Abstract
Background: The ameloblastoma is the most common odontogenic epithelial tumor, which belong to benign neoplasms that present a painless course, and usually occur in the oromaxillo-facial region. Although the histopathological manifestation of ameloblastoma is benign, it has unique biological behavior, for example local invasion and recurrence repeatedly. A few case of ameloblastoma was locally aggressive growth, and rarely metastasis to other tissue, for example the lungs, lymph nodes, and spine. Case report: A 64-year-old Chinese man, diagnosed with metastatic ameloblastoma, was treated with palliative chemotherapy consisting of cyclophosphamide, doxorubicin, and cisplatin for six cycles, and radiotherapy for 50 Gy after the last cycle chemotherapy. During the surveillance CT scan after the therapy, the tissues of the tumor were nearly complete response. Conclusion: The purpose of this study was to report a case of a patient with a right mandible ameloblastoma that recurred repeatedly and metastasized into bilateral lung. After the chemotherapy and radiotherapy, the tissues of the tumor were nearly complete response. This case is interesting because it investigated the diagnosis and treatment of the malignancy ameloblastoma, as this may help diagnose and treatment for clinician to the metastatic ameloblastoma.
Keywords: Ameloblastoma, metastasis, chemotherapy, mandible
Introduction
The ameloblastoma is the most common odontogenic epithelial tumor, which belong to benign neoplasms that present a painless course, and usually occur in the oromaxillo-facial region [1]. It has been widely accepted that the ameloblastoma have several different pathological types, including solid, multicystic, unicystic, desmoplastic, and peripheral ameloblastomas [2,3]. Each type has particular biological behavior and consequently a different prognosis and treatment. Solid/multicystic ameloblastomas have been identified as the most aggressive subtype, with a high recurrence rate following local excision [4]. This neoplasm usually does not cause pain and grows slowly, causing the distension of the bone [5]. Although the histopathological manifestation of ameloblastoma is benign, it has unique biological behavior, for example local invasion and recurrence repeatedly. In addition, a few cases of ameloblastoma were locally aggressive growth, and rarely metastasis to other tissue, for example the lungs, lymph nodes, and spine [6-8].
The treatment of primary ameloblastoma is surgical, but the treatment of metastatic ameloblastoma is not entirely uniform. In the many case reports, the diagnosis of the ameloblastoma was mainly investigated, rarely reports were about the treatment of ameloblastoma with metastasis [9,10]. The purpose of this study was to report a case of a patient with a right mandible ameloblastoma that recurred repeatedly and metastasized into bilateral lung. In addition, we specifically focused attention on evaluating the therapeutic efficacy by given six cycles of chemotherapy with “cyclophosphamide, doxorubicin and cisplatin”. Therefore, this case is interesting because it investigated the diagnosis and treatment of the malignancy ameloblastoma.
Case report
History
We present a 64-year-old Chinese man who was seen at our institution’s oncology department with a complaint of a large painless swelling over the right side of the face for two months. Twenty years ago, because of right mandible ameloblastoma, he received the first operation of partial right mandible resection in our hospital. Ten years ago, owing to local recurrence, he received the second operation of right mandibular resection and titanium reconstruction. He reported that the swelling had increased progressive during the previous two months. He also complained of one month history of left lower-limb radiating pain. His medical history and family history were unremarkable.
Physical examination
On physical examination, the bilateral lower jaw of the patient was asymmetry, the right submandibular area showed an old surgical scar, the area of right masseter and cheek was swelling significantly. A painless and hard oval mass was palpable in front of right parotid, which diameter was about 5 cm and poorly-defined with the surrounding tissue. The activity of the mass was poor. In addition, the patient’s breath sounds of right lower lung were decreased.
Imaging examination
The computerized tomography scan of maxillofacial revealed that a soft tissue mass was seen in front of parotid, which maximum diameter was about 5 × 5.6 cm (Figure 1A). The upper and lower edges of the mass were involved the right submandibular fossa. And, the left submandibular area showed multiple enlarged lymph nodes. The computerized tomography of chest showed that the multiple high-density nodules were seen in bilateral pulmonary. The posterior basal segment of right lung lower lobe was found an irregular soft tissue, about 6.2 × 4.3 cm size, which had spike and sublobe in the margin (Figure 1B).
Figure 1.
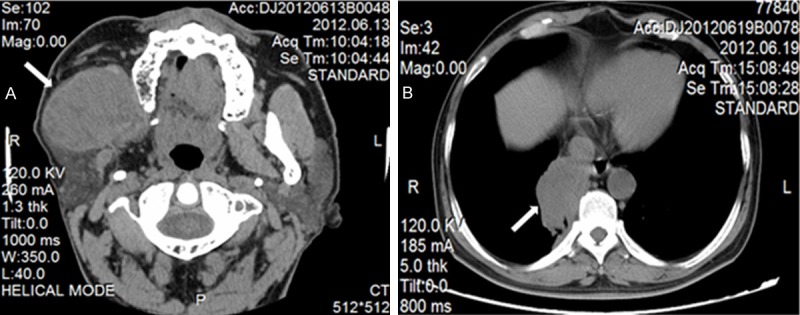
The computerized tomography images of the patient before the therapy. A. A soft tissue mass in the right maxillofacial was seen in front of parotid, which maximum diameter was about 5 × 5.6 cm (white arrow). B. A soft tissue mass was seen in the right lung, which maximum diameter was about 6.2 × 4.3 cm (white arrow).
Histological examination
The fresh tumor tissue was obtain by pneumocentesis, and then fixed in 10% neutral formalin solution for 24 hours. The fixed samples were washed with phosphate buffer solution (PBS), dehydrated with gradient of ethanol solutions at 70%, 80%, 90%, 95% and 100%, and then embedded in paraffin (melting point 56-58°C). Continuous 5-μm sections were made, and transferred onto slides. The sections were then stained with hematoxylin and eosin (HE) for histological analysis. Sequential sections were also prepared for immunohistochemistry staining. The sections of tumor samples were deparaf-finized, and rehydrated in water. The endogenous peroxidase was blocked with 3% H2O2 and epitope was retrieved under pressure sterilizer. Then, the sections were further incubated with primary antibody of anti-human CK, P63, TTF-1, CK7, CK8, Wapsin A overnight at 4°C. After being washed with PBS five times, the sections were incubated with proper horseradish peroxidase (HRP)-labeled second antibodies for 1 h at 37°C. Subsequently, the sections were developed with 3,3’-diaminobenzidine (DAB; Pierce Biotechnology, USA) and counterstained with hematoxylin. Observations were carried out at 400 × magnification by using Nikon microscope. The immunohistochemical staining showed negative staining for CK, P63, TTF-1, CK7, CK8 and Wapsin A. From the negative staining of CK/CK7/CK8 and TTF-1, we could exclude the diagnosis of the lung primary cancer. Combined with clinical history and imaging examination, the patient’s diagnosis was coincidenced pulmonary metastatic ameloblastoma (Figure 2).
Figure 2.
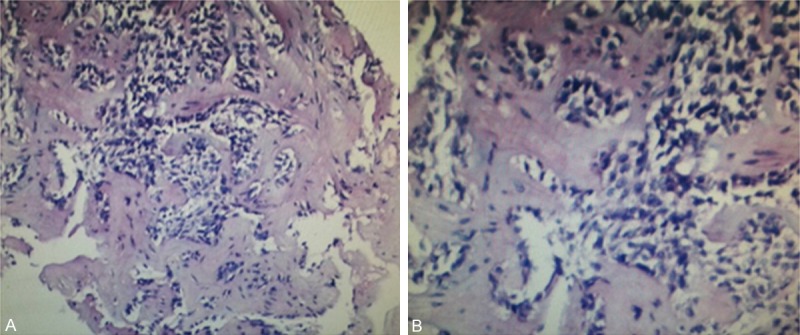
The H&E staining of tumor tissue in right lung by pneumocentesis were observed. A. Photomicrograph showing small cystic tumor islands and thin cords of ameloblastic epithelium within connective tissue stroma, original magnification × 100. B. Photomicrograph showing tumors of solid and stromal cells with infiltrative growth, original magnification × 200.
Therapy
According to tumor response and safety, the patient was given six cycles chemotherapy after the discovery of lung metastases. The regimen of chemotherapy was cyclophosphamide 750 mg/m2 (day 1), pirarubicin 50 mg/m2 (day 1), and cisplatin 75 mg/m2 over 1-hour infusion (days 1 to 3), as intravenous (i.v.) infusion every 21 days. Heart rate, respiration, blood pressure and pulse rate were monitored at the beginning of the i.v. infusions. The side-effects were evaluated using patient-reported questionnaires; there were not significant adverse reactions during chemotherapy. Treatment effects were evaluated using computerized tomography scans every two cycle’s chemotherapy. After the fourth cycle chemotherapy, the computerized tomography scan of right maxillofacial revealed that the soft tissue mass was about 5 × 4.4 cm (Figure 3A). The computerized tomography of chest showed that the irregular soft tissue in right lung was about 2.9 × 2.3 cm (Figure 3B). After the sixth cycle chemotherapy, the computerized tomography scans of maxillofacial showed that the soft tissue mass in front of right parotid was about 4.9 × 4.2 cm (Figure 3C). The computerized tomography of chest showed that the irregular soft tissue in right lung was about 0.9 × 0.7 cm (Figure 3D). Compared with the lesions before therapy, the tumor tissues in right lung reduced significantly, which achieved complete response basically. The tumor tissues in the right maxillofacial didn’t reduce up to 25% before therapy, which were evaluated stable disease.
Figure 3.
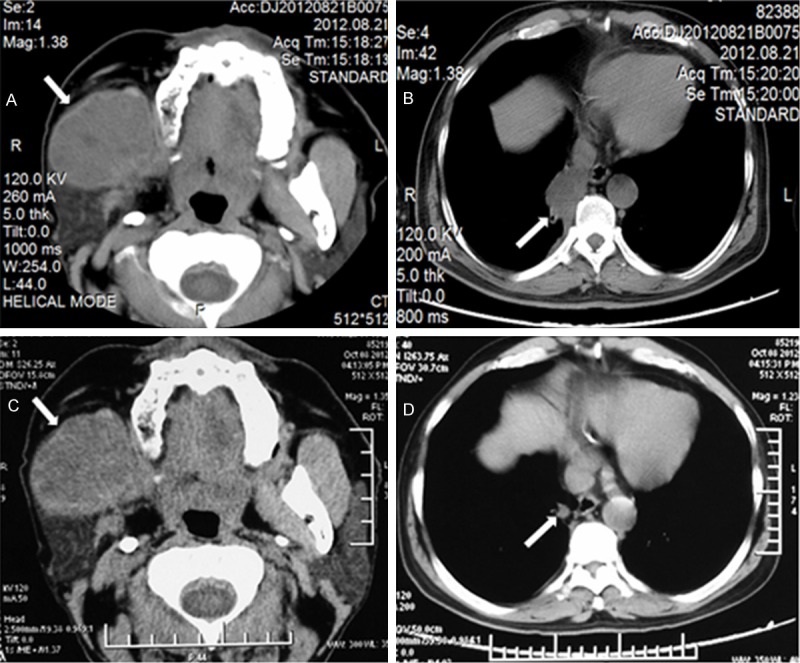
The computerized tomography images of the patient after the chemotherapy. A. After the fourth cycle chemotherapy, the soft tissue mass in the right maxillofacial was seen in front of parotid, which maximum diameter was about 5 × 4.4 cm (white arrow). B. After the fourth cycle chemotherapy, the soft tissue mass was seen in the right lung, which maximum diameter was about 2.9 × 2.3 cm (white arrow). C. After the sixth cycle chemotherapy, the soft tissue mass was seen in the right maxillofacial, which maximum diameter was about 4.9 × 4.2 cm (white arrow). D. After six cycles chemotherapy and radiotherapy, the soft tissue mass in the right lung was 0.9 × 0.7 cm (white arrow).
In order to treatment the right maxillofacial lesions, the right mandible of the patient was given 50 Gy radiotherapy after the last cycle chemotherapy, administered over 5 weeks in 25 fractions of 2.0 Gy. After the radiotherapy, the computerized tomography scans of maxillofacial were examined to evaluation the effect of the radiotherapy. The soft tissue mass in the right maxillofacial was about 4.0 × 3.2 cm, which demonstrated a light decrease (Figure 4A), but the necrotic tissue can be seen in the middle of the tissue mass. Meanwhile, the soft tissue mass in the right lung was about 0.9 × 0.6 cm (Figure 4B), which still remained complete response.
Figure 4.
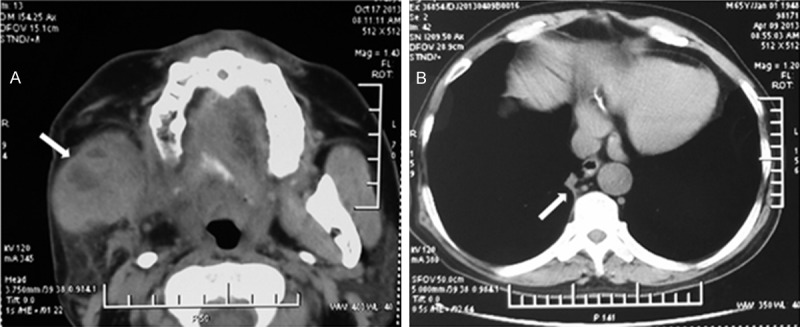
The computerized tomography images of the patient after the radiotherapy. A. The soft tissue mass in the right maxillofacial was seen in front of parotid, which maximum diameter was about 4.0 ×3.2 cm (white arrow). B. The soft tissue mass was seen in the right lung, which maximum diameter was about 0.9 × 0.6 cm (white arrow).
Discussion
Ameloblastoma is an odontogenic epithelial neoplasm which originates from the enamel organ. It has already been known as a benign, locally invasive odontogenic tumor with a limited propensity for local recurrence and metastasis after wide resection [11]. However, rarely histologically benign tumors may appear as diffusely locally infiltrating lesions and recur after mass excision, the adamantinoma is a slow-growing locally invasive epithelial tumor with a high recurrence rate (50%-72%) and rare metastasis (< 2%) [12,13]. Most ameloblastomas occur between age 30 and 60 years without gender predilection.
Ameloblastoma is seen most frequently in the maxilla which is eight times than the mandible [15]. However, because the cortical bone of maxillary is thin and porosity where blood circulation is enrich, the mandible ameloblastoma can metastasis to adjacent tissues [12]. It is a rare and slow-growing neoplasm that exhibits a low metastatic potential with tropism for the lungs [16]. This article described a rare case of adamantinoma of the right mandible which metastasized to the lungs, also investigated the therapeutic effect to this malignant adamantinoma.
In the existing literature, the diagnosis of the ameloblastoma by immunohisto-chemistry and radiology was extensively investigated. There are some studies that have used both PCNA and Ki-67 as markers of cell proliferation in ameloblastoma [17,18]. Bologna reported that the ameloblastic carcinomas displayed a significantly higher rate compared with all of the other benign ameloblastomas [19]. In our case report, the immunohistochemical staining showed negative staining for CK, P63, TTF-1, CK7, CK8 and Wapsin A. From the negative staining of CK/CK7/CK8 and TTF-1, we could exclude the diagnosis of the lung primary cancer. Combined with clinical history and imaging examination, we considered that the patient’s diagnosis was pulmonary metastatic ameloblastoma. From the lesion by H&E staining, we showed that the ameloblastoma contained small islands and thin cords of ameloblastic epithelium within a dense fibrous connective tissue stroma, and the cells of tumors were very irregular and invasive.
Because of the small number of cases reported, no therapeutic gold standard has been mentioned in the literature, and the evidence is mainly derived from case reports. Surgery is generally regarded as the best treatment approach for primary ameloblastoma. Hertog et al reported that when detecting tumor recurrence and metastasis, surgery is the only known treatment to increase disease-free survival; in the lung, wedge resection or lobectomy should be considered [20]. The effectiveness of adjuvant therapy is undecided because very few cases have been described. A case report previously demonstrated partial response of adamantinoma with lung metastases to treatment with third-line pazopanib [21], but they didn’t refer the base first-line chemotherapy. In the review of Deepali et al, they reported that adamantinoma was a biphasic tumor and characterized by epithelial and osteofibrous components that are associated with various proportions and differentiation patterns [22]. In other case report, it is common practice to treat metastatic ameloblastoma similarly to bone sarcomas. Therefore, in this case, we gave the patient with the base first-line chemotherapy and radiotherapy. The regimen of chemotherapy was “cyclophosphamide, doxorubicin and cisplatin”, which was reasonably safe, well tolerated, easy to administer. More encouraging, the efficacy of the therapy to the patient was very significant. Compared with the lesions before therapy, the tumor tissues in right lung reduced very significantly, which achieved complete response basically, and continued to the present. Although, the tumor tissues in the right maxillofacial didn’t reduce up to 25% before therapy, the effect were evaluated stable disease, which could be considered effective.
In our opinion, postoperative treatment such as chemotherapy, even radiotherapy, could be performed in malignant ameloblastoma, particularly those in whom recur repeatedly with or without distant metastasis.
Conclusion
The adamantinoma is a kind of low-grade neoplasm that rarely metastasizes to other tissues. Surgery is generally regarded as the best treatment approach for the primary adamantinoma. Given the rarity of malignant adamantinoma, there were not large scale clinical studies to the adamantinoma with lung metastases. We suggest that chemotherapy with the regimen of “cyclophosphamide, pirarubicin and cisplatin” should be considered in the metastatic adamantinoma. The efficacy for this regimen need to further reports from similar cases. Therefore, further studies, including cytogenetic or molecular biological mechanism about adamantinoma are still required to better delineate this lesion from experiment in vitro or vivo.
Acknowledgements
The authors would like to thank Dr Chen for providing radiographs data about this patient.
Disclosure of conflict of interest
None.
References
- 1.Georgios VK, Anthoula M, Anastasia SL. Ameloblastoma, a rare benign odontogenic tumour: an interesting tumour review targeting the role of radiation therapy. Clin Transl Oncol. 2011;13:793–797. doi: 10.1007/s12094-011-0735-5. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Ota Y, Sasaki M, Karakida K, Kaneko A, Sekido Y, Tsukinoki K. Peripheral ameloblastoma of the lower molar gingiva: a case report and immunohistochemical study. Tokai J Exp Clin Med. 2012;37:30–4. [PubMed] [Google Scholar]
- 3.Hunasgi S, Koneru A, Chauhan DS, Guruprasad Y. Rare giant granular cell ameloblastoma: a case report and an immunohistochemical study. Case Rep Dent. 2013;2013:372781. doi: 10.1155/2013/372781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infante-Cossio P, Prats-Golczer V, Gonzalez-Perez LM, Belmonte-Caro R, Martinez-DE-Fuentes R, Torres-Carranza E, Gacto-Sanchez P, Gomez-Cia T. Treatment of recurrent mandibular ameloblastoma. Exp Ther Med. 2013;6:579–583. doi: 10.3892/etm.2013.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temporale H, Zatoński T, Roszkowska A, Kręcicki T. Ameloblastoma of the nasal septum origin: a case report. Case Rep Otolaryngol. 2013;2013:280509. doi: 10.1155/2013/280509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves R, Saad Junior R, Dorgan Neto V, Botter M. A rare case of pneumothorax: metastatic adamantinoma. J Bras Pneumol. 2008;34:425–9. doi: 10.1590/s1806-37132008000600014. [DOI] [PubMed] [Google Scholar]
- 7.Ricard AS, Majoufre-Lefebvre C, Siberchicot F, Laurentjoye M. A multirecurrent ameloblastoma metastatic to the lung. Rev Stomatol Chir Maxillofac. 2010;111:98–100. doi: 10.1016/j.stomax.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Golubović M, Petrović M, Jelovac DB, Nenezić DU, Antunović M. Malignant ameloblastoma metastasis to the neck radiological and pathohistological dilemma. Vojnosanit Pregl. 2012;69:444–8. [PubMed] [Google Scholar]
- 9.Chawla R, Ramalingam K, Sarkar A, Muddiah S. Ninety-one cases of ameloblastoma in an Indian population: A comprehensive review. J Nat Sci Biol Med. 2013;4:310–5. doi: 10.4103/0976-9668.116984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertog D, Schulten EA, Leemans CR, Winters HA, Van der Waal I. Management of recurrent ameloblastoma of the jaws; a 40-year single institution experience. Oral Oncol. 2011;47:145–6. doi: 10.1016/j.oraloncology.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Khémiri C, Mrabet D, Mizouni H, Abbes I, Mnif E, Sellami S, Essaddem H. Adamantinoma of the tibia and fibula with pulmonary metastasis: an unusual presentation. BMJ Case Rep. 2011;4318:1–4. doi: 10.1136/bcr.06.2011.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghiam A, Al Zahrani A, Feld R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: long survival and prolonged stable disease. Ecancermedicalscience. 2013;7:323. doi: 10.3332/ecancer.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaishampayan SS, Nair D, Patil A, Chaturvedi P. Recurrent ameloblastoma in temporal fossa: A diagnostic dilemma. Contemp Clin Dent. 2013;4:220–2. doi: 10.4103/0976-237X.114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertog D, Bloemena E, Aartman IH, van-der-Waal I. Histopathology of ameloblastoma of the jaws; some critical observations based on a 40 years single institution experience. Med Oral Patol Oral Cir Bucal. 2012;17:e76–82. doi: 10.4317/medoral.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JD, Jang HS, Seo YS, Kim JS. A repeatedly recurrent desmoplastic ameloblastoma after removal and allobone graft: Radiographic features compared with histological changes. Imaging Sci Dent. 2013;43:201–7. doi: 10.5624/isd.2013.43.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo DY, Feng CJ, Guo JB. Pulmonary metastases from an Ameloblastoma: case report and review of the literature. J Craniomaxillofac Surg. 2012;40:e470–4. doi: 10.1016/j.jcms.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Ota Y, Sasaki M, Karakida K, Kaneko A, Sekido Y, Tsukinoki K. Peripheral ameloblastoma of the lower molar gingiva: a case report and immunohistochemical study. Tokai J Exp Clin Med. 2012;37:30–4. [PubMed] [Google Scholar]
- 18.Sah P, Menon A, Kamath A, Chandrashekar C, Carnelio S, Radhakrishnan R. Role of immunomarkers in the clinicopathological analysis of unicystic ameloblastoma. Dis Markers. 2013;35:481–8. doi: 10.1155/2013/517834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal. 2013;18:e174–9. doi: 10.4317/medoral.18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger AJ, Son J, Desai NK. Malignant ameloblastoma: concurrent presentation of primary and distant disease and review of the literature. J Oral Maxillofac Surg. 2012;70:2316–26. doi: 10.1016/j.joms.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Cohen Y, Cohen JE, Zick A, Orevi M, Doviner V, Rubinstein R, Goldshmidt H, Peylan-Ramu N, Katz D. A case of metastatic adamantinoma responding to treatment with pazopanib. Acta Oncol. 2013;52:1229–30. doi: 10.3109/0284186X.2013.770921. [DOI] [PubMed] [Google Scholar]
- 22.Jain D, Jain VK, Vasishta RK, Ranjan P, Kumar Y. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:1–11. doi: 10.1186/1746-1596-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


