Abstract
Heatstroke not only directly induces cell injury, but also causes large amounts of inflammatory mediators release and cells with extensive biological activities to induce a systemic inflammatory response and immune dysfunction. This study aimed to observe the effects of JAK2 inhibitor AG490 on the brain injury and inflammatory responses of rats with systemic heatstroke. Under the light microscope, the hippocampus tissues of rat with heatstroke were edema and apoptotic rate was increased. Up-regulation of malondialdehyde (MDA), nitric oxide synthase (iNOS), reactive oxygen species (ROS) and down-regulation of superoxide dismutase (SOD) were also found after heatstroke in rats, which compared with that of the control group. Heatstroke induced inflammation factors secretions and up-regulated levels of matrix metallopeptidase 2 and 9 (MMP2 and MMP-9) and systemic inflammatory response molecules including intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor-beta 1 (TNF-β1) and cyclooxygenase-2 (COX-2). However, the JAK2 inhibitor AG490 was significantly attenuated the brain injury and inflammatory responses induced by heatstroke in rats. The survival time of heatstroke rats showed that AG490 notably lived longer than heatstroke rats without AG490 treatment. These findings suggest that AG490 may prevent the occurrence of heatstroke via inhibiting the JAK2/STAT3 pathway and the systemic inflammatory responses.
Keywords: Heatstroke, AG490, JAK2/STAT3, inflammation
Introduction
Heatstroke may be defined as overheating of the body, including heatstroke caused by an over-high ambient temperature, or as an abnormally high body temperature [1]. The potential causes of heatstroke include infection, certain drugs and medications, and brain trauma [2]. High temperature may be used for tumor treatment, particularly for cancer treatment, but controversial issues remain in its clinical use [3]. Heatstroke can progress to multiple organ dysfunction or injury syndrome (MODS) and death, despite adequate lowering of the victim’s body temperature and intensive care [1]. Up to 30% of survivors may sustain permanent neurological damage [1].
Cell apoptosis in heatstroke is not well understood, which may explain the high mortality as no specific mechanisms can be targeted for treatment. Recently many studies showed the important roles of cell apoptosis in heatstroke and molecular mechanism involved in was not known [4]. In addition, increasing evidence shows that the Janus kinase 2 (JAK2)/STAT signaling pathway, especially STAT3, is the critical target and biomarker during angiogenesis and tumor growth. JAK2/STAT3-mediated apoptosis involved in protecting hepatocytes, myocardium, and heart against ischemia/reperfusion injury [5-7], especially through the phosphorylation of STAT3 to promote survival and inhibit apoptosis. JAKs and STATs are normally expressed in brain [8], and their expression increases after focal ischaemia in the rat, particularly in reactive astrocytes and microglia cells [9], indicating that JAK/STAT is an important mediator of inflammatory responses in brain ischaemia. However, the role of JAK/STAT pathway in rat heatstroke has not been known.
Studies in humans and animals suggest that the inflammatory of the host to heat stress contribute to the multiple tissue and organ injury in those who survive the initial deleterious effects of heatstroke [10]. A recent report has demonstrated that heatstroke rats display increased levels of markers for cellular ischemia and damage, and increased expression of inducible nitric oxide synthase (iNOS) [11]. In addition, various serum molecules including tumor necrosis factor-alpha (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and E-selectin have been demonstrated to be involved in the pathophysiology of systemic inflammatory response syndrome [12,13]. A study reported that heatstroke-induced elevation of the levels of heat shock protein 70 relieved the extent of the pulmonary fibrosis of rats in response to the induction of acute lung injury by lipopolysaccharide (LPS) administration [14]. However, the effects of heatstroke on the brain of rats and its mechanism of action remain unclear at present. In the clinic, cases of fatal heatstroke caused by various intraoperative factors are frequently reported, and a comprehensive treatment measure is the key to successful treatment [15,16].
In the present study, we investigated the protection of AG490, a JAK2 inhibitor, in rats with heatstroke, which induces cell apoptosis and inflammation. Our findings showed that JAK2/STAT3 pathway mediating inflammatory responses induced by heatstroke rats.
Materials and methods
Animals and AG490 treatment
A total of 36 specific pathogen-free Sprague Dawley male rats with body weights ranging from 180 to 220 g were purchased from Shanghai Laboratory Animal Company (Shanghai, China) and randomly divided into three groups, with twelve rats in each group. The groups were as follows: the control group (the rats were maintained at room temperature, without medication), the heatstroke group (the rats were placed at 42°C, without medication), and the JAK2 inhibitor AG490 treatment group (AG490 was administered before 2 h of heating). All protocols were approved by the Animal Ethics Committee of the General Hospital of Jinan Military Command (Jinan, China) in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Induction of heatstroke
The rats were anesthetized with 3% sodium pentobarbital (45 mg/kg) by intraperitoneal injection and placed into a heating chamber with a biological oxygen supply. Also, the previously examined rectal temperature was used as the basic value. Subsequently, the rats in all groups other than the control group were heated in the heating chamber at 42°C and a relative humidity of 60%. For the AG490 treatment group, AG490 was administered before 2 h of heating, respectively. After 1 h of heating, all rats were removed from the heating chamber and treated as subsequently described.
Histology
Hippocampus of rats were removed, and then fixed with 10% (v/v) neutral buffered formalin. Specimens were dehydrated and embedded in paraffin. For histological examined, 4 mm sections of fixed embedded tissues were cut on a Leica model 2165 rotary microtome (Leica Microsystem Nussloch GmbH, Wetzlar, Germany), placed on glass slides, deparaffinized, and stained sequentially with hematoxylin and eosin (H&E, Richard-Allan Scientific, Kalamazoo, MI). Stained tissue sections on slides were analyzed under identical light microscope (Axio Imager M1, Karl Zeiss, Germany) at ×200 magnification.
Terminal dUTP Nick-End labeling assay
Terminal dUTP Nick-End labeling assay was performed using a kit for programmed cell death (Medical & Biological Laboratories, Nagoya, Japan) according to the manufacturer’s directions. The fixed areas of section were examined by microscopy and the numbers of TUNEL-positive cells were counted by a pathologist at ×200 magnification, 30 fields per section. Blinding was performed for the pathologist’s grading of results.
Biochemical measurements
Hippocampus tissue was homogenized with physiological saline at a ratio of 1:9 (weight/volume) in a glass homogenizer. The homogenate was centrifuged at 1,000 g for 10 min, and the supernatant was collected to determine superoxide dismutase (SOD) and inducible nitric oxide synthase (iNOS) activity using an SOD kit and iNOS kit. To determine the malondialdehyde (MDA) and reactive oxygen species (ROS) content using a MDA kit and ROS kit. The kits used in this study were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Measurements of TNF-α, IL-1β, IL-6 and IL-8 in peripheral blood
TNF-α, IL-1β, IL-6 and IL-8 levels present in peripheral blood were determined using commercially available murine-specific sandwich enzyme-linked immunosorbent assay (ELISA) kit supplied by Santa Cruz (USA).
Western blot analysis
Tissue samples were homogenized in 13.2 mmol/l Tris-HCl, 5.5% glycerol, 0.44% SDS, and 10% β-mercaptoethanol. An equal amount of extracted soluble protein (50 μg) was fractionated by Tris-glycine-SDS polyacrylamide gel (12%) electrophoresis, and Western blotting was performed as described with use of a polyclonal rabbit antibody to recombinant rat p-JAK2 (1:500), JAK2 (1:1000), p-STAT3 (1:20000), STAT3 (1:100), MMP2 (1:500), MMP-9 (1:500), ICAM-1 (1:800), TGF-β1 (1:400), COX-2 (1:500) and GAPDH (1:1500). All the antibodies were purchased from Abcam (Jinan, China) except GAPDH (CST, Danvers, MA, USA).
Statistical analysis
The data was presented as the mean value ± S.D. The paired, two-tailed Student’s t-test was used to analyze the significance of difference between groups. Survival analysis was carried out by Kaplan-Meier method, and subjected to the log rank test. P value lower than 0.05 was considered to be statistically significant.
Results
AG490 treatment attenuates injury and apoptosis during heatstroke
We compared the survival time in heatstroke rats. The cumulative survival rate was significantly lower in heatstroke rats than that in heatstroke rats with AG490 treatment (Figure 1A). These results indicated that AG490 could represent a new prognostic factor in heatstroke rats. As shown in Figure 1B, after heatstroke in rats, the hippocampal neuron damage scores were significantly increased in rats with heatstroke compared with the control group. Histopathological verification revealed edema and the nucleus disappeared in the hippocampal neuron of heatstroke-induced rats (Figure 1B). However, AG490 treatment had neuroprotective effects. Figure 1C showed absence of or only a few scattered TUNEL-positive cells of hippocampal neuron in control group. In severe heatstroke, TUNEL-positive staining indicative of apoptotic cell death was extensive in hippocampal neuron compared with the control group (Figure 1C). However, the extensive apoptotic cells of hippocampal neuron in heatstroke-induced rats were significantly attenuated by AG490 treated rats.
Figure 1.
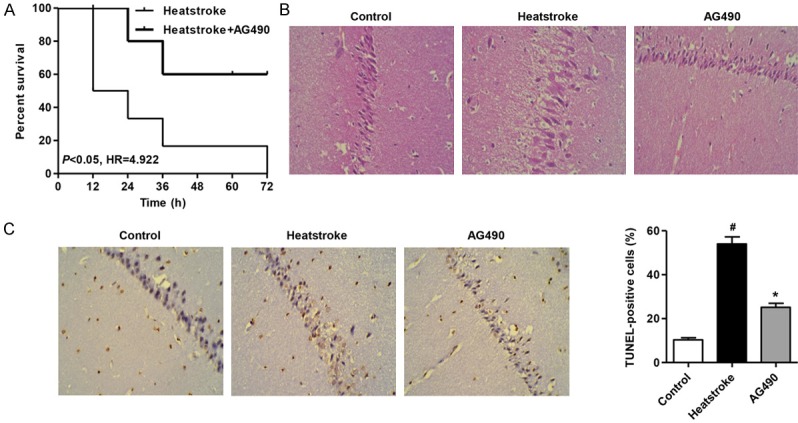
Histological examination of neuronal damage and TUNEL-positive cells. A. Survival analysis showed that heatstroke rats had a poor prognosis compared to heatstroke rats with AG490 treatment. B. After 1 h heat exposure, the AG490 treatment protected the damage of edema and disappearance of nucleus in heatstroke-induced rats. C. AG490 treatment suppressed the increase of the number of TUNEL-positive cells of hippocampal nucleus in heatstroke-induced rats. #P < 0.01 compared the control group. *P < 0.01 compared with the heatstroke group.
AG490 treatment inhibits of heatstroke-induced up-regulation of MDA, iNOS, ROS levels and down-regulation of SOD level
The comparisons of the MDA, iNOS, ROS and SOD levels in rat with heatstroke were shown in Figure 2. The MDA, iNOS and ROS levels of hippocampus tissue in rats with heatstroke were significantly higher than that in the control group (Figure 2A-D), and the SOD level of hippocampus tissue in rats with heatstroke were significantly lower than that in the control group (Figure 2B). However, the MDA, iNOS, ROS and SOD levels were reverse in the AG490 treatment group respectively.
Figure 2.
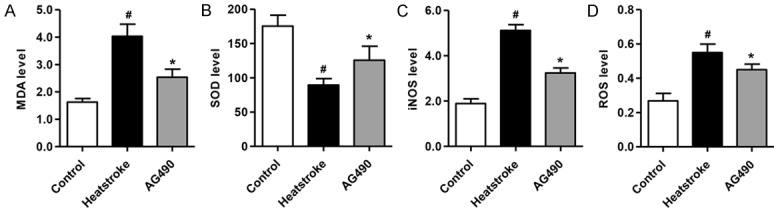
Effect of AG490 on the levels of MDA, iNOS, ROS and SOD. AG490 treatment inhibition of up-regulation of MDA (A), iNOS (C), ROS levels (D) and down-regulation of SOD level (B) induced by heatstroke. #P < 0.01 compared with the control group. *P < 0.01 compared with the heatstroke group.
AG490 treatment represses heatstroke-induced inflammation factor secretions
Next we measured TNF-α, IL-1β, IL-6 and IL-8 secretions in response to heatstroke. After exposure of rats to heat stress for 1 h, TNF-α, IL-1β, IL-6 and IL-8 secretions were significantly increased, respectively (Figure 3A-D). Pretreatment with AG490 for 2 h before exposure to heat stress markedly inhibited TNF-α, IL-1β, IL-6 and IL-8 secretions from rats, respectively. These results suggest that AG490 possesses an anti-inflammatory effect in heatstroke-induced rats.
Figure 3.
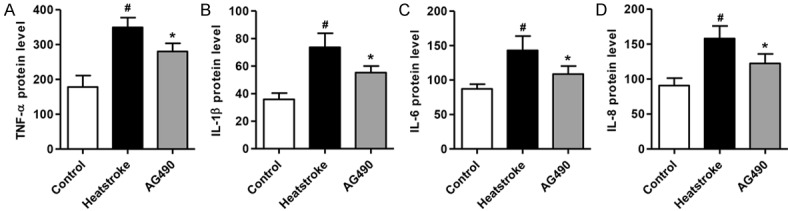
AG490 inhibits heatstroke-induced TNF-α, IL-1β, IL-6 and IL-8 secretions. Rats were exposed to heatstroke for 1 h in the absence of presence of AG490. ELISA was performed to detect the levels of TNF-α, IL-1β, IL-6 and IL-8 in peripheral blood. #P < 0.01 compared with the control group. *P < 0.01 compared with the heatstroke group.
Inhibition of injury is involved in the protection of AG490 against inflammation in heatstroke-induced rats
Since inhibitor AG490 was implicated in the inhibitory effect on JAK2, we further explored the role of AG490 against heatstroke-induced inflammatory responses. As shown in Figure 4A, pretreatment of rats with AG490 suppressed p-JAK2/JAK2 and p-STAT3/STAT3 levels. Similar to the anti-inflammatory effect of JAK2/STAT3, pretreatment of rats with AG490 suppressed heatstroke-induced increase of MMP2 and MMP-9 protein levels (Figure 4B). In addition, compared with the control group, heatstroke rats had higher levels of ICAM-1, TGF-β1, COX-2 after the onset of heatstroke (Figure 4C). The increase in the protein levels of these three markers caused by heatstroke were significantly reduced by AG490 pretreatment.
Figure 4.
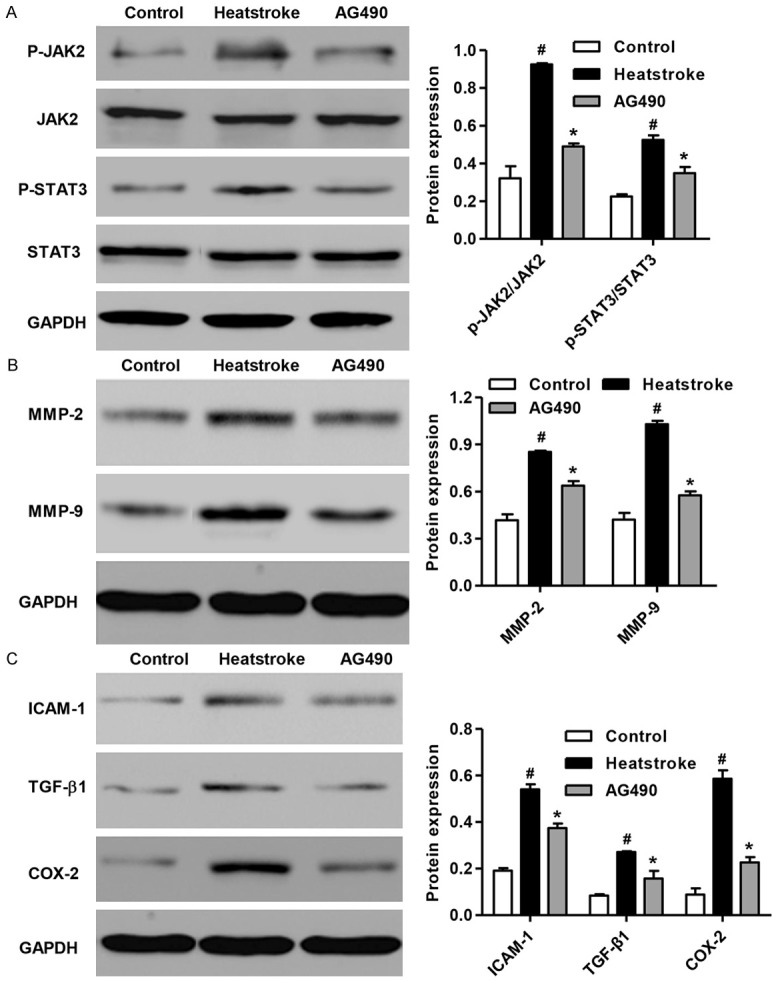
Effect of AG490 on JAK2/STAT3 pathway and inflammation. AG490 treatment suppressed activation of JAK2/STAT3 pathway and increase of inflammation related protein, MMP2 and MMP-9. And AG490 treatment also inhibition of inflammation factors secretions induced by heatstroke. #P < 0.01 compared with the control group. *P < 0.01 compared with the heatstroke group.
Discussion
Heatstroke is defined as a condition in which the core temperature is elevated to a critical level that induced multi-organ damage and dysfunction [17]. Evidence has accumulated to suggest that thermoregulatory deficits may occur during heatstroke. For example, unanesthetized, unrestrained heatstroke mice displayed hypothermia when exposed to room temperature [11,18-20]. The hypothermia that occurred after onset of heatstroke may have resulted from neuronal apoptosis and systemic imflammation in the hypothalamus [21]. In the current study, we demonstrated the protective effects of JAK2 inhibitor AG490 in reducing heatstroke-induced hippocampal neuronal apoptosis and inflammation responses.
In terms of heatstroke-induced nervous system injury, the administration of dexamethasone and mannitol in combination was associated nervous system damage in rats with heatstroke [22]. Consistent with the previous study, our data showed that the hippocampal neuron in rats with heatstroke resulted in appearance of edema and disappearance of the nuclear (Figure 1A). However, AG490 treatment attenuated the brain injury induced by heatstroke in rats. Studies in cell lines and animal models suggest that heat directly induces not only tissue injury but also cell death [17]. Rat models subjected to moderate whole body hyperthermia showed that accelerated apoptosis also contributes to cell death, but whether apoptosis is an important cause of cell death in patients with heatstroke is not known [23]. A major finding in this study was the dominance of apoptosis as a mechanism of cell death in heatstroke. Using TUNEL assay we observed significant increase in cell apoptosis of hippocampal neuron in heatstroke-induced rats (Figure 1B). However, rats with AG490 treatment showed inhibition of cell apoptosis of hippocampal neuron with heatstroke.
In the present study, the results showed that AG490 suppressed the MDA, iNOS and ROS up-regulation and SOD down-regulation induced by heatstroke in rats (Figure 2A-D). These findings were similar to those in multiple organic damages [24. 25]. Heatstroke not only directly induces cell injury of tissue, but also causes to release large amounts of inflammatory mediators to induce a systemic inflammatory response. The plasma levels of inflammatory cytokines such as TNF-α and IL-1β are elevated in persons and animals with heatstroke [26-28]. The increase in the plasma levels of these pro-inflammatory cytokines is associated with the severity of heatstroke. A number of studies have shown that AG490 is able to effectively reduce the levels of serum pro-inflammation cytokines, such as TNF-α, IL-1, IL-6 and IL-8 [29,30]. The results in the present study showed that AG490 suppressed the increase of TNF-α, IL-1, IL-6 and IL-8 in levels induced by heatstroke in rats (Figure 3A-D). To further investigate the inflammatory responses induced by heatstroke, we also detected the protein expression of MMP-2, MMP-9, ICAM-1, TGF-β1 and COX-2. MMP-2 and MMP-9 as two novel markers of inflammation were significantly increased (Figure 4B) and the expression of inflammatory molecules, including ICAM-1, TGF-β1 and COX-2 was also increased in heatstroke-induced rats (Figure 4C). However, the rats treated with AG490 inhibited the activation of JAK2/STAT3 pathway and followed by inhibited the increase of inflammation-associated proteins expression (Figure 4A-C).
In conclusion, our results demonstrate that, heatstroke-induced hippocampal neuron injury and apoptosis, and the systemic inflammatory response can be significantly prevented by JAK2 inhibitor AG490 treatment. These findings indicate that JAK2/STAT3 may improve heat tolerance by reducing the occurrence of apoptosis and the systemic inflammatory response.
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Disclosure of conflict of interest
None.
References
- 1.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 2.Varghese G, John G, Thomas K, Abraham O, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J. 2005;22:185–87. doi: 10.1136/emj.2003.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takagi M, Sakata K, Someya M, Matsumoto Y, Tauchi H, Hareyama M, Fukushima M. The combination of hyperthermia or chemotherapy with gimeracil for effective radiosensitization. Strahlenther Onkol. 2012;188:255–61. doi: 10.1007/s00066-011-0043-6. [DOI] [PubMed] [Google Scholar]
- 4.Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F, Bouchama A. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke a study in baboon model. Arterioscl Throm Vas. 2008;28:1130–36. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- 5.Yu HC, Qin HY, He F, Wang L, Fu W, Liu D, Guo FC, Liang L, Dou KF, Han H. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. 2011;54:979–88. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55:275–86. doi: 10.1111/jpi.12070. [DOI] [PubMed] [Google Scholar]
- 7.Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY, Ji Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz J Med Biol Res. 2012;45:898–905. doi: 10.1590/S0100-879X2012007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planas A, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc T. 2006;34:1267–70. doi: 10.1042/BST0341267. [DOI] [PubMed] [Google Scholar]
- 9.Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke M, Sommer C, Schwab S. Neuroprotective Effect of Granu- locyte Colony–Stimulating Factor After Focal Cerebral Ischemia. Stroke. 2003;34:745–51. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 10.Lu KC, Wang JY, Lin SH, Chu P, Lin YF. Role of circulating cytokines and chemokines in exertional heatstroke. Crit Care Med. 2004;32:399–403. doi: 10.1097/01.CCM.0000108884.74110.D9. [DOI] [PubMed] [Google Scholar]
- 11.Shen KH, Lin CH, Chang HK, Chen WC, Chen SH. Premarin can act via estrogen receptors to rescue mice from heatstroke-induced lethality. Shock. 2008;30:668–74. doi: 10.1097/SHK.0b013e31817538cb. [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J Biol Chem. 2001;276:7614–20. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T, Yoshioka H. Association between heat stress protein 70 induction and decreased pulmonary fibrosis in an animal model of acute lung injury. Lung. 2007;185:287–93. doi: 10.1007/s00408-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 15.Firstenberg M, Abel E, Blais D, Andritsos M. Delayed malignant hyperthermia after routine coronary artery bypass. Ann Thorac Surg. 2010;89:947–48. doi: 10.1016/j.athoracsur.2009.07.088. [DOI] [PubMed] [Google Scholar]
- 16.Pişkin B, Atac MS, Konca E, Yildirim M, Avsever H, Şevketbeyoğlu H. A Suspected case of malignant hyperthermia after tooth extraction: case report. J Oral Maxil Surg. 2011;69:1331–34. doi: 10.1016/j.joms.2010.05.097. [DOI] [PubMed] [Google Scholar]
- 17.Bouchama A, Roberts G, Al Mohanna F, El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R, Loualich A, Nakeeb S. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol. 2005;98:697–705. doi: 10.1152/japplphysiol.00461.2004. [DOI] [PubMed] [Google Scholar]
- 18.Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol. 2006;100:1400–1409. doi: 10.1152/japplphysiol.01040.2005. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Premachandran S, Bagewadikar RS, Bhattacharya S, Chattopadhyay S, Poduval T. Arginine metabolic pathways determine its therapeutic benefit in experimental heatstroke: Role of Th 1/Th 2 cytokine balance. Nitric Oxide. 2006;15:408–16. doi: 10.1016/j.niox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee S, Premachandran S, Sharma D, Bagewadikar RS, Poduval T. Therapeutic treatment with L-arginine rescues mice from heat stroke-induced death: physiological and molecular mechanisms. Shock. 2005;24:341–47. doi: 10.1097/01.shk.0000180983.55623.2b. [DOI] [PubMed] [Google Scholar]
- 21.Liu WS, Chen CT, Foo NH, Huang HR, Wang JJ, Chen SH, Chen TJ. Human umbilical cord blood cells protect against hypothalamic apoptosis and systemic inflammation response during heatstroke in rats. Pediatr Neonatol. 2009;50:208–16. doi: 10.1016/S1875-9572(09)60065-6. [DOI] [PubMed] [Google Scholar]
- 22.Yang TH, Ho WY, Shih MF, Leu KL, Wen YS, Liu CC. Effects of combination treatment with dexamethasone and mannitol on neuronal damage and survival in experimental heat stroke. Biol Pharm Bull. 2010;33:1522–28. doi: 10.1248/bpb.33.1522. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR, Danhauser LL, Jenkins GN, Bull JM. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res. 1995;55:5459–64. [PubMed] [Google Scholar]
- 24.Ge G, Zhang Q, Ma J, Qiao Z, Huang J, Cheng W, Wang H. Protective effect of Salvia miltiorrhiza aqueous extract on myocardium oxidative injury in ischemic–reperfusion rats. Gene. 2014;546:97–103. doi: 10.1016/j.gene.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Yan X, Qiu W, Jia B, Zhong H, Li X, Chen Z. Myocardial protection by interferon-γ late preconditioning during cardiopulmonary bypass-associated myocardial ischemia-reperfusion in pigs. Oncol Rep. 2013;30:2145–52. doi: 10.3892/or.2013.2707. [DOI] [PubMed] [Google Scholar]
- 26.Niu K, Lin K, Yang C, Lin M. Protective effects of alpha-tocopherol and mannitol in both circulatory shock and cerebral ischaemia injury in rat heatstroke. Clin Exp Pharmacol Physiol. 2003;30:745–51. doi: 10.1046/j.1440-1681.2003.03905.x. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao SH, Chang CP, Chiu TH, Lin MT. Resuscitation from experimental heatstroke by brain cooling therapy. Resuscitation. 2007;73:437–45. doi: 10.1016/j.resuscitation.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Li J, Pan X, Ding G, Cao H, Jiang W, Zheng J, Zhou H. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-α release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-κB activation. Int Immunopharmacol. 2010;10:344–50. doi: 10.1016/j.intimp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–78. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, Moilanen E. Leptin Enhances Synthesis of Proinflammatory Mediators in Human Osteoarthritic Cartilage—Mediator Role of NO in Leptin-Induced, IL-6, and IL-8 Production. Mediat Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]


