regularly for 5 years through visits to the clinic or telephone. All patients were followed for 5 years or until death. Overall survival (OS) which measured death from any case was used for prognostic analyses.
Statistical analysis
All analyses were performed using the statistical software SPSS 16.0. The x2 test or Fisher’s exact test was used to analysis the correlation of TAMs immunoreactivity with paitents’ clinicopathological variables. The Kaplan-Meier method was used to estimate overall survival. P values of <0.05 were considered statistically significant in all of the analyses.
Results
CD68 immunohistochemical pattern of TAMs in breast carcinoma
CD68 expression, the pan-macrophage marker, was found in cytoplasm mainly, positive staining was brown or brown particles. In breast cancer samples, TAMs (CD68 positive cells) diffusely or scattered exist in tumor nest, tumor stroma and peritumoral stroma. We counted the number of TAMs in 5 separated tumor nest, and the its median number was 61.14±23.76/high-power field (HFP) in 100 breast cancer cases. In order to investigate the association between the density of TAMs and clinical features, the breast cancer patients were divided into high- and low-TAMs density groups based on median density of TAMs positive cells. As shown in Figure 1.
Figure 1.
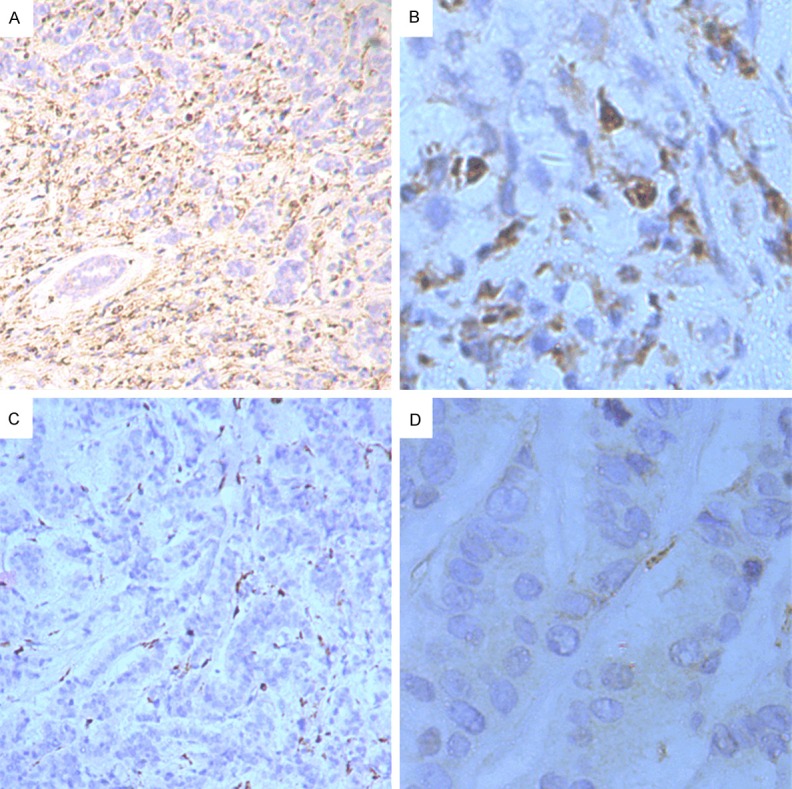
Immunohistochemical detection of TAMs (CD68+) in breast cancer tissues. CD68 expression was found in cytoplasm mainly, positive staining was brown or brown particles. In breast cancer samples, TAMs diffusely or scattered exist in tumor nest, tumor stroma and peritumoral stroma. A. TAMs high-infiltration in breast cancer specimen (×100). B. TAMs high-infiltration in breast cancer specimen (×400). C. TAMs low-infiltration in breast cancer specimen (×100). D. TAMs low-infiltration in breast cancer specimen (×400).
TAMs infiltration in scattered was observed in breast cancer adjacent tissues, and its median density was 37.44±29.26/HFP and significantly less than the breast cancer tissue one (P < 0.01, Table 1; Figure 2).
Table 1.
Comparison of TAMs infiltration density in breast cancer and adjacent tissues
| Tissue | TAMs infiltration | x2 | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Breast cancer | 64 | 36 | 9.71 | 0.002 |
| Adjacent | 42 | 58 | ||
Figure 2.
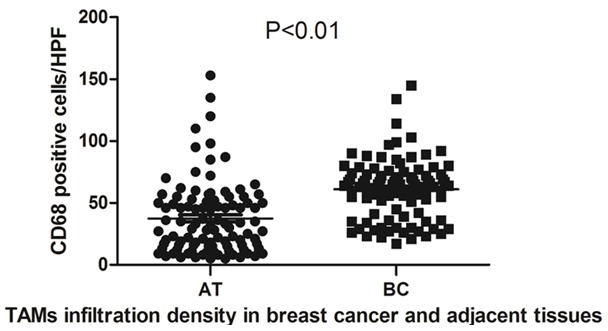
Comparison of TAMs infiltration density in breast cancer and adjacent tissues, TAMs infiltration in scattered was observed in breast cancer adjacent tissues, and its median density was 37.44±29.26/HFP. In breast cancer samples TAMs diffusely or scattered exist in tumor nest, tumor stroma and peritumoral stroma, and the its median number was 61.14±23.76/HFP. TAMs infiltration in breast cancer adjacent tissues was significantly less than the breast cancer tissue one (P < 0.01).
Correlations of TAMs status to clinicopathological features
100 patients with invasive breast carcinoma were included in the study. 60 patients were for ≥50 years of age, 40 patients were for <50 years of age. With tumor size distribution, 42 patients were for ≤2 cm, 42 patients were for >2 & ≤5 cm, 16 patients were ≥5 cm. Among the patients, 52 patients were lymph node metastasis-negative, 21 patients with 1-3 lymph node metastasis-positive, 27 patients with ≥3 lymph node metastasis-positive. At the time of diagnosis, 12 patients were at stage I, 41 patients were at stage II, 47 patients were at stage III&IV. Further detailed clinical information is presented in Table 2.
Table 2.
Correlation between TAMs expression and various clinicopathological features
| Variables | No. of Cases | TAMs infiltration | x2 | P value | |
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Age | |||||
| <50 | 40 | 18 | 22 | ||
| ≥50 | 60 | 33 | 27 | 0.96 | 0.327 |
| Tumor size | |||||
| ≤2 | 42 | 16 | 26 | ||
| >2 & ≤5 | 42 | 23 | 19 | ||
| >5 | 16 | 12 | 4 | 6.72 | 0.034 |
| Lymph node metastasis | |||||
| 0 | 52 | 23 | 29 | ||
| 1~3 | 21 | 11 | 10 | ||
| >3 | 27 | 17 | 10 | 2.52 | 0.284 |
| TNM stages | |||||
| I | 12 | 2 | 10 | ||
| II | 41 | 22 | 19 | ||
| III~IV | 47 | 27 | 20 | 6.56 | 0.037 |
TAMs infiltration was significantly associated with TNM stages and tumor size (P < 0.05, Table 2). We found no significant correlations between TAMs status and age, lymph node metastasis.
Correlations of TAMs status to survival time of breast cancer patient
100 breast cancer patients were divided into high- and low-density two groups on the basis of TAMs infiltration. The time of follow-up was 60 months, during the follow-up period 13 patients died of breast cancer; the shortest survival time was 12 months. The average survival time was 56.68 months, and five-year survival rate was 87% for all patients. 5 years survival rate was respectively 80.3% and 93.8% in TAMs high-density group and low one, and the difference was significant (P < 0.05, Table 3).
Table 3.
Comparison of TAMs infiltration density in breast cancer and 5-years survival status
| TAMs infiltration | No. of cases | Survival Status | x2 | P value | |
|---|---|---|---|---|---|
|
| |||||
| Alive | Death | ||||
| High | 51 | 41 | 10 | 4.02 | 0.045 |
| Low | 49 | 46 | 3 | ||
Using Kaplan-Meier survival analysis, among the 100 breast cancer patients, TAMs high-infiltration in tumor tissues patients experienced significantly poorer outcomes in terms of overall survival (P < 0.05) in comparison with patients who were TAMs low-infiltration (Figure 3).
Figure 3.
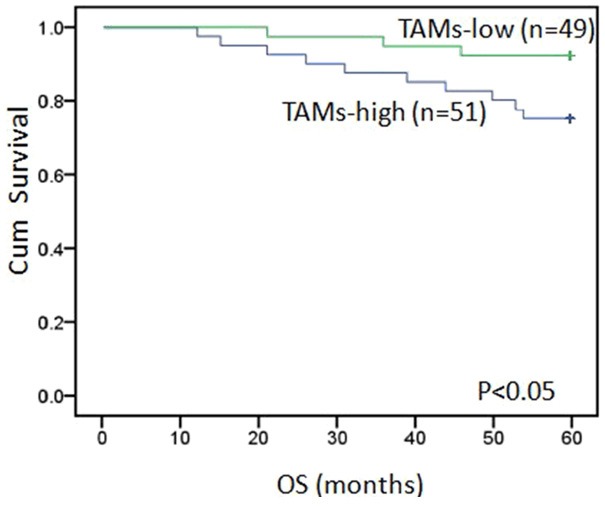
Kaplan-Meier analysis for OS based on CD68 expression in patients with breast cancer, TAMs high-infiltration in tumor tissues patients experienced significantly poorer outcomes in terms of overall survival in comparison with patients who were TAMs low-infiltration (P < 0.05). Green: TAMs low-infiltration in breast cancer specimen. Blue: TAMs high-infiltration in breast cancer specimen.
CD68 immunohistochemical pattern of TAMs in lymph nodes
CD68 expression, the pan-macrophage marker, was found in cytoplasm mainly, positive staining was brown or brown particles. In lymph node samples, TAMs (CD68 positive cells) diffusely or scattered exist in the marginal sinus and medullary cord (Figure 4). We counted the number of TAMs in 5 separated marginal sinus. The median number was 48.67±23.45/HFP in 48 metastatic lymph-node samples, and the range was 8-98 cells/HPF. TAMs infiltration in non-metastatic lymph-node samples was observed, and its median density was 119.38±40.07/HFP and the range was 44-210/HPF, which was significantly more than the cancerous metastatic lymph-node samples TAMs infiltration (P < 0.01, Table 4).
Figure 4.
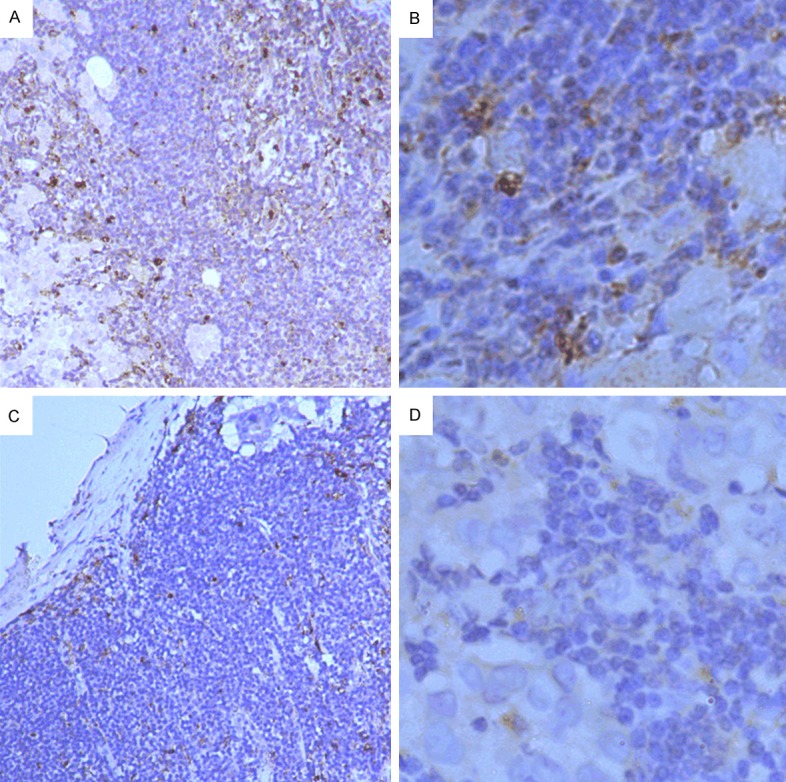
Immunohistochemical detection of TAMs (CD68+) in lymph node tissues. CD68 expression was found in cytoplasm mainly, positive staining was brown or brown particles. In lymph node samples, TAMs diffusely or scattered exist in the marginal sinus and medullary cord. A. TAMs high-infiltration in lymph node specimen (×100). B. TAMs high-infiltration in lymph node specimen (×400). C. TAMs low-infiltration in lymph node specimen (×100) D. TAMs low-infiltration in lymph node specimen (×400).
Table 4.
Comparison of TAMs infiltration density in lymph-node samples
| Tissue | No. of Cases | TAMs | F | P value |
|---|---|---|---|---|
| Metastatic | 48 | 48.67±23.45 | 108.77 | <0.01 |
| Non-metastatic | 52 | 119.38±40.07 |
Discussion
In this study, we found that TAMs infiltration in primary tumor was significantly associated with TNM stages and tumor size, and TAMs infiltration in non-metastatic lymph-node samples was significantly more than the cancerous metastatic lymph-node samples in breast carcinomas. We observed the infiltration densities of TAMs in primary tumor tissue correlate negatively with the 5-year survival rates of patients with invasive breast carcinomas, and the TAMs high-infiltration in tumor tissues patients experienced significantly poorer out comes in terms of overall survival in comparison with patients who were TAMs low-infiltration.
Breast cancer is the most common cancer among women worldwide [2]. It is has been paid more and more attention that the microevironment of breast cancer play an important role in promoting tumor invasion and metastasis. The tumor microenvironment has been increasingly considered as a therapeutic target or biomarker for diagnosis and prognosis [11]. TAMs are believed the inhibitors of antitumor immunity [12]; clinical evidences compellingly indicate that there is the association between high TAMs influx and poor prognosis in patients with breast cancers. There is a analysis has been reported that over 80% of studies on macrophage show a correlation between macrophage density and patient’s poor prognosis [13], and it have been supported by this study.
We observed that the infiltration density of TAMs in primary breast tumor tissue was significantly higher than that in breast cancer adjacent tissues, consistent with previous results. At the same time, we observed that increased infiltration of TAMs in primary breast tumor tissue along with the increased tumor stage and size. Therefore, there is a strong association between increased TMAs infiltration and tumor invasion. Although the increase of TAMs in tumor tissues could be a consequence of tumor invasion, several studies have argued that macrophage-derived factors can enhance invasion of breast cancer cells via increased angiogenesis or activation of tumorigenic signaling [14].
Recently, Mahmoud and colleagues [15] have confirmed the predictive value of TAMs using a large cohort of patients with breast cancer. In their study, higher numbers of CD68+ macrophages predict worse overall survival. In this study, we observed the TAMs in breast tumor tissue infiltration density was negatively correlated with the survival time of patients, the 5 years survival rate of high-density group was lower. Our data strongly indicated that high-infiltration of TAMs is an important factor in promoting tumor cell metastasis in breast carcinoma, which was consistent with the research results in lung cancer [16], colorectal cancer [17], ovarian cancer [18], classical Hodge lymphoma tissues [19].
Macrophages display remarkable plasticity that allows them to efficiently respond to environmental signals and change their phenotype and physiology [20]. Macrophages continuously infiltrate into the tumor microenvironment, where breast cancer cells and several other gr-owth factors including CSF-1, IL-3, CCL-2 what induce macrophages to TAMs. TAMs belongs to a class of undifferentiated macrophages, with a high degree of plasticity, in different tumors, different parts, and even the same kind of tumor in different growth stage, the TAMs phenotypic expression level of membrane molecules and cytokines are not the same.
Macrophages are generally differentiated into classically activated macrophages (M1) or alternatively activated macrophages (M2). M1 macrophages have a high bactericidal and tumoricidal capacity. M2 is involved with promotion of tissue remodeling and pro-tumor functions [21]. Both M1 and M2 can infiltrate into tumor sites and secret cytokines what regulate the tumor evolution and development direction. TAMs are heterogeneous population; distinct subsets perform distinct functions [22]. Naturally raised TAMs are biased towards the M2 type, it has been established that M2 macrophages are linked to the growth, angiogenesis, migration and invasion of a variety of cancers [23]. Either depletion of TAMs or reversion of their phenotype (M2 to M1) has been demonstrated to reject tumor progression in mouse models of breast cancer [6]. However, some macrophages exhibit both the M1 and M2 behaviors, which depend on the certain physiological and pathological conditions and the microenvironment [24].
Distant metastasis is the most important characteristics of malignant tumor; lymph node is most easily organs. It has an important significance to observe the variety of TAMs in the lymph node. Currently, the researches on the lymph mode of breast tumor are mainly concentrated in the change of the number and function of T-cells, B-cells, natural killer (NK) cells and dendritic cells (DC). DC and macrophages comprise the major types of antigen presenting cells (APC) in the lymph node, the majority of scholars believe that less numbers of DC in lymph nodes predict the more easily metastasis breast cancer [6]. In this study, we found TAMs infiltration in non-metastatic lymph-node samples was significantly more than the cancerous metastatic lymph-node samples. This outcome is to indicate that the number of TAMs in lymph nodes is associated with the tumor metastasis. Studies in mice have shown that DC in the tumor-draining lymph nodes (TDLN) is very poor stimulators of T-cells [25], its function is inhibited. In the lymph nodes, the main function of macrophage is phagocytes, and function as a kind of APC in the immune response. Since there is not result about the function of TAMs in lymph node, we hypothesis that the function of TAMs in lymph nodes was suppressed, and had different phenotype and function with the primary tumor. However, this theory need to be further investigated.
Breast cancer is a systemic disease has been a broad consensus, and current treatment mainly depends on operation excision, local treatment with radiotherapy and chemotherapy, some patients with hormone treatment, and the mortality rate has not been effectively controlled, the development of new therapeutic method is required. To investigate the role of TAMs, the tumor microenvironment main inflammatory cells, infiltrate in breast and the influence in metastasis, is of positive significance. As a matter of fact, TAMs have now been considered as potential targets for adjuvant therapy [26].
In conclusion, high-infiltration of TAMs correlated with shorter survival in patients with breast carcinoma, and high-infiltration of TAMs was associated with high-TNM stage and tumor size. TAMs infiltration in non-metastatic lymph-node samples was significantly more than the cancerous metastatic lymph-node samples. Our results suggest that TAMs are a negative predictor of breast carcinoma and that TAMs could emerge as an attractive new drug target in the treatment of breast carcinoma.
Acknowledgements
This work was supported by The Fifth People’s Hospital of Shanghai, Fudan University (project number 2013 WYQJ06).
Disclosure of conflict of interest
None.
References
- 1.Kim K, Zang R, Choi SC, Ryu SY, Kim JW. Current status of gynecological cancer in China. J Gynecol Oncol. 2009;20:72–6. doi: 10.3802/jgo.2009.20.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11–9. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Duffy SW, Tabar L. Breast cancer screening: the evolving evidence. Oncology (Williston Park) 2012;26:471–5. 479–81, 485–6. [PubMed] [Google Scholar]
- 5.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, Jordanova ES, de Gruijl TD. High and Interrelated Rates of PD-L1+CD14+ Antigen-presenting Cells and Regulatory T cells mark the Microenvironment of Metastatic Lymph Nodes from Patients with Cervical Cancer. Cancer Immunol Res. 2015;3:48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 6.Poindexterl NJ, Sahin A, Hunt KK, Grimm EA. Analysis of dendritic cells in tumor-free and tumor-containing sentinel lymph nodes from patients with breast cancer. Breast Cancer Res. 2004;6:R408–415. doi: 10.1186/bcr808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tssrfaty I, Hudson E, Jackson DG, Petillo D Chen J, Resau JH, Teh BT. Preparing the “Soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–76. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jubb AM, Soilleux EJ, Turley H, Steers G, Parker A, Low I, Blades J, Li JL, Allen P, Leek R, Noguera-Troise I, Gatter KC, Thurston G, Harris AL. Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer. Am J Pathol. 2010;176:2019–28. doi: 10.2353/ajpath.2010.090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137–48. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4 (+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–63. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, Liu S, Qin Y, Chen H. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74:188–96. doi: 10.1016/j.lungcan.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4:141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi: 10.3389/fonc.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activatio. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–12. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. 2013;4:192. doi: 10.3389/fimmu.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]


