Abstract
Objective: This study aimed to evaluate the therapeutic potential of human amniotic epithelial cell (HAEC) transplantation in the management of brain hemorrhage in an animal model. Methods: New Zealand white rabbits were induced to develop cerebral hemorrhage through autologous blood injection. Animals with confirmed brain hemorrhage were randomized to receive transplantation of, respectively, vehicle (n=15) and primary HAECs (n=15) that were expressing embryonic stem cell- and neuron-specific markers and were transfected with a retroviral vector carrying the green fluorescent protein (GFP). Behavioral and histological changes, survival of transplanted HAECs, and expression of glial fibrillary acidic protein (GFAP) and MAP-2 in transplanted perifocal tissue were assessed at various time points after transplantation. Results: At 2-3 weeks after transplantation, walking, body weight-supporting and movement coordinating capacities of limbs were improved mostly level II-III hemorrhage lesion cases in HAEC transplantation group but mostly in level I-II hemorrhage lesion cases in the vehicle control group. The Tarlov scores were significantly difference between the two groups (P<0.05). GFAP- and MAP-2-positive cells were observed in the neural tissue in animals transplanted with hAECs but not in animals in the control group (P<0.05). Conclusion: These preliminary observations suggest that hAEC transplantation possess both embryonic stem cell features and a neuron differentiation potential and thus may offer a promising treatment for hemorrhage-associated neurological damage.
Keywords: Human amniotic epithelial cell, brain hemorrhage, GFAP, MAP-2, rabbit
Introduction
Intracerebral hemorrhage (ICH) is the most common and the least treatable subtype of hemorrhagic stroke [1]. Accounting for 20%-30% of acute cerebrovascular diseases, ICH has a mortality rate of 30%-40% at the acute phase and causes severe dysfunction and loss of social and working abilities, thereby imposing a heavy burden on the family and society [2]. To date, no effective approach is available to improve functional outcomes in patients with ICH. Depending on the disease progression, surgical ablation and oral anticoagulant use is the mainstay of ICH treatment [3,4]. Recently, potential therapies targeting secondary brain injury are arousing a great deal of interest in translational studies [5].
Human amniotic epithelial cells (HAECs) have a multiple differentiation potential and a neuro-restorative effect, capable of differentiating into nerve cells [6]. Recently, the potential of HAEC transplantation in the treatment of numerous neurological diseases such as Parkinson’s disease, traumatic brain injury, cerebral ischemia, spinal cord injury and retinopathy disease has been evaluated in animal studies where HAECs are able to improve motor function significantly [7-10]. Given the unique restorative function of human amniotic epithelial cells in neurology and immunology, we hypothesize that HAECs may potentially treat cerebral hemorrhage. The objective of this study was to test this hypothesis in a rabbit model of cerebral hemorrhage.
Materials and methods
Reagents and instruments
Low glucose DMEM medium and trypsin were purchased from Gibco Life Technologies (Carlsbad, CA, USA). Mouse anti-rabbit AE5 monoclonal antibody was purchased from Chemicon International, Inc. (Temecula, CA, USA). Trizol reagent was purchased from Invitrogen Life Technologies (NY, USA). Fetal bovine serum was purchased from Evergreen Biological Engineering Materials Ltd. (Hangzhou, Zhejiang, China). Rabbit mononuclear cell separation medium was purchased from PeproTech Inc. (Rocky Hill, NJ, USA). SP and DAB kits were purchased from Boster Biological Products Co., Ltd. (Wuhan, Hubei, China). Stereotactic was purchased from General Instrument Corporation (Chicago, IL, U.S.). Fluorescence microscope was from Leica Instrument (Buffalo Grove, IL, USA).
Primary HAEC culture
Term placentae were obtained from women with uncomplicated pregnancies and negative tests for HIV, hepatitis A, hepatitis B, chlamydia and syphilis. HAECs were isolated as previously described [11]. Briefly, amnion was dissected from choriocarcinoma, washed three times with PBS and then digested in a solution containing 0.25% trypsin and 0.02% EDTA at room temperature for 15min. Dispersed cells were collected and cultured in RPMI-1640 supplemented with 10% FBS. The viability of the cells was assessed by 0.4% trypan blue staining. The expression of neural stem cell-specific markers OCT-4 and CD29, CD34 was determined. The protocols for placenta collection and HAEC isolation were approved by the Institutional Review Board of the Anqing Hospital, Anhui Medical University. Written informed consent was obtained from all placenta donors.
Retroviral vector infection
Primary HAECs were infected by a retroviral vector carrying the green fluorescent protein (GFP). The expression of GFP was assessed under a fluorescence microscope 24 hours after infection. GFP-expressing HAECs were used in transplantation experiments as described below.
Establishment of rabbit cerebral hemorrhage model
A total of 30 New Zealand white rabbits, weighing 2.0-2.5 kg, were purchased from Taizhou Experimental Animal Center, Jiangsu, China. They were housed according to China’s Laboratory Animal Management Regulations. Animals were divided into HAEC transplantation group and a vehicle (physiological saline) group by using a random table. The animal experimental protocols were reviewed and approved by the Animal Management Committee of Animal Resource Center Institute of Anhui Medical University.
For cerebral hemorrhage induction, animals were fixed in a stereotaxic apparatus and anesthetized with chloral hydrate (10% chloral hydrate, 0.3 ml/100 g, intraperitoneal injection). After the surgical area was cleaned with iodine, a skin incision was made. According to rabbit stereotaxic atlas, a hole, 5.5 mm deep, was drilled at the right ventricle wall (3.5 mm behind bregma and 2 mm from the side opening). Autologous arterial blood was slowly injected into the brain, using a micro-injector within 3 minutes. The needle was withdrawn 5 min later to ensure no reflux through the needle. Following closure of the drilling hole with denture acrylic resin, the skin incision was sutured and disinfected with iodine and intramuscular penicillin was given to protect against infection. Animals were then placed in an incubation chamber (37°C) for 10 min until their body temperature turned to normal. Heart rate, blood pressure and body temperature were monitored during the entire surgical procedure.
HAEC transplantation
Twenty-four hours after surgical induction of cerebral hemorrhage, 2×106 HAECs in 10 µl PBS were slowly injected under stereotactic guidance into the right ventricle wall of animals in the experimental group while an equal amount of PBS was injected into animal in the control group.
Behavioral and histological assessments
Behavioral assessment was performed on postoperative days 7, 14, 21 and 30 respectively. The assessment parameters including left limb movement (e.g., walking), support and coordination of movement were evaluated using the modified Tarlov scale as follows: level 0: complete limb paralysis, scoring 0-1 points; level I: partial limb paralysis and possible limb movement, scoring 2-5 points; level II: normal joint movement, but failure to stand, scoring 5-8 points; level III: normal standing, but failure to jump, scoring 9-11 points; level IV: normal limb functions, scoring 12-13 points. Two staff blind to the study design performed the assessment and independent scores were averaged for each animal.
For histological assessment, animals were euthanized by decollation on postoperative day 30. Brains were collected immediately by craniotomy. Surviving transplanted HAECs in the intraventricular nervous tissue were identified under a Leica fluorescence microscope. GFAP- or MAP-2-expressing cells in the neural tissue around hemorrhage were assessed with the SP method after immunohistochemical staining of paraffin sections of the collected specimens. Four slides for each animal and 10 random fields in each slide were examined under a light microscope (×200), and the average of the 10 fields was considered as the number of GFAP- or MAP-2-positive cells.
Statistical analysis
Data were expressed as mean ± SD analyzed by independent t-test and X2 test when appropriate or ANOVA analysis. The statistical software SAS 8.0 was used. Difference was considered significant when P<0.05.
Results
Morphology of cultured HAECs
Primary HAECs formed a confluent monolayer on the dish surface when cultured in serum-containing medium. As revealed by light microscopy and H&E staining, cultured cells displayed a typical polygonal shape with a round nucleus, a large nucleus-cytoplasm ratio, and clear uniform cytoplasm. Immunofluorescence analysis showed OCT-4 protein in the cytoplasm (Figure 1A) and flow cytometry analysis showed that primary HAECs were positive for stem cell markers CD29 and CD34 (Figure 2B, 2C).
Figure 1.
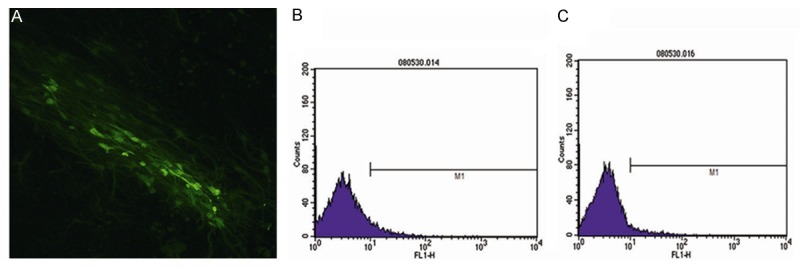
Representative photos (×200) of cultured HAECs, showing immunofluorescence of OCT-4 in the cytoplasm (A), and stem cell markers CD29 (B) and CD34 (C) revealed by flow cytometry.
Figure 2.
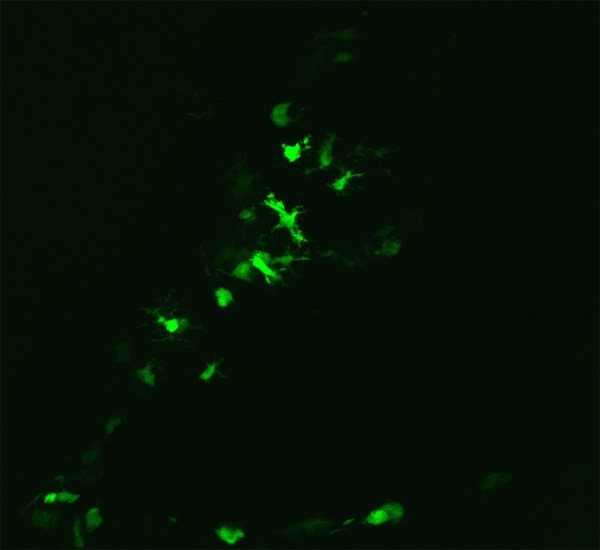
Integration of transplanted HAECs in the peripheral neural tissue in rabbits 30 days after transplantation (×200).
Behavioral and functional changes in rabbits after HAEC transplantation
Autologous blood-induced cerebral hemorrhage resulted in contralateral limb paralysis. Compared with the control, HAECs significantly improved limb functions including body weight support, movement coordination and walking at 2-3 weeks after transplantation. The results of the limb function assessment by the modified Tarlov scale are presented in Table 1; limb function recovery occurred mostly in level II-III dysfunction cases in the HAEC transplantation group but mostly in I-II dysfunction cases in the control group (P<0.05).
Table 1.
Modified Tarlov score evaluation for motor function in rabbits (mean ± SD)
| Group | N | Feeding time | |||
|---|---|---|---|---|---|
|
| |||||
| 7 d | 14 d | 21 d | 30 d | ||
| hAECs treated | 15 | 4.16±0.23 | 7.38±1.02 | 8.45±2.18 | 9.96±1.83 |
| Control | 15 | 3.88±0.31 | 4.35±0.78 | 5.27±1.36 | 5.63±1.57 |
| T value | 2.81 | 9.14 | 4.79 | 6.96 | |
| P value | 0.0091 | 0.0036 | 0.0087 | 0.0053 | |
Changes in GFAP and MAP-2 in nerve cells after HAEC transplantation
The peripheral neural tissue in the brain wall was assessed after behavioral and functional assessment. Strong fluorescence was observed along the lateral wall of the brain (Figure 2). The immunohistochemical analysis showed brown cytoplasmic staining of GFAP (Figure 3A) and MAP-2 (Figure 4A) in the perifocal tissue in the HAEC transplantation group but not in the control group (Figures 3B, 4B). Presented in Table 2 quantitative results of GFAP, MAP-2-positive neurons in peripheral tissue of the brain in both groups.
Figure 3.
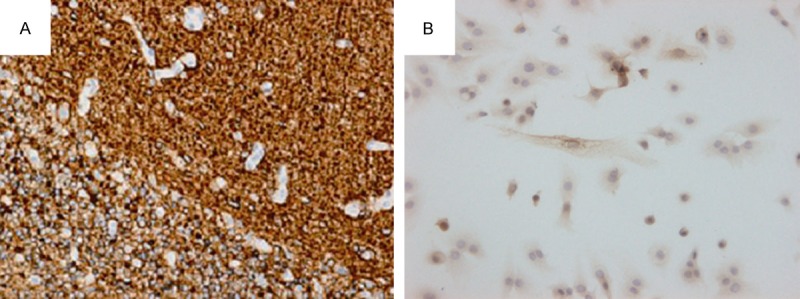
Cytoplasmic GFAP-positive (A) and GFAP-negative (B) neurons in the peripheral nervous tissue, respectively, in the HAEC transplantation group and the control group (×200).
Figure 4.
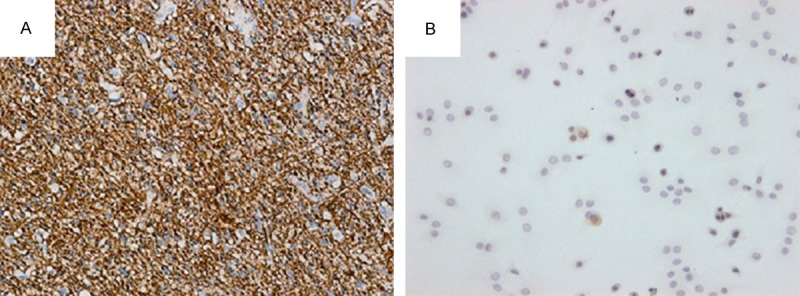
Cytoplasmic MAP-2-positive (A) and MAP-2-negative (B) neurons in the peripheral nervous tissue, respectively, in HAEC transplantation group and the control group.
Table 2.
Comparison results of positive GFAP, MAP-2 neurons in peripheral ventricular wall (mean ± SD, N/view)
P<0.05, compared with control group.
Discussion
Cerebral hemorrhage may cause inevitable damage to the brain. The underlying mechanisms include direct mechanical injury, ischemia, toxins, inflammation and apoptosis [12,13]. Mature nerve cells lack regenerative capacity. There are neural stem cells in the nervous system, but they have limited ability of generating new neurons after damage [14,15]. Transplantation provides a suitable platform of the central nervous system extracellular matrix generation where various neurotrophic factors may enhance the regeneration of central neurons. With the advance of stem cell engineering in recent years, cell transplantation therapy has shown a great therapeutic potential for injury, hereditary and degenerative diseases, thus attracting widespread attention [16,17]. HAECs that originate from the inner cell mass-derived embryonic ectoderm are pluripotent and capable of potentially differentiating into nerve cells. HAECs secrete a variety of cytokines. Similar with stem cell, amnion cells express stem cell-specific markers like OCT-4, Nanog, SOX-2, and Rex-1. Moreover, HAECs express neuron-specific markers GFAP, MAP-2, Nestin and NSE [18,19]. Both the stem cell-like features and high similarities to neural cells make transplantation of HAECs a promising approach to treat neurological disorders. As previously demonstrated in animal models of various neurological diseases such as Parkinson’s disease, traumatic brain injury, cerebral ischemia, spinal cord injury and retinopathy, HAEC transplantation may significantly improve neurological function and prognosis [7-10].
In our study, in vitro experiments showed that cultured HAECs expressed embryonic stem cell-specific marker molecules OCT-4, CD29 and CD34. This observation suggests that HAECs have some of the embryonic stem cell features and may potentially differentiate into neurons. EGFP emitting strong and stable fluorescence but not interfering cell function has been widely used as a live cell marker in investigations of cell morphology, physiology, biochemistry, molecular biology and genetics [20]. To evaluate the integration, localization and survival of transplanted HAECs, we infected with lentiviruses carrying EGFP and we observed strong fluorescence in the neural tissue in the lateral ventricle wall in rabbits transplanted with EGFP-tagged primary HAECs but not rabbits in the control group. This indicates that HAECs were integrated into the brain 30 days after transplantation where they might provide a good repair matrix platform with a variety of nerve nutritional factors to support the regeneration of nerve cells.
GFAP is a cytoplasmic filamentous protein, which is expressed in mature astrocytes cells and constitutes the major component of intermediate filaments in the central nervous system [21]. Nerve injury increases GFAP in astrocytes cells, thus promoting astrocyte cell mitosis and release of NGF, bNGF, IL-1, IL-3, IL-6, TNF and other neurological factors, which in turn promote nerve cell and fiber repair [22]. MAP-2 is a heat-stable phosphoprotein, which is structurally associated with microtubules. Mainly expressed in the cell body and dendrites in the central nervous system, MAP-2 plays an important role in promoting neuronal development, microtubule structure and stability, and dendrite formation [23,24]. In this study, strong expression of GFAP and MAP-2 was observed in neural tissue of rabbits in the HAEC transplantation group but not the control group. We speculate that the transplanted HAECs were integrated into the hemorrhage region where they might differentiate into neurons and nerve cells and secrete a variety of growth factors, thus promoting hemorrhage-associated damage repair and reconstruction.
In conclusion, this preliminary observational study has demonstrated that: 1) primary HAECs derived from healthy term pregnancies express both stem cell- and neuron-specific markers, thus possessing a neuron differentiation potential; 2) HAECs transplanted into the brain of rabbits with experimentally induced cerebral hemorrhage are effective to attenuate the hemorrhage-induced neurological damage and improve limb functions. These observations strongly suggest that HAEC transplantation may have a significant clinical implication in managing cerebral hemorrhage-related neural deficits. Nevertheless, more extensive and intensive investigations are warranted to further evaluate the clinical benefits of HAECs to patients with cerebral hemorrhage.
Disclosure of conflict of interest
None.
References
- 1.Sussman ES, Connolly ES Jr. Hemorrhagic transformation: a review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front Neurol. 2013;4:69. doi: 10.3389/fneur.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mook-Kanamori BB, Fritz D, Brouwer MC, van der Ende A, van de Beek D. Intracerebral hemorrhages in adults with community associated bacterial meningitis in adults: should we reconsider anticoagulant therapy? PLoS One. 2012;7:e45271. doi: 10.1371/journal.pone.0045271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG Investigators of the Registry of the Canadian Stroke Network and the Stroke Outcomes Research Canada Working Group. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104. doi: 10.1161/STROKEAHA.112.676551. [DOI] [PubMed] [Google Scholar]
- 4.Trabert J, Steiner T. Medical versus surgical management of intracerebral hematomas. Curr Atheroscler Rep. 2012;14:366–72. doi: 10.1007/s11883-012-0259-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Yang D, Han Y, Zhang J, Seyda A, Chopp M, Seyfried DM. Therapeutic effect of human umbilical tissue-derived cell treatment in rats with experimental intracerebral hemorrhage. Brain Res. 2012;1444:1–10. doi: 10.1016/j.brainres.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Yang XX, Xue SR, Dong WL, Kong Y. Therapeutic effect of human amniotic epithelial cell transplantation into the lateral ventricle of hemiparkinsonian rats. Chin Med J (Engl) 2009;122:2449–54. [PubMed] [Google Scholar]
- 8.Yawno T, Schuilwerve J, Moss TJ, Vosdoganes P, Westover AJ, Afandi E, Jenkin G, Wallace EM, Miller SL. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev Neurosci. 2013;35:272–82. doi: 10.1159/000346683. [DOI] [PubMed] [Google Scholar]
- 9.Castillo-Melendez M, Yawno T, Jenkin G, Miller SL. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Front Neurosci. 2013;7:194. doi: 10.3389/fnins.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh DH, Seo MS, Choi HS, Park SB, Han HJ, Beitz AJ, Kang KS, Lee JH. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22:1577–90. doi: 10.3727/096368912X659907. [DOI] [PubMed] [Google Scholar]
- 11.Marongiu F, Gramignoli R, Sun Q, Tahan V, Miki T, Dorko K, Ellis E, Strom SC. Isolation of amniotic mesenchymal stem cells. Curr Protoc Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc01e05s12. Chapter 1: Unit 1E 5. [DOI] [PubMed] [Google Scholar]
- 12.Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat. 2013;2013:394036. doi: 10.1155/2013/394036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zinnanti WJ, Lazovic J, Housman C, Antonetti DA, Koeller DM, Connor JR, Steinman L. Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I. Acta Neuropathol Commun. 2014;2:13. doi: 10.1186/2051-5960-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Y, Huang Q, Liu T, Guo L. Human amnion epithelial cells can be induced to differentiate into functional insulin-producing cells. Acta Biochim Biophys Sin (Shanghai) 2008;40:830–9. [PubMed] [Google Scholar]
- 15.Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955–68. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 16.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–88. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Ma J, Shatos MA, Chen H, Cyr D, Lashkari K. Application of human persistent fetal vasculature neural progenitors for transplantation in the inner retina. Cell Transplant. 2012;21:2621–34. doi: 10.3727/096368912X647153. [DOI] [PubMed] [Google Scholar]
- 18.Wichayacoop T, Briksawan P, Tuntivanich P, Yibchok-Anun S. Anti-inflammatory effects of topical supernatant from human amniotic membrane cell culture on canine deep corneal ulcer after human amniotic membrane transplantation. Vet Ophthalmol. 2009;12:28–35. doi: 10.1111/j.1463-5224.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- 19.Dong W, Chen H, Yang X, Guo L, Hui G. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int. 2010;34:573–7. doi: 10.1042/CBI20090248. [DOI] [PubMed] [Google Scholar]
- 20.Giaretta I, Madeo D, Bonaguro R, Cappellari A, Rodeghiero F, Giorgio P. A comparative evaluation of gene transfer into blood cells using the same retroviral backbone for independent expression of the EGFP and deltaLNGFR marker genes. Haematologica. 2000;85:680–9. [PubMed] [Google Scholar]
- 21.Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, Peters B, Shen ZT, Vanguri V, Liggitt D, Huseby ES. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J Immunol. 2014;192:3029–42. doi: 10.4049/jimmunol.1302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto Y, Yamasaki T, Tanaka N, Mochizuki Y, Kajihara H, Ikuta Y, Ochi M. Differential activation of astrocytes and microglia after spinal cord injury in the fetal rat. Eur Spine J. 2006;15:223–33. doi: 10.1007/s00586-005-0933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang L, Lu Y, Zheng W, Li Y. Overexpression of MAP-2 via formation of microtubules plays an important role in the sprouting of mossy fibers in epileptic rats. J Mol Neurosci. 2014;53:103–8. doi: 10.1007/s12031-013-0204-4. [DOI] [PubMed] [Google Scholar]
- 24.Mondello S, Gabrielli A, Catani S, D’Ippolito M, Jeromin A, Ciaramella A, Bossu P, Schmid K, Tortella F, Wang KK, Hayes RL, Formisano R. Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Inj. 2012;26:1629–35. doi: 10.3109/02699052.2012.700083. [DOI] [PubMed] [Google Scholar]


