Abstract
Luteolin can be found in many traditional Chinese medicines, it’s a falconoid compound derived from Lonicera japonica Thunb. This study aims to investigate the neuroprotective effects of luteolin against cognitive impairment induced by amyloid-β (Aβ) peptide and the underlying mechanisms in rats. The animal behavioral tests showed that luteolin could ameliorate Aβ-induced learning and memory impairment. In hippocampal tissue, the activity of choline acetyl transferase (ChAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) increased after treated by luteolin. Luteolin also reversed the increased activity of acetylcholine esterase (AchE). In hippocampi homogenate, the content of acetylcholine (Ach) increased, but malondialdehyde (MDA) reduced. Moreover, luteolin can increase Bcl-2/Bax ratio. This study demonstrated that luteolin could protect Alzheimer’s disease (AD) rats against Aβ-induced cognitive impairment through regulating the cholinergic system and inhibiting oxidative injuries. The results suggesting that luteolin may have potential as a therapy for AD.
Keywords: Luteolin, Aβ peptide, Alzheimer’s disease, antioxidant activity, neuroprotection
Introduction
Alzheimer’s disease (AD), an irreversibly progressive neurodegenerative disorder, is more likely to affect older people. It associate with memory loss and its typical symptoms are memory impairment and cognitive decline [1-3]. At present, the pathogenesis of AD remains unclear and what we can do just symptomatic alleviation [4], so seeking for effectively therapeutic agent becomes urgently.
The previous study showed that cholinergic therapies produced effective improvement in AD patients [5]. Enhance the levels of acetylcholine (Ach) in the brain is very important in disease prevention. It could help prevent neuronal degeneration by compensating the loss of cholinergic neuron. Moreover, oxidative damage and formation of oxidized lipids and proteins have been observed in the brain of patients with AD [6]. Antioxidants can help prevent neuronal degeneration by preventing the generation of free radicals [7-10], and antioxidant therapy can prevent the learning and memory deficits [11]. According to these researchers, we can surmise that adjusting the balance of cholinergic system and reducing oxidative stress, could be an effective measure to treat AD.
Luteolin (3,4,5,7-tetra-hydroxylflavone) can be found in many traditional Chinese medicines. It has shown multiple functions including anti-inflammatory, anti-oxidative and anti-carcinogenic effects, etc [12-15]. In recent years, it has been applied in pharmacological and clinical practice. This study has examined if luteolin could improve learning and memory impairment induced by infusion of Aβ (1-40) peptide in rats, further to determine the effect of luteolin on cholinergic system and oxidative injury.
Numerous studies have demonstrated that soluble amyloid-β (Aβ) oligomers can induce neuronal damage [16-18]. In this study, we establish cognitive impairment model through continuous intracerebroventricular infusion of Aβ (1-40) peptide in rats.
Materials and methods
Materials
Luteolin was obtained from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China), the purity of the chemical was more than 98.0%. Aβ (1-40) peptide was purchased from Sigma (Saint Louis, USA). Bcl-2, Bax and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Animals
Sprague-Dawley rats (250 ± 20 g) were obtained from the Experimental Animal Center of Suzhou Aiermaite technology Co. Ltd. (SPF grade, Certificate No. SCXK20140007). The rats were housed in a room under temperature (23 ± 2°C), relative humidity (50 ± 10%), 12 h light/dark cycle and free access to water and food.
Ethics statements
All animal experiments were performed in accordance with the Institutional Animal Care Committee of Yantaishan hospital.
Experimental procedure
Rats were randomly assigned to five groups: (I) sham control group; (II) Aβ (1–40); (III) Aβ (1–40) + luteolin 50 mg/kg; (IV) Aβ (1–40) + luteolin 100 mg/kg; (V) Aβ (1-40) + luteolin 200 mg/kg. Each group consisted of 10 rats.
Aβ (1-40) peptide was dissolved in 0.9% saline solution at a concentration of 1 nM/µl and incubated at 37°C for one week [19]. Rats were anesthetized with 10% chloral hydrate (300 mg/kg), Aβ (1-40) (2 µl) was slowly infused into the right lateral ventricle (A-3.0, L-2.0, V-3.3) [20]. The rats in the luteolin treatment groups (groups III, IV and V) received luteolin orally once daily for 17 days, respectively. I and II groups received the same volume of physiologic saline.
Morris water maze test
The Morris water maze test [21] was carried out after the last administration and was evaluated blind to the treatments by the observer.
The apparatus consisted of a circular water tank (130 cm diameter and 50 cm high). The water temperature was kept at 24 ± 1°C. Animals were tested for place-learning acquisition with the escape platform (11 cm diameter) located in the middle of the southeast quadrant, 1.5 cm below water surface. Swimming paths of the rats were monitored by a video camera linked to a computer through an image analyzer (SMART, Panlab SL, Barcelona, Spain). Training were performed four times daily for 5 days, introducing the rats randomly from each of the four starting positions while facing the wall, and allowing them to swim freely until they found the platform. At the end of each trial, the rat was allowed to stay on the platform for 10 s. The rats were guided to the platform and left there for 10 s if failing to find the platform within 120 s.
On the sixth day, the platform was removed and allowing each rat to swim freely for 120 s inside the pool, escape latency to find the hidden platform was measured.
Single-trial passive avoidance test
The Single-trial passive avoidance test was evaluated by the method of Bagheri and the minor modlifications [22]. The apparatus consisted of an illuminated box (20 cm × 30 cm × 40 cm) and a dark box (20 cm × 30 cm × 40 cm). On the first and second days, rats were placed in the apparatus for 15 min to habituate. On the third day, rats were placed in the illuminated compartment for 5 min. Then the guillotine door was lifted, as soon as the rat had entered the dark chamber through the guillotine door, a single electric (1 mA, 1 s) was applied. The interval between placement in the illuminated chamber and entry into the dark chamber was measured as step-through latency (STL). 24 h after the shock, the rat was placed in the illuminated box again and the STL was determined. The test was concluded when the animal entered into the dark compartment, the observation period was regarded as 5 min.
Biochemistry measurement
After behavioral tests, the rats were anesthetized with 10% chloral hydrate (300 mg/kg), the hippocampus were isolated quickly. Then weighed the hippocampus and prepared as a 5% (w/v) tissue homogenate in 0.9% saline solution. The homogenate was centrifuged and the supernatant was collected. The levels of acetylcholine esterase (AchE), choline acetyl transferase (ChAT), Ach, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and malondialdehyde (MDA) were measured according to the manufacturer’s instructions (Nanjing Jiancheng Technology Co., Ltd).
Western blot analysis
The levels of Bcl-2 and Bax were detected by western blot analysis. The rat hippocampus was homogenized in lysis buffer containing complete protease inhibitor cocktail (1 M Tris–HCl (pH 8.0), 5 M NaCl, 10% Nonidet P-40 and 1 M 1,4-dithio-dl-threitol (DTT)). After quantitated with the bicinchoninic acid (BCA) protein assay kit (Beyotime Biotech, Shanghai, China), proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into polyvinylidene difluoride (PVDF) membrane. After the blots were blocked with 5% fat-free dried milk for 2 h at room temperature, the membranes were incubated overnight at 4°C with corresponding primary antibodies. Subsequently, the membranes were incubated with the corresponding secondary antibodies at room temperature for 2 h. The blots were visualized with enhanced chemiluminescence (ECL) detection system (Amersham), and the results were analyzed by LabImage version 2.7.1 (Kapelan GmbH, Halle, Germany).
Statistical analysis
Statistical analysis was performed by using SPSS 17.0, data were reported as the mean ± SD. Differences between groups were analyzed by one-way analyses of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered to indicate statistically significant.
Results
Luteolin significantly improved Aβ-induced impairments in spatial learning and spatial working memory
The escape latency to find the hidden platform in all rats during the water maze test is shown in Table 1. The results suggested that Aβ-injected rats had spatial learning and memory impairment. While, administration of luteolin caused a significant improvement in the longer escape latencies compared to relevant data for the Aβ group (P < 0.05, P < 0.01). These results indicate that Aβ injection leaded to the impairment in spatial learning and memory, and treatment with luteolin significantly attenuated the impairment of performance in the test trials.
Table 1.
Effects of luteolin on the escape latency to find the hidden platform in water maze test
| Groups | 1 day | 2 day | 3 day | 4 day | 5 day | 6 day |
|---|---|---|---|---|---|---|
| sham | 34.17 ± 6.12 | 29.11 ± 5.73 | 20.09 ± 4.46 | 13.31 ± 1.99 | 8.43 ± 1.72 | 8.31 ± 2.41 |
| Aβ | 73.41 ± 12.10 | 61.71 ± 10.47 | 43.44 ± 9.93 | 39.93 ± 10.03 | 30.41 ± 7.01 | 25.72 ± 6.03 |
| Aβ + luteolin 50 mg/kg | 66.76 ± 10.03 | 53.91 ± 11.12 | 40.03 ± 9.97 | 30.11 ± 8.83 | 22.12 ± 5.13 | 18.92 ± 4.41 |
| Aβ + luteolin 100 mg/kg | 58.61 ± 6.41* | 40.11 ± 9.41* | 28.37 ± 6.13* | 21.51 ± 4.81* | 16.16 ± 3.71* | 13.93 ± 2.86* |
| Aβ + luteolin 200 mg/kg | 41.07 ± 6.35# | 30.15 ± 6.92# | 22.17 ± 7.69# | 15.16 ± 4.31# | 13.93 ± 3.17# | 10.33 ± 2.86# |
Values are mean ± SD.
P < 0.05 vs. Aβ group;
P < 0.01 vs. Aβ group.
Luteolin significantly improved Aβ-induced impairments in cognitive capacities
The data of STL of rats in the single-trial passive avoidance test are presented in Figure 1. No significant differences were observed between the groups in initial latency. The Aβ-injected rats developed a marked impairment in STL compared with the sham-operated rats (43.04 ± 4.21 s and 98.12 ± 2.13 s, respectively). However, treatment with luteolin reversed this impairment. The results showed that treatment with luteolin did produce a significantly improvement in cognitive capacities in Aβ-injected rats.
Figure 1.
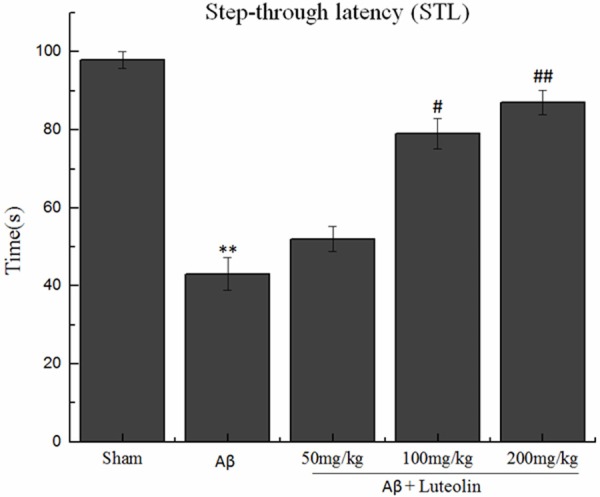
Effects of luteolin on step-through latency in the single-trial passive avoidance test. **P < 0.01 vs. sham group, #P < 0.05, ##P < 0.01 vs. Aβ group.
Effect of luteolin on the levels of Ach, AchE and ChAT in hippocampi homogenate
We observed the effects of luteolin on the content of Ach, and the activities of AchE and ChAT in hippocampus of rats. The finding showed (Figure 2) that the contents of Ach and the activity of AchE increased in luteolin treated groups (P < 0.05, P < 0.01), but ChAT activity decreased in the hippocampus. We could deduce that luteolin could significantly influence the cholinergic system.
Figure 2.
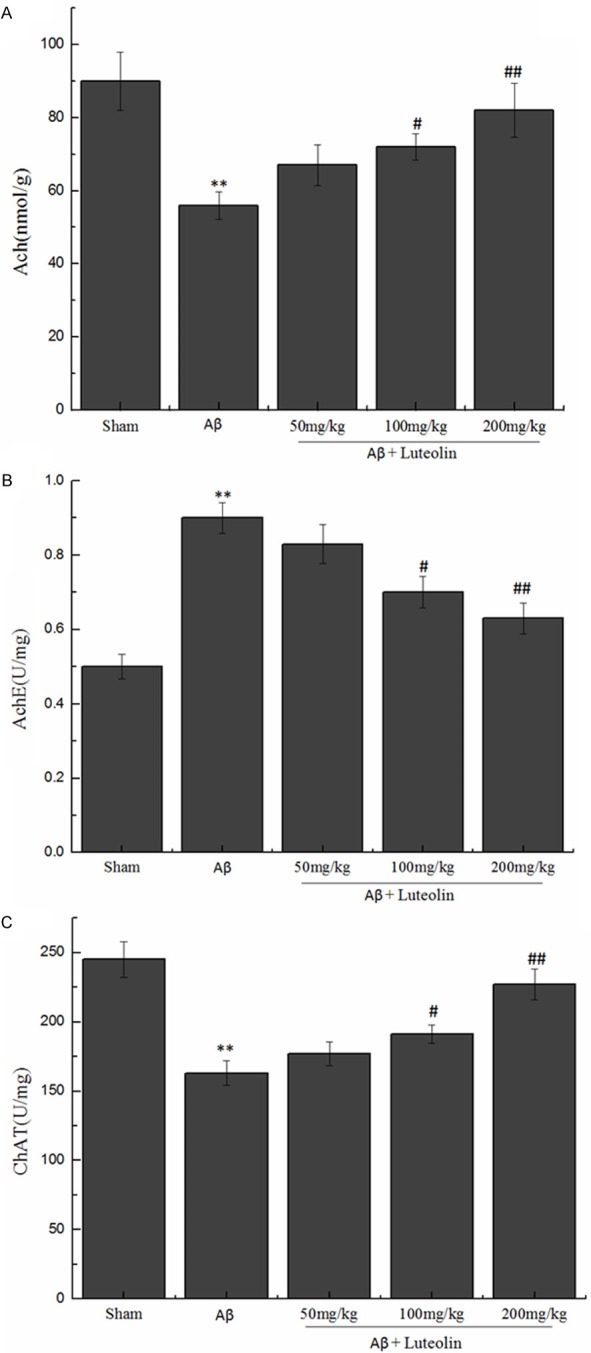
Effects of luteolin on the levels of Ach, AchE and ChAT in hippocampi homogenate. **P < 0.01 vs. sham group, #P < 0.05, ##P < 0.01 vs. Aβ group.
Effect of luteolin on the levels of SOD, GSH-Px and MDA in hippocampi homogenate
In this study, we tested the homogenate levels of the antioxidant enzymes (SOD and GSH-Px) and end product of oxidation (MDA). The results showed a significant decline in the level of SOD and GSH-Px (104.42 ± 8.81 U/mg protein and 7.41 ± 1.84 nmol/mg protein, respectively), along with the noticeable rise of the level of MDA (8.33 ± 0.48 nmol/mg protein). Those dates were observed in Aβ-injected rats compared with sham-operated rats, while treatment with luteolin increased the homogenate level of SOD and GSH-Px, simultaneously decreased the homogenate MDA level (P < 0.05, P < 0.01) (Figure 3).
Figure 3.
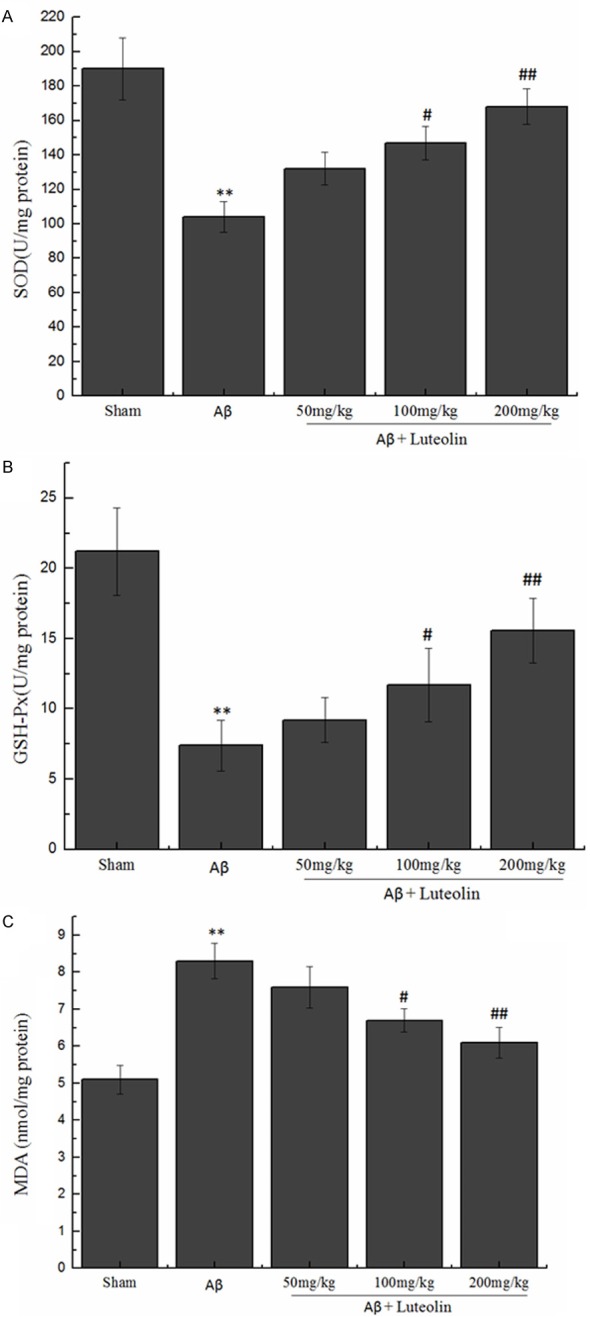
Effects of luteolin on the levels of SOD, GSH-Px and MDA in hippocampi homogenate. **P < 0.01 vs. sham group, #P < 0.05, ##P < 0.01 vs. Aβ group.
Effect of luteolin on Bcl-2/Bax ratio in hippocampi homogenate
To confirm the effects of luteolin on apoptosis in Aβ-injected rat model of AD, we tested Bcl-2/Bax ratio. The results showed that the Bcl-2/Bax ratio decreased in Aβ-injected alone groups compared with sham group (Figure 4). While treatment with luteolin this decrease was greatly blocked. The results suggested that luteolin protected Aβ-injected rat model of AD via regulating the Bcl-2 family proteins.
Figure 4.
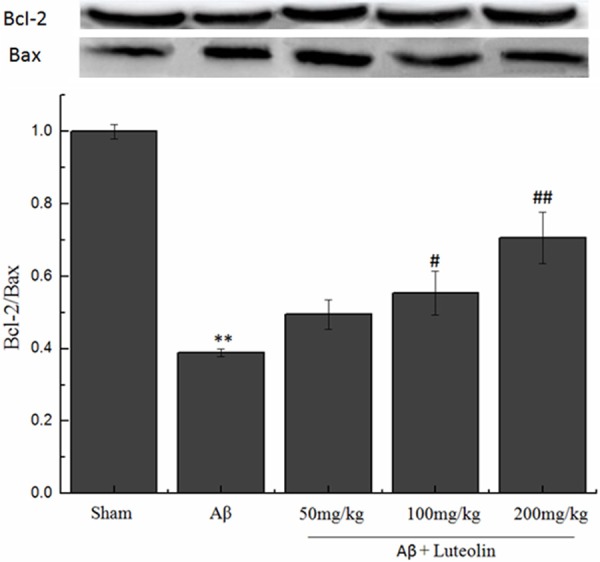
Effects of luteolin on apoptosis induced by infusion of Aβ peptide. **P < 0.01 vs. sham group, #P < 0.05, ##P < 0.01 vs. Aβ group.
Discussion
Aβ is the key player in neuronal damage and dementia in AD patients, in this study, we established cognitive impairment model through continuous intracerebroventricular infusion of Aβ (1-40) peptide in rats. Our study confirmed that continuous infusion of Aβ (1-40) into the cerebral ventricle induced spatial learning and spatial working memory deficits in the rats. The findings showed that luteolin significantly ameliorated the impairment of spatial learning and working memory in Morris water maze test and single-trial passive avoidance test. These results demonstrate that luteolin could protect AD rats against Aβ-induced cognitive impairment.
Previous studies have showed that Aβ (1-40) can lead to cholinergic dysfunction and cognitive impairment [23,24]. Ach is a key transmitter in brain which influences learning and memory. Enhance the levels of Ach in the brain is very important in disease prevention, it could help prevent neuronal degeneration by compensate the loss of cholinergic neuron. ChAT and AchE are important for maintaining a stable level of Ach in brain. In our study, the cholinergic system was slightly impaired in the Aβ-infused rats. The results showed a significant increased activity of AchE and decreased activity of ChAT, as well as a significant decrease in the content of Ach in hippocampus of rats. While, treated with luteolin could reverse this abnormality. It was demonstrated that luteolin could significantly influence the cholinergic system, also explain the behavioral improvement we observed in the treated rats.
Oxidative damage plays an important role in the development and progression of AD [25], which was associated with Aβ-induced neuronal damage. In the present study, we found that luteolin reversed Aβ-induced oxidative injuries. There are some enzymes in cellular protection against damage from oxygen-derived free radicals, such as GSH-Px and SOD. The main physiological function of GSH-Px is free radical scavenging, antioxidant and anti-aging. SOD is able to transform intracellular superoxide anion to H2O2. MDA is the end-product of the oxygen-derived free radicals and lipid oxidation, which reflects the damage caused by reactive oxygen species. Our study showed that the injection of Aβ (1-40) in rats decreased the activities of SOD and GSH-Px, but enhanced MDA production. However, luteolin could significant ameliorate these abnormalities. These biological parameters demonstrated the luteolin appears to be an effective antioxidant, and the neuroprotective effects of luteolin might because of its antioxidant capacity.
Some studies have provided that neurodegeneration in AD is associated with apoptosis [26,27]. The apoptosis always link with genes under physiological and pathological conditions. The Bcl-2 and Bax genes are suggested to play a major role in determining cell’s survival or death under the condition of apoptotic stimulate [28]. Bcl-2 plays an important role in the maintenance of cell survival, while the main role of Bax is to accelerate the apoptotic. Bcl-2 and Bax have been thought to implicate in the process of apoptosis [29]. In the present study, the injected of Aβ (1-40) leaded to the expression of Bcl-2 protein was reduced, while the expression of Bax protein was increased. These changes finally leaded to the decrease of Bcl-2/Bax ratio. However, treatment with luteolin, the ratio was obviously improved. The results showed that luteolin could attenuate the Aβ-induced apoptosis in AD rats. Therefore, we speculated that luteolin protected Aβ-injected rat model of AD via regulating the Bcl-2 family proteins.
In conclusion, we have demonstrated that luteolin improved Aβ-induced learning and memory deficits in AD model rats. The mechanisms of action for luteolin include regulating the cholinergic system and inhibiting oxidative injuries. Our findings suggest, luteolin has a strong potential for neuroprotection. Thus, it may be effective as a treatment for AD. This study provides basis for further research of effective substance and mechanism.
Disclosure of conflict of interest
None.
References
- 1.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka J, Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem. 2002;81:1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 4.Jakob-Roetne R, Jacobsen H. Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 5.Ruan CJ, Li Z, Zhang L, Chen DH, Du GH, Sun L. Protective effects of trans-2,4-dimethoxystibene on cognitive, impairments induced by Aβ25-35 in hypercholesterolemic rats. Brain Res. Bull. 2010;82:251–258. doi: 10.1016/j.brainresbull.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxylterminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 7.Floyd RA. Antioxidants, oxidative stress and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 8.Sultana R, Newman S, Mohmmad-Abdul H, Keller JN, Butterfield DA. Protective effect of the xanthate, D609, on Alzheimer’s amyloid betapeptide (1-42)-induced oxidative stress in primary neuronal cells. Free Radical Res. 2004;38:449–458. doi: 10.1080/1071576042000206478. [DOI] [PubMed] [Google Scholar]
- 9.Choi SJ, Kim MJ, Heo HJ, Hong B, Cho HY, Kim YJ, Kim HK, Lim ST, Jun WJ, Kim EK, Shin DH. Ameliorating effect of Gardenia jasminoides extract on amyloid beta peptide-induced neuronal cell deficit. Mol Cells. 2007;24:113–118. [PubMed] [Google Scholar]
- 10.Zhang HY, Liu YH, Wang HQ, Xu JH, Hu HT. Puerarin protects PC12 cells against beta-amyloid-induced cell injury. Cell Biol Int. 2008;32:1230–1237. doi: 10.1016/j.cellbi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid β (1-40) rat model of Alzheimer’s disease. Neurobiol. Learn Mem. 2011;95:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Pandurangan AK, Dharmalingam P, Ananda Sadagopan SK, Ganapasam S. Effect of luteolin on the levels of glycoproteins during azoxymethane-induced colon carcinogenesis in mice. Asian Pac J Cancer Prev. 2012;13:1569–1573. doi: 10.7314/apjcp.2012.13.4.1569. [DOI] [PubMed] [Google Scholar]
- 13.Jung HA, Jin SE, Min BS, Kim BW, Choi JS. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol. 2012;50:2171–2179. doi: 10.1016/j.fct.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Manju V, Nalini N. Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochem Funct. 2007;25:189–194. doi: 10.1002/cbf.1305. [DOI] [PubMed] [Google Scholar]
- 15.Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu HB, Meng XB, Qin M, Sun J, Wang HW, Sun XB. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol Appl Pharmacol. 2012;265:229–240. doi: 10.1016/j.taap.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 17.Malaplate-Armand C, Florent-Béchard S, Youssef I, Koziel V, Sponne I, Kriem B, Leininger-Muller B, Olivier JL, Oster T, Pillot T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Vieira MN, Forny-Germano L, Saraiva LM, Sebollela A, Martinez AM, Houzel JC, De Felice FG, Ferreira ST. Soluble oligomers from a non-disease related protein mimic Abeta-induced tau hyperphosphorylation and neurodegeneration. J Neurochem. 2007;103:736–748. doi: 10.1111/j.1471-4159.2007.04809.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng MQ, Yin DZ, Zhang L, Lei B, Cheng DF, Cai HC, Han YJ, Wu MX, Zhang H, Wang J. Biological characters of [F-18] O-FEt-PIB in a rat model of Alzheimer’s disease using micro-PET imaging. Acta Pharmacol Sin. 2008;29:548–554. doi: 10.1111/j.1745-7254.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 20.Han M, Liu Y, Tan Q, Zhang B, Wang W, Liu J, Zhang XJ, Wang YY, Zhang JM. Therapeutic efficacy of stemazole in a beta-amyloid injection rat model of Alzheimer’s disease. Eur J Pharmacol. 2011;657:104–110. doi: 10.1016/j.ejphar.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid (1-40) rat model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;95:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Kar S, Quirion R. Amyloid beta peptides and central cholinergic neurons: functional interrelationship and relevance to Alzheimer’s disease pathology. Prog Brain Res. 2004;145:261–274. doi: 10.1016/S0079-6123(03)45018-8. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Miyashita H, Tsunekawa H, Mouri A, Kim HC, Saito K, Matsuno T, Kawashima S, Nabeshima T. Effects of a novel cognitive enhancer, spiro [imidazo-[1,2-a] pyridine-3,2-indan] -2 (3H)-one (ZSET1446), on learning impairments induced by amyloid-beta1-40 in the rat. J Pharmacol Exp Ther. 2006;317:1079–1087. doi: 10.1124/jpet.105.098640. [DOI] [PubMed] [Google Scholar]
- 25.Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer’s disease: colocalization with c-Jun immunoreactivity, relationship to brain area and effect of postmortem delay. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 28.Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Zhang HY, Tang XC. Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by betaamyloidprotein-(1-40) in rat. Eur J Pharmacol. 2001;421:149–156. doi: 10.1016/s0014-2999(01)01030-5. [DOI] [PubMed] [Google Scholar]


