Abstract
Purpose: To investigate the effect of β, β-dimethylacryloyl alkannin, a main component of Lithospermum erythrorhizon, on activated dendritic cells (DCs) in a psoriasis mouse model. Methods: BALB/c mice were used to establish the animal model for psoriasis-like skin lesion; alkannin at 10 mg/kg (high), 5 mg/kg (medium), 2.5 mg/kg (low), respectively, were intragastrically administered. Psoriasis area and severity index (PASI) was used to evaluate the skin lesions. Histological changes, the thickness of epidermis, and the quantity of interleukin (IL)-23 in skin lesion were measured. In in vitro experiments, mononuclear cells in peripheral blood from healthy people were isolated, and monocytes were obtained. DCs with a mature state in differentiation and function were obtained through in vitro induction with several cytokines, and identified by flow cytometry. The influence of DCs on proliferation of allogenic lymphocytes was analyzed. The influence of alkannin on messenger ribonucleic acid (mRNA) expression of pro-inflammatory factors by mature DCs was evaluated using reverse transcriptase polymerase chain reaction. Results: Mice treated with alkannin at varying concentration showed obvious remission in psoriasis-like skin lesion compared to control group, with decreased PASI score, obviously reduced vertical thickness of epidermis. Besides, alkannin treatment decreased the expression of IL-23 in skin lesion. Alkannin (12.5 μg/mL) suppressed the ability of DCs to stimulate the proliferation of allogenic lymphocytes, and suppressed the expression and secretion of IL-6, IL-12 p40, IL-23, IL-1β, tumor necrosis factor-α mRNA and proteins, respectively. Conclusions: β, β-dimethylacryloyl alkannin could suppress the function of activated DCs in imiquimod-induced psoriasis mouse model.
Keywords: β, β-dimethylacryloyl alkannin, dendritic cells, psoriasis, Lithospermum erythrorhizon, L-shikonin
Introduction
Psoriasis is a kind of inflammatory disease mediated by cellular immunity. Current evidences indicated that antigen presentation cells such as dendritic cells (DCs) play an important role in the morbidity and development of psoriasis [1]. DCs can regulate adaptive immune system through activation and proliferation of T cells, and thereby organism might react to the environment. In immune system, immature DCs develop into mature DCs after stimulation, and then secrete inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-12 p70, and IL-23. These cytokines can potentiate the activation and proliferation of T cells; abnormally activated T cells as well as secreted cytokines can in turn promote the maturation of DCs [2]. As a result, after activation of DCs, the secreted cytokines can activate T cells. Abundant cytokines, chemokines, and growth factors altogether constitute a “cytokine storm”. A vicious circle occurs in adjacent Malpighian cells, endothelial cells, neutrophils, and immune cells, which together lead to erythema in psoriasis [3].
Lithospermum erythrorhizon, a traditional Chinese medicine with a common clinical use as collected in Chinese pharmacopoeia, is an important and effective medicine to cure psoriasis. Pharmacological researches indicated that L. erythrorhizon showed promising antibacterial, anti-inflammatory, anticancer, contraceptive, antithyroid, anti-immunodepressive, and hypoglycemic effects, as well as protection of liver [4]. The active chemical components of L. erythrorhizon, naphthoquinones family with high-fat-solubility, are L-shikonin and β,β-dimethylacryloyl alkannin. Recently, numerous studies have focused on shikonin and its derivatives, which have been found to show immune regulatory functions. A few studies have revealed that shikonin have immunosuppressive properties: Kyong Nyon Nam et al have found that shikonin can reduce the inflammatory reaction in microgliocytes through inhibition of inflammatory pathways such as extracellular-signal-regulated kinase, protein kinase B (PKB or Akt), or nuclear factor-κB pathway [5]; Ting Li et al have demonstrated that shikonin can suppress lymphocytes activation in human through inhibition of IKK-β activation and c-Jun N-terminal kinase phosphorylation [6]. There were reports concerning the potentiation of immune function by shikonin: Long S et al studied the influence of shikonin on mice with tumors and found that shikonin protected the immune system of the mice, with improved immune reaction, and potentiated the anti-tumor ability [7].
Currently, studies concerning pharmacological action of alkannin are mainly focused on its antitumor effect. Yoshihisa et al have found that alkannin can induce the expression of heat shock protein 70, which might protect Malpighian cells from apoptosis induced by Ultraviolet B [8]. Interestingly, Dent et al [9] have reported that derivative of alkannin, SYUNZ-16, can induce the apoptosis of tumor cells and suppress the growth of tumor cells through impaired activation of PKB/AKT kinase. However, few reports existed concerning the immuneregulatory function of alkannin.
In preliminary studies, it was found that L. erythrorhizon could impair the release of cytokines such as TNF-α, IL-8, and IL-6 in patients with psoriasis. Moreover, it was demonstrated that shikonin could significantly suppress the activity of activated T cells, decrease the expression of CD69, and efficiently reduce the secretion of interferon-gamma, IL-2, and TNF-α, implicating immunoregulatory cells and cytokines are one of the targets through which shikonin can cure psoriasis [10].
In the present study, to further explore the pharmacological effects of the active chemical components in L. erythrorhizon, animal model of psoriasis-like skin lesion was established, and the effect of alkannin on skin lesion as well as the changes in number of DCs located in skin lesion was examined. In in vitro experiments, monocytes from peripheral blood were isolated, differentiated into DCs after induction, and stimulated with R484, an agonist of toll-like receptor 8 (TLR8), to obtain mature DCs, which can imitate the pathological state in the pathogenesis of psoriasis. Subsequently, the influence of β, β-dimethylacryloyl alkannin on secretion of cytokines by activated DCs was observed, to clarify the mechanism underlying its therapeutic effect on psoriasis.
Materials and methods
Animals and grouping
Male BALB/c mice, with a body weight between 18 and 20 g, were bought from Beijing Huafukang Biotechnology Stock Company Ltd (License No: SCXK (Jing) 20090007). Mice were housed under specific pathogen-free conditions and were given free access to food and water. All animal experimentations were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals, (Beijing University of Chinese Medicine), People’s Republic of China. This animal study was approved by the Animal Care and Scientific Committee of (Beijing University of Chinese Medicine).
Mice were anaesthetized via intraperitoneal injection of pentobarbital sodium (80 mg/kg). Hair on the back was shaved, and an area of 2 cm×3 cm was exposed. After been raised in an individual cage for one day, each mouse was treated as follows. Mice were randomly allocated into control group, model group, positive drug group [methotrexate (MTX)], alkannin-high dose group (DMA-H), alkannin-moderate dose group (DMA-M), and alkannin-low dose group (DMA-L). In control group, naked skin on the back of mice was daubed with appropriate amount of Vaseline each day at a regular time; meanwhile, mice were intragastrically administered with 0.4 mL physiological saline once a day. In MTX group, naked skin on the back of mice and right ear were daubed with 4% imiquimod cream (Med Shine Pharma Co., China) at a dose of 42 mg per day at a regular time; meanwhile, mice were intragastrically administrated with 0.4 mL MTX (Shanghai Pharmaceutical Group Pharmaceutical Co., Ltd., China) once a day (MTX was dissolved in physiological saline and was administered at a dose of 1 mg/kg/day, which was equivalent to the dose used in human). In DMA-H group, naked skin on the back of mice was daubed with 4% imiquimod cream at a dose of 42 mg per day at a regular time; meanwhile, mice were intragastrically administrated with 0.4 mL alkannin (10 mg/kg, bought from the National Institute for the Control of Pharmaceutical and Biological Products) once a day. In DMA-M group, naked skin on the back of mice was daubed with 4% imiquimod cream at a dose of 42 mg per day at a regular time; meanwhile, mice were intragastrically administrated with 0.4 mL alkannin (5 mg/kg) once a day. In DMA-L group, naked skin on the back of mice was daubed with 4% imiquimod cream at a dose of 42 mg per day at a regular time; meanwhile, mice were intragastrically administrated with 0.4 mL alkannin (2.5 mg/kg) once a day. Each treatment lasted for 6 days continuously.
Eyeballs were removed for blood collection. Mice were executed through cervical vertebra dislocation and their skin was collected. The collected blood samples were kept at room temperature for a while, and then subjected to centrifugation at 3500 rpm for 10 min, and stored at -80°C until use. The skin was separated into two parts for hematoxylin and eosin (HE) staining after paraffin section preparation and reverse transcriptase polymerase chain reaction (RT-PCR), respectively.
Psoriasis area and severity index (PASI) analysis
Digital camera was used to record the state of the skin, and erythema (E), scales (S), and incrassation (I) in each sample of the skin was scored according to PASI criteria. The three index scores were added together to obtain a total score (T). The trend line for scores of the skin lesion was obtained (to identify the changes in the skin of mice) using the average value of scores in each group. The PASI scores were follows: 0 (none): no visible erythema or scales on the skin surface, and the skin lesion was comparable to normal healthy skin; 1 (low-grade): part of skin lesion was covered by scales, mainly small pieces of danders, and the skin lesion appeared slightly advanced compared to normal skin, with a light red in color; 2 (moderate): majority of the skin was partially or completely covered by sheeted scales, with moderate ridges, and the edge of the plaque was red in color, with round or slope shape; 3 (severe): almost the whole surface of the skin lesion was covered by thick and layered scales, and the skin lesion was thick, deep red, with obvious ridges; 4 (extremely severe): the whole surface of the skin lesion was covered by very thick and layered scales, and the skin lesion was very thick, extremely red in color, with extremely obvious ridges.
HE staining and pathological changes of skin of mice in each group
The obtained skin was fixed in 10% formaldehyde for 72 h, dehydrated using automatic dehydration machine, embedded with paraffin, cut into 5-µm slices after repairmen, and attached to glass slides. After been baked at 60°C for 30 min, each slice was subjected to HE staining to observe the pathological changes and then photographed. The thickness of epidermis layer at three randomly selected sites in each HE stained slice was measured, and the averaged value was used to evaluate the changes in thickness of epidermis layer.
Cell culture
Concentrated white blood cells (bought from Beijing Red Cross Blood Center, China) were diluted at the ratio of 1:1 with cell dilution buffer, and added onto human lymphocytes isolation reagent along the wall of tubes at the ratio of 1:1. After centrifugation at 1500 rpm for 30 min, the cloud-like cells in the middle layer were collected, washed with Wash buffer two times, and centrifuged at 1500 rpm for 20 min. The peripheral blood mononuclear cells (PBMCs) were isolated and were subjected to magnetic-activated cell-sorting (MACS, Miltenyi Biotec, Germany) to isolate CD14+ cells. The supernatant liquid after centrifugation was discarded and PBMCs were re-suspended in 160 µl buffer and 80 µl microbeads. After incubation at 4°C for 15 min and centrifugation at 1200 rpm for 15 min, cells were re-suspended in 500 µl buffer and filtered using a screen mesh to get single cell suspension. LS Column was placed in the magnetic field of a MACS separator and rinsed with 500 µl buffer. The cell suspension was then applied onto the column, which was washed three times with 3 mL buffer thereafter. Cells which passed through the column were collected and frozen. The LS column was then removed from the separator, and the cells in the column were flushed out and collected into a tube. Cells were counted and seeded in a six-well plate at a density of 1×106/ml with 2 mL Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with granulocyte-macrophage colony-stimulating factor (100 ng/L) and IL-4 (100 ng/L, PeproTech, USA), and cultured at 37°C with 5% carbon dioxide. Half of the medium was replaced every other day, and the total amount of cytokines was added. On the 7th day, R848 (10 ng/mL, Sigma, USA) was added, and the cells as well as supernatant were collected on the 8th day.
Grouping of cells and treatment with drugs
CD14- cells were seeded onto 96-well plate and treated with L-shikonin or β, β-dimethylacryloyl alkannin for 48 h. Cell Counting Kit-8 (CCK-8, 10 µl) was then added, and the cells were incubated for 3 h. Later, optical density (OD) was measured using a microplate reader. Cells were divided into three groups: Unstimulated DCs group: no stimulation was carried out during cell culture; Stimulated group: cells were stimulated with R848 (10 ng/mL) for 24 h on the 7th day; and β, β-dimethylacryloyl alkannin group: cells were treated with β, β-dimethylacryloyl alkannin (12.5 μg/ml) for 24 h before the addition of stimulation factors on the 7th day.
Identification of DCs by flow cytometry
On the 7th day of culture, cells in unstimulated group and R848 (10 ng/mL) stimulated DCs group were suspended in 100 µl phosphate-buffered saline (PBS), and various antibodies (mouse anti human FITC-CD80, PE/Cy7-CD83, and PE-CD86 antibodies, BD Biosciences, USA) were added. Isotype control group and compensation group were established. After incubation at 4°C for 30 min away from light, cells were washed once with 1 mL PBS (centrifugation was carried out at 1500 rpm for 5 min) and analyzed using a flow cytometer (BD Biosciences, USA).
Mixed homogenous lymphocytes reaction
DCs in unstimulated group and R484 stimulated group were treated with 25 µg/mL mitomycin C, incubated at 37°C for 45 min, and washed three times with PBS. Later, cells were suspended in RPMI 1640 medium and seeded at the density of 1×102/well, 1×103/well, as well as 1×104/well in round-bottomed 96-well plates. Homogenous lymphocytes (4×105) were added into each well to make up a final volume of 200 µl. Three replicates were performed for each group. Cells were cultured for 5 days, and then 10 µl CCK-8 (Cell Counting Kit-8, Dojindo, Japan) was added into each well for further incubation of 2 h. The OD was measured using a microplate reader, and the average value of three wells was calculated.
Detection of IL-6, IL-12 p40, IL-23, IL-1β, TNF-α, and IL-10 messenger ribonucleic acid (mRNA) expression in DCs
Fluorescence quantitative RT-PCR was used in this analysis. Total RNA from each sample was extracted according to manufacturer’s instruction. The PCR amplification was carried out with fluorescent dye UltraSYBR Mixture through chimeric fluorescence method. The primers used were as follows: Glyceraldehyde 3-phosphate dehydrogenase F, 5’-CCTCTGACTTCAACAGCGACAC-3’, R 5’-TGGTCCAGGGGTCTTACTCC-3’, product length 174 bp; IL-6 F 5’-GAGAGTAGTGAGGAACAAGCCAGAG-3’, R 5’-CATTTGTGGTTGGGTCAGG-3’, product length 118 bp; IL-12 p35 F 5’-ATGCCTTCACCACTCCCAAAACCTG-3’, R 5’-TGGTTAATTCCAATGGTAAACAGGC-3’, product length 167 bp; IL-12 F 5’-GAGGAGAGTCTGCCCATTGAGGTC, R 5’-GGGTGGGTCAGGTTTGATGATGTC-3’, product length 108 bp; IL-23A F 5’-TGCTCCCTGATAGCCCTGTGG-3’, R 5’-TGGAGGCTGCGAAGGATTTTG-3’, product length 166 bp; IL-1β F 5’-AGTGGTGTTCTCCATGTCCTTTGTA-3’, R 5’-AGCTTGTTATTGATTTCTATCTTGT-3’, product length 139 bp; TNF-α F 5’-CTCCTCACCCACACCATCAGCCGCA-3’, R 5’-ATAGATGGGCTCATACCAGGGCTTG-3’, product length 135 bp; IL-10 F 5’-CCGAGATGCCTTCAGCAGAGTG-3’, R 5’-GCCTTGATGTCTGGGTCTTGGTT-3’, product length 156 bp. PCR was carried out as follows: 95°C 10 min; 50 cycles of 95°C, 15 s, and 60°C 60 s. Relative quantitative analysis was carried out using 2-ΔΔCt method.
Detection of inflammatory factors in supernatant using cytometric bead array (CBA) method
Supernatant in each group was collected after 48-hour stimulation with β, β-dimethylacryloyl alkannin, and a subsequent stimulation was carried with R848 for 24 h. The inflammatory factors including IL-6, IL-12 p40, IL-23, IL-1β, TNF-α, and IL-10 were detected using flow cytometry, according to manufacturers’ instructions supplied by CBA Kit (BD Biosciences, USA).
Statistical analysis
Data were presented as mean ± standard derivation. The statistical analyses were performed using SPSS15.0 software. T-test was used for comparison of data between two groups, while one-way analysis of variance was used for comparison of data between several groups. The values of α = 0.05 and P < 0.05 represent the differences with statistical significance, while P < 0.01 represent the differences with obviously statistical significance.
Results
Influence of alkannin on psoriasis mice model
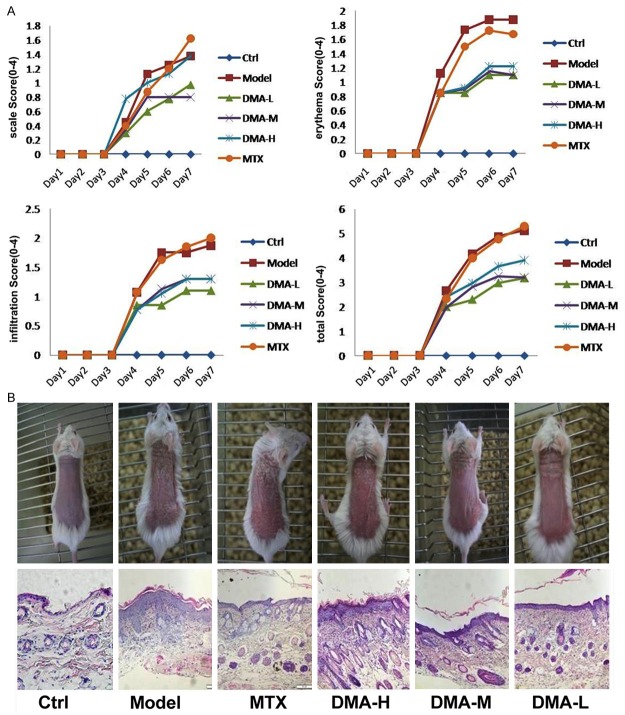
The morphological observations and pathological slices were shown in Figure 1. After 6-day treatment with drugs, mice in control group could move freely, with increased body weight, and they had rosy and smooth skin, as well as clear vessels under the skin. Mice in model group showed limited movement, with slowly increased or even decreased body weight when compared to control group. Moreover, mice in the model group had slightly increased temperature of skin, with obviously prominent erythema or flaky erythema in the skin of back. Almost all the naked skin was covered with layered and thick scales, which was easy to fall off, resulting in needle-shape bleeding point; the skin was obviously thickened, with apophysis. In MTX group, mice could move freely, with slightly increased body weight. Obviously improved symptoms were noted including small erythema, light color, less scales, and less-thickened skin.
Figure 1.
Morphological changes of skin lesions and psoriasis area and severity index scores of mice in treatment, model, and control groups.
The epidermal layer thickness of each mouse in all groups was measured after 5 days of treatment and the data were listed in Table 1. The epidermal thickness was significantly reduced after alkannin treatment at all tested concentrations, as well as after MTX treatment. The expression of IL-23 levels in mouse skin was detected using PCR method and the results were shown in Figure 2. The relative IL-23 mRNA expression was increased in model group compared to control, and this increased IL-23 mRNA expression was significantly suppressed in all alkannin-treated groups and in MTX group.
Table 1.
Epidermal layer thickness of each mouse from all groups was measured after 5 days of treatment (n = 6, x̅ ± s)
| Groups | Epidermis thickness (μm) |
|---|---|
| Control | 10.43±0.91* |
| Model | 78.26±5.64 |
| MTX | 47.61±3.38* |
| DMA-H | 53.1±4.01* |
| DMA-M | 45.46±2.60* |
| DMA-L | 31.30±1.43* |
P < 0.05 vs model.
MTX, methotrexate; DMA-H, alkannin-high dose group; DMA-M, alkannin-moderate dose group; DMA-L, alkannin-low dose group.
Figure 2.
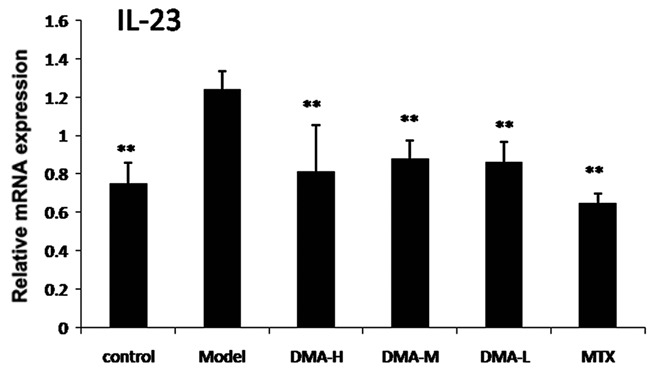
Expression of interleukin-23 mRNA levels in skin lesion.
Influence of alkannin on the impact of stimulated DCs on lymphocytes
After stimulation DCs with 10 ng/ml R848, the surface marker molecules of DCs including CD80, CD83, and CD86 were detected, and up-regulation of these markers indicated stimulation of DCs (Figure 3A). After treatment with 10 ng/ml R848 or 10 ng/ml R848+12.5 µg/ml alkannin for 24 h, 4 x 105 allograft lymphocytes were co-cultured with DCs of each group, and the proliferation rate of lymphocytes were measured using CCK-8 Kit. As shown in Figure 3B, 12.5 µg/ml alkannin treatment suppressed the activation of DCs induced by R848.
Figure 3.
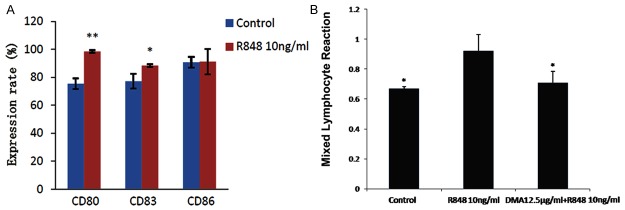
Influence of alkannin on the impact of stimulated dendritic cells on lymphocytes. A, the surface marker molecules of DCs including CD80, CD83 and CD86 in control and R848 treated group were detected by flow cytometry. *P < 0.05,**P < 0.01 vs Control; B, after treatment with 10 ng/ml R848 or 10 ng/ml R848+12.5 μg/ml alkannin for 24 h, 4 × 105 allograft lymphocytes were co-cultured with DCs of each group, and the proliferation rate of lymphocytes were measured using CCK-8 Kit. *P < 0.05 vs R848 group.
Influence of alkannin on the cytokines of DCs
The mRNA and protein levels of cytokines including IL-6, IL-12 p40, IL-10, IL-1β, TNF-α, and IL-23 were measured using RT-PCR and CBA methods. As shown in Figure 4A, the mRNA expression of IL-6, IL-12 p40, IL-1β, TNF-α, IL-23, and TLR8 were significantly suppressed after alkannin treatment; meanwhile, the mRNA expression of IL-10 was increased after alkannin treatment. Similarly, as shown in Figure 4B, the secretion of IL-6, IL-12 p40, IL-1β, TNF-α, IL-23, and IL-10 proteins were significantly suppressed after alkannin treatment.
Figure 4.
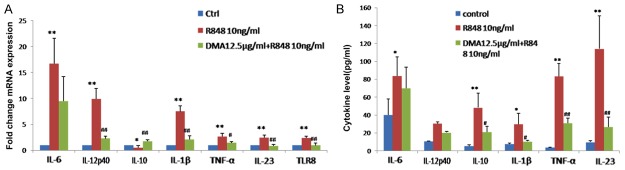
Effect of alkannin on mRNA expression and secretion of pro-inflammatory cytokines. After treatment with 10 ng/ml R848 or 10 ng/ml R848 + 12.5 μg/ml alkannin for 24 h, the mRNA (A) and protein (B) levels of cytokines including IL-6, IL-12 p40, IL-10, IL-1β, TNF-α, and IL-23 were measured using RT-PCR and CBA methods. *, # P < 0.05; **, ## P < 0.01 vs. Control.
Discussion
Psoriasis, commonly known as lepra alphos, is also called Bai Bi in traditional Chinese medicine. Psoriasis is a common skin disease with erythema and scales, and numerous researches in skin surgery are focused on psoriasis. The morbidity rate of psoriasis is 2-5%, which increases progressively with age. Currently, psoriasis has been defined in modern medical science as a kind of organ-specific autoimmune disease mediated via immune system [11]. DCs, kind of antigen-presenting cells with powerful functions, trigger the initiation of psoriasis. Once DCs are activated, cytokines secreted by DCs can activate Th1 and Th17 cells, which then produce abundant cytokines, chemokines, and growth factors, which constitute a “cytokine storm”. A vicious circle occurs in adjacent Malpighian cells, endothelial cells, neutrophils, and immune cells, which altogether lead to erythema in psoriasis. The activation of DCs is mainly mediated by signal pathways activated by TLRs [8]. TLRs are a kind of important proteins participating in nonspecific immunity (innate immunity); meanwhile, TLRs can bridge nonspecific immunity and specific immunity. During the pathogenesis of psoriasis, abundant autogenous deoxyribonucleic acid (DNA) and RNA are produced in erythematosus skin [12]. Antibacterial peptide LL37 can destroy the immune tolerance to autogenous DNA/RN and form an immune complex with autogenous DNA/RNA; after the immune complex is transported to DCs, plenty of inflammatory cytokines are produced via TLR8-mediated signaling pathway such as IL-6, IL-12, and TNF-α, which might lead to the pathogenesis and persistence of psoriasis [13].
Studies have indicated that the quantity of DCs was increased in acute skin lesion of patients with early psoriasis. Abundant CD123+/BDCA-2+/ChemR23+ pDCs have been found in skin lesions as well as in epidermis adjacent to skin lesions of patients with early psoriasis; whereas in chronic or long-term skin lesions, the amount of pDCs was low, implicating that abnormal DCs existed in the early stage or acute period of psoriasis [14,15]. Moreover, the amount of DCs in the peripheral blood of patients with psoriasis in acute period was significantly higher than that in normal patients; along with the improvement in clinical symptoms and histopathology after therapy, the expression of surface markers on DCs, CD1a, and human leukocyte antigen-DR, was also reduced [16]. Moreover, it was effective, to some extent, to treat psoriasis with cytokines secreted by DCs as targets. As reported, ustekinumab is a humanized monoclonal antibody against IL-12 and IL-23, which can bind to the p40 subunit of IL-12 and IL-23 and inhibit the biological activity of IL-12 and IL-23. In Krueger et al study, 320 patients with moderate to severe psoriasis were treated using ustekinumab, a monoclonal antibody against IL-12 and IL-23, which showed good therapeutic effect [17].
Traditional Chinese medicine showed advantages in therapy of psoriasis, especially in terms of cooling the blood and removing toxins in psoriasis therapy showed promising therapeutic effects; however, the targets and mechanism of actions were still unclear. L. erythrorhizon is a commonly used drug in therapy of psoriasis, with L-shikonin and β, β-dimethylacryloyl alkannin as the main chemical components; studies have indicated that shikonin have some immunosuppressive effects such as inhibition of the proliferation of lymphocytes and suppression of the cytokine secretion by activated lymphocytes [10]. However, fewer reports existed concerning the effects of β, β-dimethylacryloyl alkannin. Xia et al have demonstrated that β, β-dimethylacryloyl alkannin is the most abundant chemical component in L erythrorhizon, and hence, it may infer that β, β-dimethylacryloyl alkannin might also have some immunosuppressive effects [18].
The present study results indicated that alkannin could improve imiquimod cream induced psoriasis-like skin lesion in mice, as well as the suppression of expression of IL-23 in skin lesion. IL-23 is mainly secreted by mature DCs, and hence, it is inferred that the alkannin has shown some suppressive effect on DCs. In the in vitro experiment, 12.5 µg/mL alkannin could inhibit the proliferation of lymphocytes stimulated by R848 in mature DCs derived from human peripheral blood monocytes, suppress the expression of IL-6, IL-12, IL-23, IL-1β, and TNF-α mRNA by DCs, and promote the expression of IL-10 mRNA; meanwhile, it also impaired the secretion of IL-6, IL-12 p40, IL-1β, IL-23, and TNF-α, and promoted the secretion of IL-10. These data implicated that alkannin can suppress, to some extent, the activated DCs. As a result, it could be inferred that L erythrorhizon, the commonly used Chinese medicine in therapy of psoriasis, showed its therapeutic effect through inhibition of activated DCs. However, the influence of L erythrorhizon on other functions of activated DCs is still unclear, including phenotype of cells and the influence on phagocytosis and antigen presentation, which might be explored further in future. Moreover, the effect on activated DCs by other Chinese herbal monomers, which are effective in therapy of psoriasis, would be explored further in future to clarify the theoretical basis of these monomers on therapy of psoriasis.
Acknowledgements
The study was supported by National Natural Youth Fund.
Disclosure of conflict of interest
None.
References
- 1.Pietrzak AT, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, Osemlak P, Paszkowski T, Rolinski JM. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008;394:7–21. doi: 10.1016/j.cca.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 3.Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Wang J, Wang P, Li P, Wang L. Clinical observation of Liangxue Jiedu decoction for white facial skin disease (Psoriasis vulgaris) of blood heat syndrome. World J Integr Trad West Med. 2010;5:17–21. [Google Scholar]
- 5.Nam KN, Son MS, Park JH, Lee EH. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: neuroprotective implications. Neuropharmacology. 2008;55:819–825. doi: 10.1016/j.neuropharm.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Yan F, Wang R, Zhou H, Liu L. Shikonin Suppresses Human T Lymphocyte Activation through Inhibition of IKK beta Activity and JNK Phosphorylation. Evid Based Complement Alternat Med. 2013;2013:379536. doi: 10.1155/2013/379536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long S, GuangZhi Y, BaoJie G, Wei X, YanYong H, YingLi W, Yang Z, LiHua L. Shikonin derivatives protect immune organs from damage and promote immune responses in vivo in tumour-bearing mice. Phytother Res. 2012;26:26–33. doi: 10.1002/ptr.3503. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihisa Y, Hassan MA, Furusawa Y, Tabuchi Y, Kondo T, Shimizu T. Alkannin, HSP70 inducer, protects against UVB-induced apoptosis in human keratinocytes. PLoS One. 2012;7:e47903. doi: 10.1371/journal.pone.0047903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng R, Tang J, Xie BF, Feng GK, Huang YH, Liu ZC, Zhu XF. SYUNZ-16, a newly synthesized alkannin derivative, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of AKT/FOXO signal pathway. Int J Cancer. 2010;127:220–229. doi: 10.1002/ijc.25032. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Li P, Zhao JX, Wang Y. Effects of Lingxue Huoxue Capsule on Jurkat T Lymphocytes Proliferation, Activation and Cytokine Production. Chinese Journal of Experimental Traditional Medical Formulae. 2012;22:060. [Google Scholar]
- 11.Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 12.Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova JL, Su B, Li X. TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- 13.Monteleone G, Pallone F, MacDonald TT, Chimenti S, Costanzo A. Psoriasis: from pathogenesis to novel therapeutic approaches. Clin Sci (Lond) 2011;120:1–11. doi: 10.1042/CS20100163. [DOI] [PubMed] [Google Scholar]
- 14.Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen JD, Parmentier M, De Pita O, Girolomoni G, Sozzani S. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albanesi C, Scarponi C, Bosisio D, Sozzani S, Girolomoni G. Immune functions and recruitment of plasmacytoid dendritic cells in psoriasis. Autoimmunity. 2010;43:215–219. doi: 10.3109/08916930903510906. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Huang LM, McNutt NS, Krueger JG, Lowes MA. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. J Clin Immunol. 2009;29:247–256. doi: 10.1007/s10875-009-9278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M, Group CP. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, YJ Y. Determination of Radix Arnebiae Chemical ingredients. Strait Pharmaceutical Journal. 2007;19:35–37. [Google Scholar]