Abstract
Follicle-stimulating hormone (FSH) is associated with the pathogenesis of ovarian cancer. We sought to explore whether desmocollin 3 (Dsc3) mediates FSH-induced ovarian epithelial cancer cell proliferation and whether the EGFR/Akt signaling pathway may be involved in this process. Dsc3 positivity in ovarian tissue specimens from 72 patients was assessed by immunohistochemistry. The positive expression rates of Dsc3 were similar in ovarian cancer tissues (24/31:77.4%) and borderline ovarian tumor tissues (18/22:81.8%) (P>0.05), but were significantly higher in these cancerous tissues than in benign ovarian cyst tissues (3/19:15.8%) (P<0.05). Consistently, the expression of Dsc3 in four out of five ovarian cancer cells (HO8910, Skov3ip, Skov and Hey cells, but not ES-2 and in borderline ovarian MCV152 tumor cells was higher than in the immortalized ovarian epithelial cell line, Moody. FSH up-regulated the expression of Dsc3 and EGFR in a dose- and time-dependent manner. Furthermore, a converse relationship between the expression of Dsc3, EFGR and PI3K/Akt signaling was elucidated using RNA interference and PI3K/Akt inhibitor in the absence and presence of FSH. A role for these proteins in FSH-induced cell proliferation was verified, highlighting their interdependence in mediating ovarian cancer cell function. These results suggest that Dsc3 can mediate FSH-induced ovarian cancer cell proliferation by activating the EGFR/Akt signaling pathway.
Keywords: Ovarian cancer, follicle-stimulating hormone (FSH), Dsc3, EGFR/Akt signaling pathway, cell proliferation
Introduction
Ovarian cancer is a malignant tumor of the female reproductive system that severely threatens women’s health. Ovarian cancer, which is the most lethal cancer of all gynecological cancers, approximately causes 14000 deaths each year [1]. Follicle-stimulating hormone (FSH) is a contributing factor to the pathogenesis of ovarian cancer. Therefore, increased understanding of the molecular mechanisms of FSH has an important guiding significance for the treatment of ovarian cancer.
Desmocollin 3 (Dsc3) of the cadherin superfamily, is an important component of cell desmosomes [2]. Recent studies show that Dsc3 plays a role in the development of certain tumors [3-7]; however, no reports have assessed its expression in ovarian cancer. The loss of Dsc2, a related protein, has recently been shown to promote the proliferation of colonic epithelial cells in vitro through the activation of the epidermal growth factor receptor/serine/threonine protein kinase signaling pathway (EGFR/Akt signaling pathway) [8]. Studies suggest that the EGFR signaling way promotes the proliferation and resistance to apoptosis of cancer cells through PI3K/AKT signal transduction pathway [9]. We aimed to determine whether Dsc3 is expressed in ovarian cancer and whether it may mediate FSH-induced ovarian epithelial cancer cell proliferation through the activation of the EGFR/Akt signaling pathway. These results elucidate a new pathway of tumor growth activation, which increases the understanding of the mechanisms of pathogenesis that are prevalent in ovarian cancer.
Material and methods
Clinical specimens
Paraffin sections of ovarian tissue specimens were collected from 72 patients at the Department of Pathology in the Shanghai First People’s Hospital from 2007-2011. The specimens represent 31 epithelial ovarian cancer tissues, 22 borderline ovarian tumor tissues, and 19 benign epithelial ovarian tumor tissues. All patients provided complete clinical and pathological data. The pathological diagnosis and grading of the specimens were determined by two experienced pathologists who were blinded to patient identity. All patients signed informed consent before surgery. This experiment was approved by the Shanghai Changzheng Hospital Ethics Committee (Number: CZEC (2007)-02).
Cell lines
Epithelial ovarian cancer cell lines ES-2, HO8910, Skov3ip, Skov3, and Hey; borderline ovarian cystadenoma cell line MCV152; and the immortalized ovarian epithelial cell line Moody were preserved by the Youji Feng group of the Department of Obstetrics and Gynecology at the Shanghai First People’s Hospital.
Reagents and materials
Normal goat serum was from Shanghai Sun Biotech Co. Ltd. SSLABEL Polymer-HRP was from BioGenex. MCDB109/M199, DMEM-F12 medium, and fetal bovine serum were from Hyclone. FSH, thiazolyl tetrazolium (MTT). And dimethylsulfoxide (DMSO) were from Sigma. Immunohistochemical kits were from Santa Cruz Biotechnology. Dsc3 polyclonal antibody (mouse anti-human), Dsc3 monoclonal antibody (rabbit anti-human), EGFR monoclonal antibody (rabbit anti-human), Akt monoclonal antibody (rabbit anti-human), pAkt monoclonal antibody (rabbit anti-human), and GAPDH monoclonal antibody (rabbit anti-human) were from eBioscience, Abcam, EPITOMICS, R&D, and Cell Signaling Technology. Lipofectamine 2000 was from Invitrogen Corporation. siRNA was synthesized by Zimmer Technology Pharmaceutical Co. Ltd. ECL-emitting agents were from PerkinElmer.
Immunohistochemistry
The expression of Dsc3 protein was detected by S-P staining. The specimens were routinely deparaffinized, and the antigens were retrieved by high temperature heating: the sections were immersed in sodium citrate buffer (pH 6.0), boiled for 15 minutes in a pressure cooker and cooled at room temperature. After blocking in normal goat serum, the samples were incubated with the first antibody overnight and then incubated with the secondary antibody. DAB staining was performed under the microscope for 5 to 10 minutes, followed by hematoxylin counterstaining for 2 minutes. The specimens were then dehydrated and mounted after alcohol hydrochloride differentiation. Dsc3 mouse anti-human polyclonal antibody was diluted 1:10. Experimental procedures were performed in strict accordance with immunohistochemical reagent instructions. Each section was counted in five randomly selected horizons. Light yellow to brownish yellow or brown colored ovarian epithelial cells were considered positive. According to Remmele’s methods [10], scoring for the percentage of positive cells was as follows: 1 point for ≤30% positive cells, 2 points for 30%~70% positive cells, and 3 points for >70% positive cells. Scoring for the color depth was as follows: 0 points for no staining, 1 point for yellow staining, and 2 points for brownish yellow to brown staining. The final specimen score was calculated by multiplying the positivity score by the color depth score. According to the final score, the expression level of each index value was divided into the following categories: 0 points, negative, 1~3 points, weakly positive; 4~6 points, strongly positive. Representative images were captured using a photomicrography system.
Cell culture and treatment
Moody cells were cultured in MCDB109/M199 medium containing 15% fetal bovine serum. The other cells were cultured in DMEM-F12 medium containing 10% fetal bovine serum, 100 U/L penicillin and 100 U/L streptomycin. All cells were grown at 37°C in a 5% CO2 incubator. Experiments were performed using cells in the logarithmic growth phase. Cells were collected at confluence for the detection of Dsc3 expression by Western blotting. To assess the effects of FSH, Hey and HO8910 cells were serum starved for 24 hours, and then treated with FSH at a concentration of 0, 20, 40, or 80 IU/L for 0, 24, 48, or 72 hours. Effects on the expression of Dsc3 and EGFR were assessed by Western blotting. To determine the role of these proteins, the expression was silenced by the siRNA interference method. The role of the PI3K/Akt pathway was determined by treating cells with the specific PI3K/Akt pathway inhibitor LY294002 (10 μmol/L; Selleck, USA) for 1 hour, followed by treatment with FSH for 48 hours. Cells were then collected and the expression of cell signaling pathway modulators was determined by Western blotting or the effects on cell proliferation were determined by MTT assay.
Western blot analysis
After treatment and collection of cells, lysates were prepared and protein concentrations were normalized by the BCA protein determination method. 60 μg protein per well was loaded onto 10% SDS-PAGE gels. After electrophoresis, proteins were transferred to PVDF membranes, and the membranes were incubated with antibodies against Dsc3 (1:5000), EGFR (1:1000) (1:1000), Akt (1:1000), pAkt (1:1000), or GAPDH (1:1000) at 4°C overnight. After washing with TBST, the membranes were incubated with appropriate secondary antibodies for 1 hour and then washed with TBST. The expression of the targeted proteins was detected by ECL chemiluminescence. Experiments were repeated three times.
MTT analysis of cell proliferation
Hey or HO8910 cells were seeded in 96-well plates at a density of 1000~3000 cells/well. The edge wells were filled with sterilized 1×PBS and each well was added to 200 μL with DMEM medium containing 10% fetal bovine serum. The cells were incubated in 5% CO2 at 37°C until the cells adhered to the wells. Dsc3 or EGFR expression was silenced by the siRNA interference method and PI3K/Akt was inhibited with specific pathway inhibitor (10 μmol/L) for 1 hour, and then the cells were treated with FSH for 48 hours. The cells were harvested, and then put into 6 repetitive wells with the volume of 200 μL. 20 μL MTT solution (5 mg/ml; 0.5% MTT final concentration) was added into each well. Then the cells were incubated with 5% CO2, at 37°C for 4 hours. Then the culture medium was carefully Aspirated. 150 μL DMSO was added per well. The plates were oscillated for 10 min at a low speed to fully dissolve the crystals, and the optical density (OD) was measured by enzyme-linked immunosorbant assay at 490 nm.
siRNA interference
Cells were divided into the following groups: the siCon group: transfected with negative control (non-silencing) siRNA; the siDsc3 or siEGFR group: transfected with siRNA specifically targeting Dsc3 or EGFR; the FSH+siCon group: siCon group treated with FSH; the FSH+siDsc3 or FSH+siEGFR group: siDsc3 or siEGFR group treated with FSH. Hey cells or HO8910 cells in 6-well plates were starved for 24 h in serum-free OPTI-MEM. When the cell density reached 30% to 50%, the appropriate siRNA-liposome mixture was transfected cells according to the Lipofectamine 2000 instructions. After 12 hours transfection, the cells were treated with 40 IU/L FSH and then proteins were extracted proteins after 48 hours and the expression of Dsc3, EGFR, Akt, and pAkt was measured by Western blotting. MTT assays were performed 48 h after FSH treatment for assessing the effect of Dsc3 and EGFR on cell proliferation.
Statistical analysis
The data were statistically analyzed by SPSS17.0 software. Immunohistochemistry data were analyzed by the Kruskal-Wallis Test. The data from MTT assays were analyzed by single factor analysis of variance. The inspection level (α) was 0.05.
Results
Expression of Dsc3 is elevated in ovarian tumor tissues and cell lines
To determine whether Dsc3 is expressed at elevated levels in ovarian cancer, we assessed the Dsc3 expression in 72 specimens from surgically excised benign and cancerous ovarian tumor tissues (Figure 1A-C). The Dsc3 positive cells were located primarily at the cell membrane, and the Dsc3 positive expression rate was 77.4% (24/31) in ovarian cancer tissues and 81.8% (18/22) in borderline ovarian tumors, which is statistically higher than the rate in benign ovarian tumor tissues (15.8% [3/19]; P<0.05). The difference between the Dsc3 positive expression rate of borderline ovarian tumors and ovarian cancer was not statistically significant (P>0.05). These findings suggest that Dsc3 positivity is associated with cancerous ovarian tumors, but is not significantly different for ovarian cancer and borderline ovarian cancer types of tumors.
Figure 1.
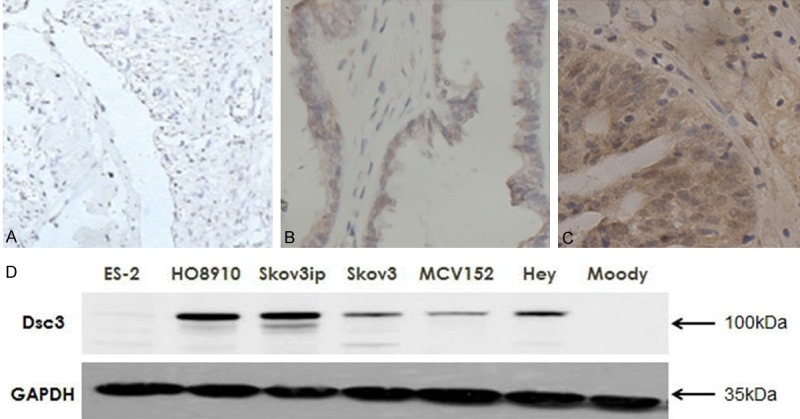
A-C. Expression of Dsc3 in different ovarian tissues determined by immunohistochemical staining (SP method). A. Benign ovarian cyst tissues; B. Borderline ovarian tumor tissues; C. Ovarian cancer tissues. Original magnification: ×100. D. Dsc3 expression was higher in the ovarian cancer cell lines HO8910, Skov3ip, Skov3, Hey and the borderline ovarian cystadenoma cell line MCV152 than the immortalized epithelial ovarian cells Moody.
To verify these findings, we assessed Dsc3 expression in seven different ovarian tissue cell lines by Western blotting. Dsc3 expression was higher in the ovarian cancer cell lines HO8910, Skov3ip, Skov3, Hey and the borderline ovarian cystadenoma cell line MCV152 than the immortalized epithelial ovarian cells Moody (Figure 1D), which supports a trend of higher Dsc3 positivity in ovarian cancer cells. The exception was the ovarian cancer cell line ES-2, which had levels that were similar to those of Moody cells. However, the relatively high expression in five out of six ovarian cancer cell lines is consistent with a correlation of Dsc3 expression with the cancerous state.
FSH induces dose- and time-dependent Dsc3 and EGFR expression in ovarian cells
To determine whether Dsc3 expression is regulated by FSH and whether Dsc3 expression correlates with EGFR expression, we performed Western analysis of Hey and HO8910 cells. Treatment with different concentrations of FSH for 48 hours led to a dose-dependent increase in the expression is at the FSH concentration of 40 IU/LDsc3 and the expression decreases slightly at the concentration of EGFR, which peaked at about 40-80 IU/L FSH (Figure 2A, 2B).
Figure 2.
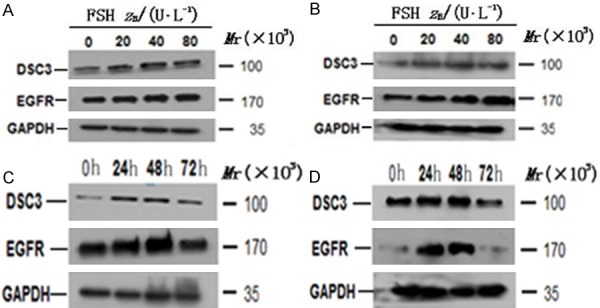
A and B. Effect of FSH of different concentrations on Dsc3 and EGFR expression detected by Western blotting analysis. A. Hey cells; B. HO8910 cells. C and D. Dsc3 and EGFR expression in Hey cells and HO8910 cells after treatment with FSH at the optimal concentration of 40 IU/L. C. Hey cells; D. HO8910 cells.
To establish a time course of induction, we treated Hey cells and HO8910 with the FSH optimal concentration of 40 IU/L for 24 hours, 48 hours, and 72 hours. The expression of Dsc3 and EGFR was the highest at 48 hours and was down regulated by 72 hours (Figure 2C, 2D), suggesting that the induction of these proteins is both dose-dependent and time-dependent.
FSH activates an autoregulatory pathway of Dsc3, EGFR and AKT signaling in ovarian cells
To determine whether Dsc3 expression is necessary for the activation of EGFR expression and downstream Akt signaling, we transfected specific siRNA (siDsc3) or a non-targeting control siRNA (siCon) into Hey and HO8910 cells. Our results showed that Dsc3 siRNA specifically inhibits the expression of EGFR in both untreated and FSH-treated cells. Furthermore, levels of pAkt were reduced with no significant effect on total Akt levels, suggesting an effect on Akt signaling (Figure 3A, 3B). These results suggest that Dsc3 positively regulates the activation of the EGFR and Akt signaling pathways, which are enhanced by FSH.
Figure 3.
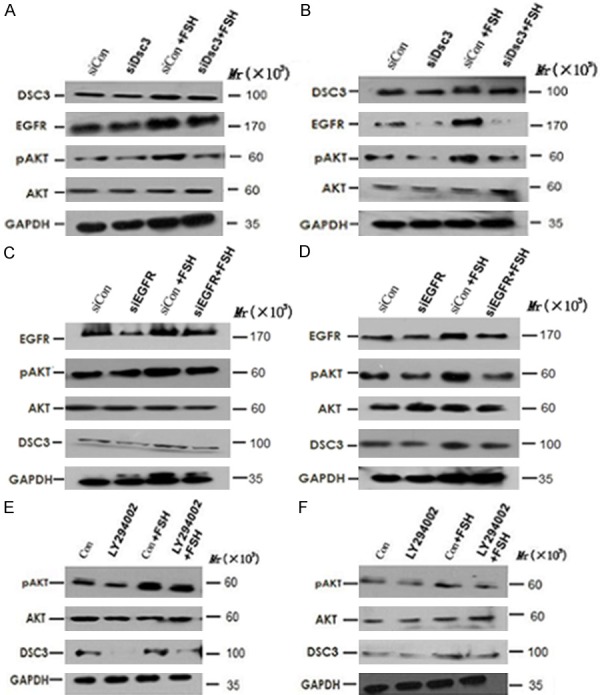
A and B. Specific siRNA (siDsc3), a non-targeting control siRNA (siCon) and their combination with FSH were transfected into Hey and HO8910 cells. These results suggest that Dsc3 positively regulates the activation of the EGFR and Akt signaling pathways, which are enhanced by FSH. A. Hey cells; B. HO8910 cells. C and D. siRNA targeting EGFR (siEGFR) and their combination with FSH were transfected into Hey and HO8910 cells. These results suggest that Dsc3 and EGFR are converse positive regulators and that each of these proteins is upstream of Akt signaling. C. Hey cells; D. HO8910 cells. E and F. Hey and HO8910 cells were treated with LY294002, which is a specific inhibitor of the PI3K/Akt pathway. These results suggest that Dsc3, EGFR and Akt are activated by FSH within a positive autoregulatory loop. E. Hey cells; F. HO8910 cells.
To determine whether EGFR can also regulate the expression of Dsc3, we transfected Hey and HO8910 cells with siRNA targeting EGFR (siEGFR). As expected, siEGFR transfection resulted in reduced Akt signaling, as evidenced by the reduced pAkt levels in siEGFR-transfected cells as compared to siCon-transfected cells without or with FSH treatment. Furthermore, Dsc3 expression was reduced upon siEGFR transfection (Figure 3C, 3D). These results suggest that Dsc3 and EGFR are converse positive regulators and that each of these proteins is upstream of Akt signaling.
To further determine whether Dsc3 expression is regulated by PI3K/Akt signaling, we treated Hey and HO8910 cells with LY294002, which is a specific inhibitor of the PI3K/Akt pathway. As expected, LY294002 reduced the activation of pAkt both with and without FSH treatment. Furthermore, LY294002 led to decreased levels of Dsc3 expression in both cell lines (Figure 3E, 3F). These results suggest that Dsc3, EGFR and Akt are activated by FSH within a positive autoregulatory loop.
FSH activation of ovarian cell proliferation is dependent on Dsc3, EGFR and PI3K/Akt signaling
The above results suggest that Dsc3 functions in a regulatory loop that affects EGFR and Akt signaling by FSH. To determine whether this regulatory loop mediates the function of FSH in stimulating the proliferation of ovarian cancer cells, we performed MTT assays. Proliferation was reduced by siRNA-mediated reduction of Dsc3 or EGFR and by PI3K/Akt pathway inhibitor treatment, both with or without FSH treatment (Figure 4; P<0.05). These results suggest that the Dsc3/EGFR/Akt axis functions in mediating ovarian cell proliferation, which is enhanced by FSH.
Figure 4.
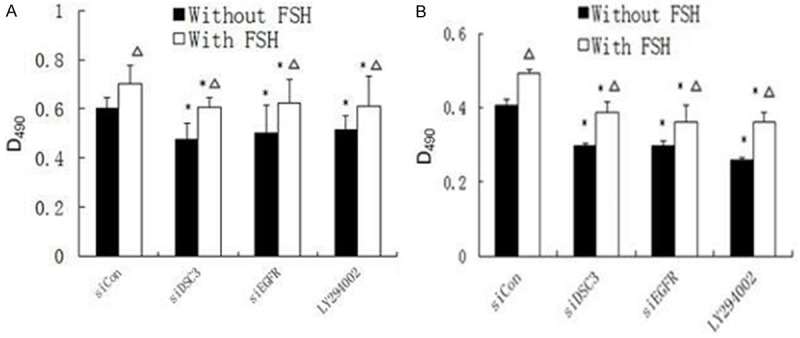
MTT assay showed that proliferation was reduced by siRNA-mediated reduction of Dsc3 or EGFR and by PI3K/Akt pathway inhibitor treatment, both with or without FSH treatment. These results suggest that the Dsc3/EGFR/Akt axis functions in mediating ovarian cell proliferation, which is enhanced by FSH. A. Hey cells; B. HO8910 cells.
Discussion
Ovarian cancer has the highest mortality rate of all gynecologic malignancies, mainly due to its residency deep in the pelvic cavity. Its localization makes ovarian cancer difficult to detect early, so that the majority of ovarian cancer patients are diagnosed at advanced stages. Consequently, an understanding of the molecular basis of ovarian cancer will help to develop drugs that target specific sites toward the development of effective treatments. Ovaries are the main targets of FSH [11], which promotes the growth of normal ovarian cells, but also is associated with ovarian cancer cell proliferation. Epidemiological studies have shown that the incidence of ovarian cancer in postmenopausal women is significantly higher than in women of reproductive age [12]. Furthermore, high serum FSH levels in perimenopausal and postmenopausal women are closely related to the development of ovarian cancer. One study [13] showed that the concentrations of FSH are significantly different between benign and malignant epithelial ovarian and borderline ovarian tumors and also varies with the extent of malignancy (P<0.05). On the basis of this association, many reports have focused on the mechanism of FSH in the progression of ovarian cancer. For example, Lin et al. [14] showed that FSH can stimulate the proliferation of epithelial ovarian cancer through a signal transduction pathway mediated by protein kinase C, while Yiwen et al. [15] showed that FSH regulates proliferation and invasion of epithelial ovarian cancer ES-2 cells through specific AT sequence binding protein 1.
To further understand the molecular mechanisms of ovarian cancer and the function of FSH, we explored a potential association of Dsc3 expression with ovarian cancer. Recent studies show that Dsc3 plays a role in the development of certain tumors [16,17]. For example, Dsc3 DNA methylation may lead to reduced expression in colorectal cancer [4]; and loss of Dsc3 leads to increased incidence rate of proto-oncogene K-Ras-mediated skin cancer [4]. Dsc3 is highly expressed in nearly half of undifferentiated large cell lung cancers [6], as well as in poorly differentiated pancreatic ductal cancer (99.1%) [8]. However, there are few reports about Dsc3 expression in ovarian cancer. We sought to determine whether Dsc expression may also correlate with ovarian cancer and whether its expression might be associated with specific pathways of tumor cell proliferation. To this end, we selected paraffin sections of ovarian cancer and normal ovarian epithelial tissues from the Shanghai First People’s Hospital for immunohistochemical experiments. Results show that Dsc3 is highly expressed in ovarian cancer and borderline ovarian tumors, compared to normal ovarian epithelial cells. Furthermore, Dsc3 expression is elevated in four out of five ovarian cancer cell lines and one borderline ovarian tumor cell line as compared immortalized ovarian epithelial cells. Each of these differences is statistically significant (P<0.05), which emphasizes the potential role of Dsc3 in ovarian cancer.
It has been reported that loss of Dsc2, a cognate molecule of Dsc3, can promote colon epithelial cell proliferation by activating the EGFR/Akt signaling pathway and resultant transformation into a malignant phenotype [8]. Consequently, we explored whether Dsc3 promotes FSH-regulated ovarian cancer cells proliferation through the EGFR/Akt signaling pathway, thereby promoting tumor development. Our results show that FSH up regulates the expression of Dsc3 and EGFR in a time and dose dependent manner. Epidemiological investigations have found that the incidence of ovarian cancer is increased in patients with FSH elevation [18]. In normal menstruation, the level of FSH content in the blood is about 5~20 IU/L before ovulation and increases to no more than 30 IU/L. However, in postmenopausal or ovarian function-declined patients, the serum level of FSH can be greater than or equal to 40 IU/L [19]. Furthermore, the serum concentration of FSH is (24.74±18.7) IU/L in ovarian cancer patients [19]. Two previous studies have determined that the cell proliferation of ovarian cancer cell peaks at the FSH concentration of 40 IU/L [20,21]. As a result, in this study, we selected the 20, 40, and 80 IU/L concentrations of FSH to treat ovarian cancer cells. Previous studies have determined 48 h as optimal time for treatment with FSH [22], and we therefore selected 24, 48, 72 hours as the time range of FSH effects. Consistent with the latter studies, we observed that a concentration of 40 IU/L and treatment time of 48 hours was optimal for the induction of Dsc3 and EGFR expression.
To determine a possible effect of Dsc3 and EGFR/Akt in regulating Dsc3/EGFR expression and Akt signaling, we employed RNA interference and specific inhibitor to block the expression and activity of each pathway. The results demonstrate that Dsc3 and EGFR regulate each other and the activation of the Akt pathway, and that Akt mediates FSH-dependent Dsc3 expression. These findings are suggestive of an autoregulatory loop that is enhanced by FSH. MTT assays also showed that each of these proteins is important in the proliferation of ovarian cancer cells, which suggest a functional outcome of the Dsc/EGFR/Akt axis that could contribute to the pathology of ovarian cancer. Few reports have determined whether Dsc3 can affect the invasion or apoptosis of ovarian cancer or other tumor cell, and there is little information about the role that Dsc family proteins may play in the progression of cancer [23]; however, this study provides a step forward to the understanding of this family of proteins. The characterization of Dsc3 and other cancer-promoting genes may help to identify new tumor therapeutic targets for the treatment of human tumors.
Disclosure of conflict of interest
None.
References
- 1. WHO, IARC GLOBOCAN, Cancer Incidence and Mortality Worldwide in 2008 at http://globocan.iarc.fr/
- 2.Aoyama Y, Yamamoto Y, Yamaguchi F, Kitajima Y. Low to high Ca2+-switch causes phosphorylation and association of desmocollin 3 with plakoglobin and desmoglein 3 in cultured keratinocytes. Exp Dermatol. 2009;18:404–408. doi: 10.1111/j.1600-0625.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- 3.Cui T, Chen Y, Yang L, Knösel T, Zöller K, Huber O, Petersen I. DSC3 expression is regulated by p53, and methylation of DSC3 DNA is a prognostic marker in human colorectal cancer. Br J Cancer. 2011;104:1013–1019. doi: 10.1038/bjc.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, O’Shea C, Fitzpatrick JE, Koster MI, Koch PJ. Loss of desmocollin 3 in skin tumor development and progression. Mol Carcinog. 2012;51:535–545. doi: 10.1002/mc.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Liu T, Wang Y, Cao L, Nishioka M, Aguirre RL, Ishikawa A, Geng L, Okada N. Altered expression of desmocollin 3, desmoglein 3, and β-catenin in oral squamous cell carcinoma: correlation with lymph node metastasis and cell proliferation. Virchows Archiv. 2007;451:959–966. doi: 10.1007/s00428-007-0485-5. [DOI] [PubMed] [Google Scholar]
- 6.Monica V, Ceppi P, Righi L, Tavaglione V, Volante M, Pelosi G, Scagliotti GV, Papotti M. Desmocollin-3: a new marker of squamous differentiation in undifferentiated large-cell carcinoma of the lung. Mod Pathol. 2009;22:709–717. doi: 10.1038/modpathol.2009.30. [DOI] [PubMed] [Google Scholar]
- 7.Hamidov Z, Altendorf-Hofmann A, Chen Y, Settmacher U, Petersen I, Knösel T. Reduced expression of desmocollin 2 is an independent prognostic biomarker for shorter patients survival in pancreatic ductal adenocarcinoma. J Clin Pathol. 2011;64:990–994. doi: 10.1136/jclinpath-2011-200099. [DOI] [PubMed] [Google Scholar]
- 8.Kolegraff K, Nava P, Helms MN, Parkos CA, Nusrat A. Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/β-catenin signaling. Molecul Biol Cell. 2011;22:1121–1134. doi: 10.1091/mbc.E10-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mc-Cubrey JA, Steelman LS, Chappel WH, Abrams SL, Wong E, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathologe. 1987;8:138. [PubMed] [Google Scholar]
- 11.Viqar S, Gregory U, Samuel CM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. [PubMed] [Google Scholar]
- 12.Stadel BV. Letter: The etiology and prevention of ovarian cancer. Am J Obstet Gynecol. 1975;123:772. doi: 10.1016/0002-9378(75)90509-8. [DOI] [PubMed] [Google Scholar]
- 13.Li LX, Chen LH, Wang YQ, Guo J. Expression and significance of FSH in epithelium ovarian tumor. Modem Oncolog. 2009;17:927–928. [Google Scholar]
- 14.Sheng L, Liu DY, Shen K. Preliminary study on pathway of follicle-stimulating hormone on human epithelial ovarian cancer cell proliferation. Zhonghua Fu Chan Ke Za Zhi. 2004;38:752–755. [PubMed] [Google Scholar]
- 15.Feng YW, Wei YN, Zhu Y, Zhou DM, Wu L, Yang T, Xi XW, Zhang JB, Feng YJ. Follicle stimulating hormone controls proliferation and invasion activity of epithelial ovarian cancer es-2 cells by specialat-rich sequence-binding protein 1. Progress in Modern Biomedicine. 2011;11:2801–2805. [Google Scholar]
- 16.Tsuta K, Tanabe Y, Yoshida A, Takahashi F. Utility of 10 immunohistochemical markers including novel markers (desmocollin-3, glypican 3, S100A2, S100A7, and Sox-2) for differential diagnosis of squamous cell carcinoma from adenocarcinoma of the Lung. J Thorac Oncol. 2011;6:1190–1199. doi: 10.1097/JTO.0b013e318219ac78. [DOI] [PubMed] [Google Scholar]
- 17.Oshiro MM, Kim CJ, Wozniak RJ, Junk DJ, Muñoz-Rodríguez JL, Burr JA, Fitzgerald M, Pawar SC, Cress AE, Domann FE, Futscher BW. Epigenetic silencing of DSC3 is a common event in human breast cancer. Breast Cancer Res. 2005;7:R669. doi: 10.1186/bcr1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng YZ. Chinese Obstetrics and Gynecoly. 1st edition. Beijing: People’s Medical Publishing; 1999. pp. 1755–1761. [Google Scholar]
- 19.Halperin R, Hadas E, Angre R. Peritonealfluid gonadotropins and ovarian hormones in patients with ovarian cancer. Int J Gynecol Cancer. 1999;9:502–507. doi: 10.1046/j.1525-1438.1999.99075.x. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Lu JJ, Luo F, Zheng Y, Feng Yj, Felix JC, Lauchlan SC, Pike MC. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the efect by luteinizing hormone. Gynecol Oncd. 2000;76:80–88. doi: 10.1006/gyno.1999.5628. [DOI] [PubMed] [Google Scholar]
- 21.Song GH, Liu XS. Effects of Human Follicle Stimulating Hormone on Cell Proliferation and E-cadherin Expression in Human Epithelial Ovarian Cancer Cell Lines. Journal of Practical Obstetrics and Gynecology. 2004;20:294–297. [Google Scholar]
- 22.Hu ZY, Deng XG, Yao ZW. Study of the regulatory effects of reproductive hormones on growth of ovarian cancer cell Iine H08910 in vitro. Journal of Chong Qing Medical University. 2004;29:148–152. [Google Scholar]
- 23.Syed SH, Trinnaman B, Martin S, Major S, Htchinson J, Magee AI. Molecular interactions between desmosomal cadherins. Biochem J. 2002;362:317–327. doi: 10.1042/0264-6021:3620317. [DOI] [PMC free article] [PubMed] [Google Scholar]


