Abstract
The oncogenic transcription factor forkhead box protein M1 (FOXM1) plays critical roles in gastric cancer (GC) development and progression. However, the underlying mechanisms has not fully demonstrated. Lactate dehydrogenase A (LDHA) is widely overexpressed in a series of cancers and is one of the two subunits of Lactate dehydrogenase (LDH), which is the key glycolytic enzyme and catalyzes the interconversion of pyruvate and lactate. In this study, we characterized the regulation of aerobic glycolysis by FOXM1 via transactivation of LDHA in GC. We found that LDHA was overexpressed GC cells, and the expression of LDHA was transcriptionally regulated by FOXM1. Furthermore, FOXM1 regulated GC cells glycolytic phenotype, proliferation, migration and invasion via LDHA. Thus, FOXM1-LDHA signaling functioned as a stimulator of glycolysis and promoted GC progression.
Keywords: FOXM1, LDHA, gastric cancer, progression, glycolysis1
Introduction
Although the incidence of gastric cancer (GC) is decreasing worldwide, it is still relatively high in Eastern-Asia area, particularly in China and Japan. GC remains the fourth most frequently diagnosed malignant cancer in the world and ranks the third most common causes of cancer-related deaths [1]. In China, GC was predicted to rank as the second most common cancer, with 0.3 million deaths and 0.4 million new cases reported [2]. Thus, a better understanding of the molecular mechanisms which promote the pathogenesis of GC is urgently needed.
A lot of researches have demonstrated that development and progression of tumors depends on glycolysis even under normal oxygen concentrations, and this phenotype has been defined as “Warburg effect” or “aerobic glycolysis” [3]. Recently, studies have revealed that “Warburg effect” might result from genetic or epigenetic changes of oncogenes, as most glycolytic enzymes are regulated by oncogenes such as hypoxia inducible factor (HIF), Ras, Myc, and etc [4]. Lactate dehydrogenase (LDH) is the key glycolytic enzyme and catalyzes the interconversion of pyruvate and lactate. LDH is composed of two types of subunits LDHA (muscle-type, M subunit) and LDHB (heart-type, H subunit), and results in five isozymes: LDH5 (A4), LDH4 (A3B1), LDH3 (A2B2), LDH2 (A1B3), and LDH1 (B4) [5]. It has been published that LDHA is overexpressed in a series of cancers, including GC, and high expression of LDHA in GC was associated with shorter overall survival (OS) [6-10]. However, the mechanism of increased expression of LDHA in GC is not demonstrated. It has been reported in pancreatic cancer that Forkhead box protein M1 (FOXM1) transcriptionally upregulated LDHA and promoted pancreatic cancer growth and metastasis.
FOXM1, an oncogenic transcription factor, shares the winged-helix DNA-binding domain and belongs to Forkhead transcription factors superfamily. FOXM1 plays critical roles in regulating cancer cell proliferation, angiogenesis, invasion and metastasis by transcriptionally regulating its downstream target genes and networking with other factors [11]. It has been reported that FOXM1 is overexpressed in GC and promotes GC formation, proliferation, angiogenesis, epithelial-mesenchymal transition (EMT), metastasis and chemo-resistance [12-17]. Previously, it has been reported that FOXM1 promoted pancreatic cancer progression via increasing glycolytic phenotype [18]. However, the roles and mechanism of FOXM1 in regulating glycolysis in GC has not been demonstrated.
In this study, we sought to determine the role and mechanism of the increased expression of LDHA in GC. We discovered that LDHA was transcriptionally regulated by FOXM1 (FOXM1B) and FOXM1-LDHA signaling promoted glycolytic phenotype (increased lactate production and glucose utilization) and progression of GC.
Materials and methods
Cell lines and reagents
The human GC cell lines NCI-N87, AGS, HTB135, SNU1 cells were purchased from the American Type Culture Collection. The SK-GT15 cell line was obtained from Gary K. Schwartz (Memorial Sloan-Kettering Cancer Center, New York, NY), and the TMK-1 cell lines was obtain from Masashi Kanai (Kyoto University, Kyoto, Japan). All of these cell lines were maintained in plastic flasks as adherent monolayers in Eagle’s minimal essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution (Flow Laboratories). The cell lines were obtained directly from ATCC that performs cell line characterizations or authentication by the short tandem repeat profiling and passaged in our laboratory for fewer than 6 months after receipt. A competitive LDHA inhibitor oxamate sodium was purchased from Sigma-Aldrich Corp. (St. Louis, MO, US) [19].
Plasmids and small interfering RNAs
The plasmids pcDNA3.1-FOXM1 (pFOXM1B) and control vector pcDNA3.1 were described previously [20]. The pLuc-LDHA-1480, pLuc-LDHA-1031 and pLuc-LDHA-493 of LDHA promoter reporter plasmids were reported previously [18]. The small interfering RNA (siRNA) targeting FOXM1 (siFOXM1) was a pool of 3 target-specific 20-25nt siRNAs designed to knock down FOXM1 expression (Santa Cruz Biotechnology).
Transient transfection
Transfection of plasmids and siRNAs into GC cells was performed using Lipofectamine LTX and Lipofectamine 2000 CD (Invitrogen, Grand Island, NY, US) transfection reagents, respectively. For transient transfection, cells were transfected with plasmids or siRNA at different doses as indicated for 48 hours before the performance of functional assays. GC cells treated with transfection reagent alone were included as mock controls.
Western blot analysis
Standard Western blot was carried out using whole-cell protein lysates and primary antibodies against FOXM1 and LDHA (Santa Cruz Biotechnology); and a secondary antibody (anti-rabbit IgG or anti-mouse IgG; Santa Cruz Biotechnology). Equal protein sample loading was monitored using an anti-α-tublin antibody (Oncogene, Rockville, MD, US).
LDH activity, lactate production and glucose utilization assay
Tumor cells were transfected with plasmids and siRNAs, 1 × 106 cells were prepared for LDH activity and lactate production assay with the Lactate Dehydrogenase Activity Assay Kit and Lactate Assay Kit (Sigma, Louis, MO, US) according to the manufacturer’s protocol. For glucose utilization assay, tumor cells were transfected with plasmids and siRNAs, and the cultures were incubated for 24 hours. The media were replaced with phenol-red free RPMI with 1% FBS or phenol-red free RPMI with 1% FBS and 20 mmol/L oxamate to continuous culture for 3 days. Glucose concentrations in media were measured using a colorimetric glucose assay kit (Biovision, US) and normalized with cell number [21].
Promoter reporter and dual luciferase assay
GC cells were transfected with the indicated LDHA promoter reporters, FOXM1 siRNA or expression plasmid. The LDHA promoter activity was normalized by cotransfecting a β-actin/Renilla luciferase reporter containing a full-length Renilla luciferase gene [22]. The luciferase activity in the cells was quantified using a dual luciferase assay system (Promega) 24 hours after transfection.
Colony-formation assay
Two hundred cells from each group as indicated were plated in 24-well plates and allowed to grow for 14 days in culture medium; the medium was changed twice a week. Cells were then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution for 10 minutes. Colonies (> 20 cells) were counted using an inverted microscope at 40× magnification. The results were calculated as the percentage of proper control. All experiments were performed in triplicate and repeated twice.
Tumor-cell migration/invasion assay
NCI-N87 cells were transfected for 6 hours with different reagents respective to different groups (control or pFOXM1). Cells in each group were trysinized and 2 × 104 to 5 × 104 cells in a 300l volume of serum-free medium were in each group were placed in the upper parts of modified Boyden chambers with a Matrigel-coated/uncoated membrane (Millipore). DMEM with 10% FBS or with 10% FBS and 20 mmol/L oxamate were used as chemoattractant. 500 ml of respective conditioned media was added to the lower chamber. After 24 hours of incubation, invasive/migrated cells were fixed, stained and counted under a microscope in five randomly selected fields at a magnification of 200×.
Statistical analysis
The significance of the data was determined using the Student t test (two-tailed) or one way ANOVA. In all of the tests, P values less than 0.05 were considered statistically significant. The SPSS software program (version 13.0; SPSS Inc, New York, US) was used for statistical analysis.
Result
Association of elevated expression of FOXM1 with LDHA overexpression in GC
It has reported that the expression of LDHA and FOXM1 were both elevated in GC tissues and LDHA was transcriptionally regulated by FOXM1 in pancreatic cancer [18]. We then evaluated the relationship between FOXM1 and LDHA expression in GC. First, we analyzed the expression of FOXM1 and LDHA in GC cancer cell lines. The result showed that the expression of FOXM1 was positively associated with LDHA in GC cell lines (Figure 1A). Then the impact of altered expression of FOXM1 on LDHA expression was further investigated. We knockdown FOXM1 in SK-GT5 and SNU1 cells which expressed relatively higher levels of FOXM1 and LDHA, whereas overexpressed FOXM1 in AGS and NCI-N87 cells which has lower levels of FOXM1 and LDHA. The result revealed that knockdown of FOXM1 led to decreased expression of LDHA, but overexpression of FOXM1 resulted in increased expression of LDHA (Figure 1B).
Figure 1.
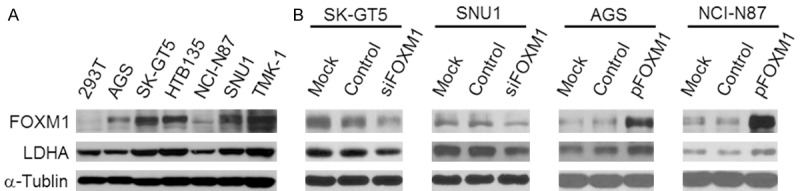
Regulation of LDHA expression by FOXM1 in GC. A. Western blot analysis of FOXM1 and LDHA expression in GC cell lines. B. Regulation of LDHA expression by FOXM1. SK-GT5 and SNU1 cells were transfected with siFOXM1 or control siRNA, and AGS and NCI-N87 cells were transfected with pFOXM1 or control vector. The culture were incubated for 48 hours. Total protein lysates were harvested to measure the FOXM1 and LDHA expression using western blot.
Transcriptional activation of LDHA by FOXM1 in GC cells
The above results revealed that FOXM1 regulated the expression of LDHA. We then further analyzed the effect of FOXM1 on LDHA promoter activity by dual luciferase assay. The three promoter reporters of LDHA were previously reported [23]. pLcu-LDHA-1480 contains 4 potential FOXM1-binding sites, pLuc-LDHA-1031 contains 2 potential FOXM1-binding sites, and pLcu-LDHA-493 does not contain potential FOXM1-binding sites. Transfection of GC cells with FOXM1 siRNA significantly decreased the promoter activity of LDHA (pLcu-LDHA-1480 and pLuc-LDHA-1031), whereas overexpression of FOXM1 increased this activity. However, altered expression of FOXM1 had little effect on pLcu-LDHA-493 (Figure 2). These result indicated that the FOXM1-binding sites were positive regulatory elements in the LDHA promoter and FOXM1 regulated the expression of LDHA on transcriptional level.
Figure 2.
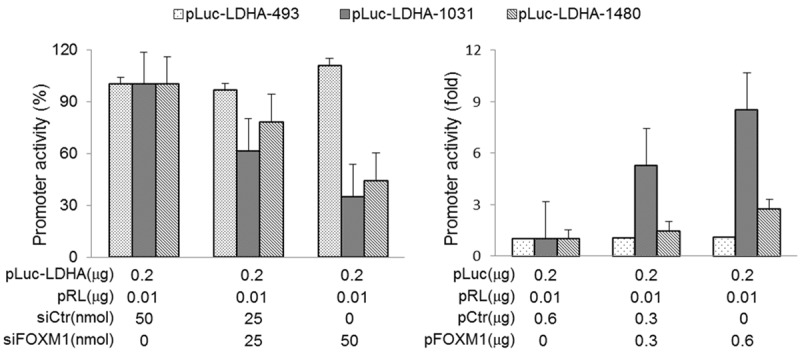
Transcriptional regulation of LDHA by FOXM1 in GC. Measurement of the LDHA promoters activity. SK-GT5 cells were co-transfected with 0.2 μg of the LDHA promoter-luciferase construct pLuc-LDHA-493, pLuc-LDHA-1031 or pLuc-LDHA-1480, and 0, 25, and 50 nmol/L siFOXM1or control siRNA. Promoter activity was examined using a dual luciferase assay kit. NCI-N87 cells were co-transfected with 0.2 μg of the LDHA promoter-luciferase constructs pLuc-LDHAs and 0, 0.3, and 0.6 μg of pFOXM1 or control vector. Promoter activity was examined using a dual luciferase assay kit (*, P < 0.01).
FOXM1 promotion of the Warburg effect in GC cells via LDHA
Given that FOXM1 regulated the expression of LDHA which was a key enzyme of aerobic glycolysis (Warburg effect), we then investigated the role of FOXM1 in regulating aerobic glycolysis via LDHA by analysis the correlation of altered the expression of FOXM1 with LDH activity, glucose utilization, and lactate production. We found that knockdown of FOXM1 in SK-GT5 significantly decreased LDH activity, glucose utilization, and lactate production (Figure 3A). In comparison, overexpression of FOXM1 in NCI-N87 increased the LDH activity, glucose utilization, and lactate production (Figure 3B). Furthermore, elevated LDH activity, glucose utilization, and lactate production induced by FOXM1 were dependent on LDHA, as specific inhibition of LDHA activity by oxamate sodium attenuated the increasing effect of FOXM1 on LDH activity, glucose utilization, and lactate production. These data indicated that FOXM1 affected LDH activity, glucose utilization, and lactate production, which were the main features of Warburg effect, via transcriptionally regulating the expression of LDHA.
Figure 3.
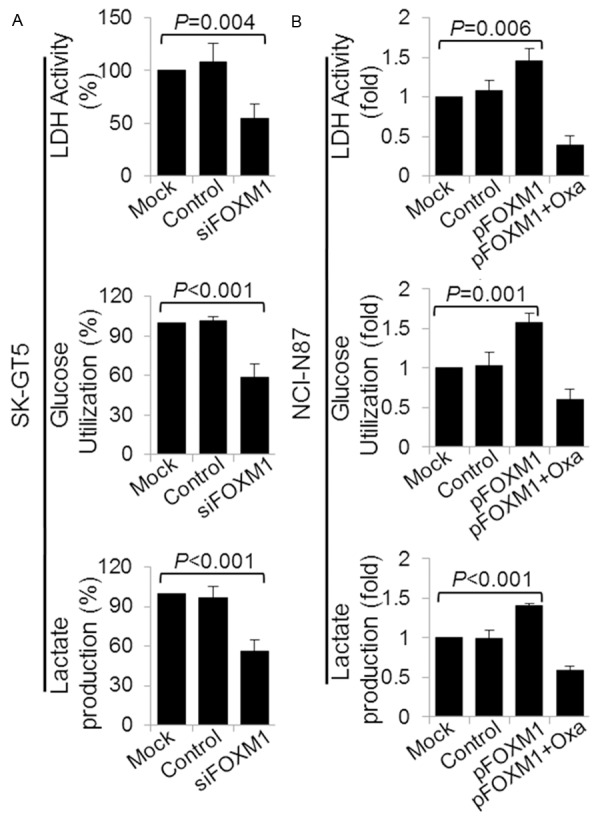
Regulation of LDH activity, glucose utilization and lactate production by FOXM1-LDHA signaling. SK-GT5 cells were transfected with siFOXM1 or control siRNA (A), and NCI-N87 cells were transfected with control vector, pFOXM1 or pFOXM1 and treated with 20 mmol/L oxamate sodium (B). The cultures were incubated for 48 hours. An LDH Activity Assay Kit was used to analyze the LDH activity (upper panels), a Glucose Utilization Assay Kit was used to analyze the use of glucose (middle panels), and a Lactate Assay Kit was used to analyze the lactate production (lower panels).
Direct impact of altered FOXM1-LDHA signaling on GC cell proliferation, migration and invasion
To investigate the role of altered FOXM1-LDHA signaling on GC biology, we overexpressed FOXM1 in NCI-N87 cells and evaluated the proliferation, migration and invasion of the cells. The colony-formation assay results revealed that overexpression of FOXM1 led to increased colony formation via LDHA, as oxamate sodium attenuated the increasing effect of FOXM1 (Figure 4A and 4B). Furthermore, elevated expression of FOXM1 resulted in increased cell migration and invasion, but inhibited LDHA by oxamate sodium decreased the promoting effect of FOXM1 on cell migration and invasion. These result demonstrated that FOXM1 promoted GC growth and metastasis at least partly through transcriptionally regulating the expression of LDHA.
Figure 4.
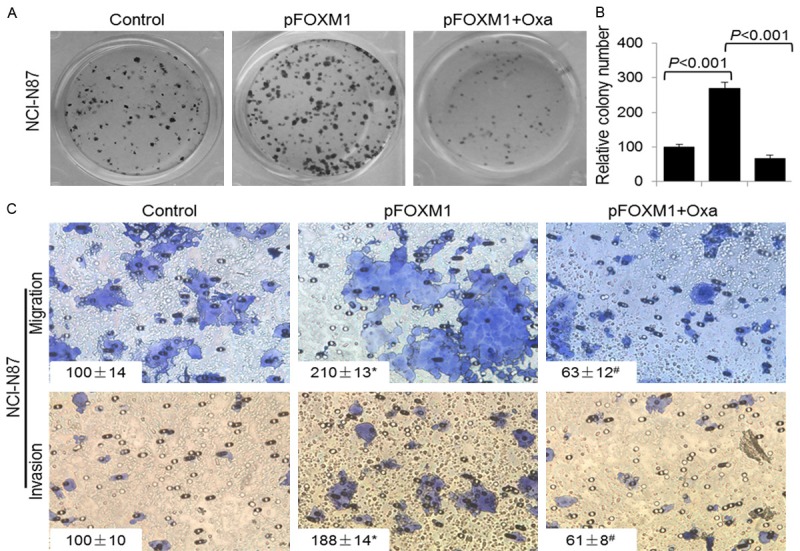
FOXM1-LDHA signaling promoted GC cells proliferation, migration and invasion. NCI-N87 cells were transfected with control vector, pFOXM1, or pFOXM1 and treated with 20 mmol/L oxamate sodium. A, B. Colony formation assay was performed in 24-well plates and numbers of colonies were counted 14 days after retroviral infection. C. The migration and invasion of NCI-N87 cells were determined as described in Materials and Methods. Representative tumor cell migrated or invaded were photographed, data represent mean ± SD of triplicates. (*, P < 0.05, a comparison of the pFOXM1 groups with the control groups; #, P < 0.05, a comparison of the pFOXM1 + Oxa groups with the pFOXM1 groups).
Discussion
In the present study, we determined the critical roles of FOXM1-LDHA signaling in regulating aerobic glycolysis in GC for the first time, demonstrated the underlying mechanism. We revealed that increased FOXM1-LDHA signaling promoted GC cells proliferation, migration and invasion.
FOXM1, an oncogenic transcription factor, has reported to be overexpressed in many kinds of cancer cells, including GC, and is associated with tumor growth, angiogenesis, EMT and metastasis [14,20,24-26]. Recently, it has been reported to regulate Warburg effect in pancreatic cancer. However, the regulating role of FOXM1 in the Warburg effect of GC has not been reported. In this study, we showed that knocking down the expression of FOXM1 decreased LDH activity, glucose utilization, and lactate production, whereas overexpression of FOXM1 increased LDH activity, glucose utilization, and lactate production via transcriptionally regulating the expression of LDHA which is the key enzyme of glycolysis.
LDHA is a major subunit of LDH, which is comprised of the subunits A and B [18]. Studies have demonstrated that LDHA favored catalysis of the conversion of pyruvate to lactate, but LDHB preferred converting lactate to pyruvate, and the shift from LDH1 to LDH5 promoted cancer progression [9,27,28]. LDHA has been reported to overexpress in a series of cancer, including GC, and promoted cancer development and progression [6,8,9]. However, the mechanism of elevated expression of LDHA in GC has not been demonstrated. In the present study, we found that the expression of LDHA was positively correlated with FOXM1 in GC cell lines. We then investigated the effect of altered FOXM1 expression on LDHA and found that FOXM1 overexpression led to increased LDHA expression, whereas knockdown of FOXM1 expression did the opposite. Furthermore, altered the expression of FOXM1 resulted in changed LDHA promoter activity. These data revealed that FOXM1 transcriptionlly regulated the expression of LDHA.
FOXM1-LDHA signaling played important roles in GC aerobic glycolysis which was one of the hallmarks of cancer, we then further determined the impact of FOXM1-LDHA signaling on GC biology [29]. We found that elevated FOXM1-LDHA signaling promoted GC cells proliferation, migration and invasion. And LDHA played critical roles in mediating the effect of FOXM1 in GC biology, as inhibited LDHA attenuated the increasing effect of FOXM1 on cancer cells proliferation, migration and invasion.
In summary, this study identified the roles of FOXM1 in GC metabolism for the first time. Four lines of evidence were provided to support the role for FOXM1 in GC glycolysis via transcriptionally regulating LDHA. Firstly, FOXM1 and LDHA were concomitantly overexpressed in GC cell lines. Secondly, the expression of LDHA was regulated by FOXM1. Furthermore, FOXM1 regulated the transcriptional activity of LDHA promoters. Thirdly, altered expression of FOXM1 affected the LDH activity, lactate production and glucose utilization. Fourthly, increased FOXM1-LDHA signaling promoted GC cells proliferation, migration and invasion. Collectively, these findings not only revealed the critical roles of FOXM1 in GC metabolism, but also indicated that FOXM1-LDHA signaling might be a new therapeutic target of GC, which required further studies.
Acknowledgements
The authors gratefully thank Jiujie Cui for her excellent technical support and provide plasmid mentioned in the paper.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32:106–112. doi: 10.5732/cjc.013.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta. 2012;1826:370–384. doi: 10.1016/j.bbcan.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RD, Kaplan NO, Hall TC. Lactic dehydrogenase in human neoplastic tissues. Cancer Res. 1964;24:389–399. [PubMed] [Google Scholar]
- 7.Koukourakis MI, Kontomanolis E, Giatromanolaki A, Sivridis E, Liberis V. Serum and tissue LDH levels in patients with breast/gynaecological cancer and benign diseases. Gynecol Obstet Invest. 2009;67:162–168. doi: 10.1159/000183250. [DOI] [PubMed] [Google Scholar]
- 8.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J. Clin. Oncol. 2006;24:4301–4308. doi: 10.1200/JCO.2006.05.9501. [DOI] [PubMed] [Google Scholar]
- 9.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL Tumour and Angiogenesis Research Group. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, Sun Z, Zhu Z, Guan H, Zhang J, Zhang Y, Xu H, Sun M. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS One. 2014;9:e91068. doi: 10.1371/journal.pone.0091068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo CY, Muir KW, Lam EW. FOXM1: From cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Hu CJ, Wang B, Tang B, Chen BJ, Xiao YF, Qin Y, Yong X, Luo G, Zhang JW, Zhang D, Li S, He F, Yang SM. The FOXM1-induced resistance to oxaliplatin is partially mediated by its novel target gene Mcl-1 in gastric cancer cells. Biochim Biophys Acta. 2015;1849:290–299. doi: 10.1016/j.bbagrm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Jia Z, Wang L, Kong X, Li Q, Guo K, Tan D, Le X, Wei D, Huang S, Mishra L, Xie K. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology. 2012;142:531–542. doi: 10.1053/j.gastro.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, Huang S, Tan D, Xie K. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69:3501–3509. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao L, Xiong X, Lin Y, Cheng Y, Lu J, Zhang J, Cheng N. Down-regulation of FoxM1 leads to the inhibition of the epithelial-mesenchymal transition in gastric cancer cells. Cancer Genet. 2014;207:75–82. doi: 10.1016/j.cancergen.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Okada K, Fujiwara Y, Takahashi T, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori M, Doki Y. Overexpression of forkhead box M1 transcription factor (FOXM1) is a potential prognostic marker and enhances chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol. 2013;20:1035–1043. doi: 10.1245/s10434-012-2680-0. [DOI] [PubMed] [Google Scholar]
- 17.Qian J, Luo Y, Gu X, Zhan W, Wang X. Twist1 promotes gastric cancer cell proliferation through up-regulation of FoxM1. PLoS One. 2013;8:e77625. doi: 10.1371/journal.pone.0077625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595–2606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30:2983–2991. doi: 10.3892/or.2013.2735. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, Sawaya R, Huang S. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593–3602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 21.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, Huang S. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J, Zhu Z, Gao Y, Xie K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20:4370–4380. doi: 10.1158/1078-0432.CCR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalinina OA, Kalinin SA, Polack EW, Mikaelian I, Panda S, Costa RH, Adami GR. Sustained hepatic expression of FoxM1B in transgenic mice has minimal effects on hepatocellular carcinoma development but increases cell proliferation rates in preneoplastic and early neoplastic lesions. Oncogene. 2003;22:6266–6276. doi: 10.1038/sj.onc.1206640. [DOI] [PubMed] [Google Scholar]
- 25.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–2161. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 26.Madureira PA, Varshochi R, Constantinidou D, Francis RE, Coombes RC, Yao KM, Lam EW. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. J Biol Chem. 2006;281:25167–25176. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 27.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim EL, Lee YK, Park CB, Kim BW, Wang HJ, Yoon CH, Lee SJ, Yoon G. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects. Exp Cell Res. 2011;317:1108–1118. doi: 10.1016/j.yexcr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]


