Abstract
Objectives: To investigate the therapeutic effects of OM-85 BV as an adjunctive treatment on experimental chronic rhinosinusitis (CRS) in mice. Methodology: Female BALB/c mice aged 8-12 weeks were sensitized and administrated by intranasal Aspergillus fumigatis (AF) three times per week for 1 week, 3 weeks, 2 months and 3 months (n = 10 each time point). The mice were randomly and equally assigned to four groups: normal control group, model group, OM-85-BV plus amoxicillin group, and isolated amoxicillin group. Inflammatory changes were determined by hematoxylin-eosin (HE) staining. The expression levels of suppressor of cytokine signaling (SOCS) 1, SOCS3, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ in samples were assessed by using real-time PCR (RT-PCR) and Western blotting. Results: There were significantly inflammatory and structural changes between the model and other groups. Compared to the model group, the mRNA expression levels of SOCS1, SOCS3, TNF-α, and IFN-γ were significantly decreased in OM-85-BV plus amoxicillin group and isolated amoxicillin group, along with the protein levels. Conclusion: The bacterial extract OM-85 BV is a low-cost alternatively adjunctive drug to treat CRS with simple oral administration, good safety, and few side effects.
Keywords: OM-85-BV, SOCS1, SOCS3, TNF-α, IFN-γ
Introduction
Chronic rhinosinusitis, a chronic inflammation of the nose and nasal sinuses, is characterized by manifestation of two or more common nasal symptoms, such as purulent nasal discharge, nasal obstruction, head/facial pain post, nasal drip and olfactory impairment. These symptoms will significantly affect quality of life. Patients who suffer from CRS have obviously higher bodily pain and reduced social function compared with other chronic diseases (e.g. chronic obstructive pulmonary disease, back pain) [1]. In addition, the prevalence of CRS has been estimated to affect 10.9% to 15.5% of the total population in different countries [2,3], and a Europe research team has confirmed this common chronic disease as a burden and pointed out the underestimation of this disease [3]. Moreover, it has been reported that patients with CRS have higher risk for stroke occurrence, chronic periodontitis [4,5].
Therefore, an adequate therapy is essential.
Although the etiology and pathophysiology of CRS are still remains unclear, cytokine and chemokine signaling has been considered as important inflammatory factors [6]. The suppressor of cytokine signaling (SOCS) family is one of the major mechanisms for regulations of cytokine signaling [7]. Among the members of SOCS family, SOCS1 and SOCS3 are the best-characterized members. It has been reported that the increased SOCS1 and SOCS3 expression is associated with CRS [8]. Hence, we supposed that inhibition the expression of SOCS1, SOCS3, and other related cytokines may have therapeutic effect on CRS.
Treatment of CRS includes antibiotics, nasal decongestants, antihistamines, topical nasal steroids and/or oral steroids, and saline irrigation, or topical or systemic corticosteroids. Mast cell stabilizers and leukotriene inhibitors are adjuvant treatments, which may also help alleviate symptoms in some patients, but severe patients need allergy immunotherapy and/or sinus surgery. However, some patients do not respond with the tailored therapy. Hence, novel therapies should be required to improve the disease symptoms and quality of life of CRS patients. Broncho-Vaxom (OM-85 BV) is an alternative novel therapeutic agent. It is an immunostimulant constituted from eight different bacterial species which are frequently responsible for respiratory infections. Recent studies have shown their efficacy for treatment of recurrent respiratory tract infections, asthma, chronic obstructive pulmonary disease (COPD), chronic bronchitis, subacute sinusitis and allergic rhinitis [9-11]. However, rare studies are focused on the treatment of CRS.
The aim of this study was to investigate the therapeutic effects of OM-85 BV as an adjunctive treatment on experimental CRS in mice. Our experimental results may provide efficient supporting evidences for clinical treatment of CRS.
Materials and methods
Experimental animals and model building
Forty female BALB/c mice aged 8-12 weeks were purchased from Experimental Animal Center of Weifang Medical University. Each animal was housed in a single clean cage and provided with liquid diet before the procedure. The protocol of using animal was complied with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals (NIH Publication 85-23). Culture filtrate and combined with mycelial extracts of Aspergillis fumigatus (AF) (Hollister-Stier Laboratories, Spokane, WA) was applied for intraperitoneal and intranasal use [12].
The method of CRS model was refereed to by Lindsay, et al., and later modified by Khalid, et al. [13,14]. The experimental mice received an intraperitoneal injection of AF extract, 200 μg absorbed into 2 mg of alum in 5 mL of phosphate buffered saline solution. After sensitization by intraperitoneal injection one week later, a nasal antigen challenge of AF extract was performed 3 times per week for 12 consecutive weeks to the experimental mice. The animals of model group animals were provided with nasal saline solution at the same time intervals. Untreated mice was used as normal controls. Mice were lightly anesthetized with methoxyfluorane inhalation, and then the mice were held in the supine position, followed by 10 μg (5 μL) of AF antigen or 5 μL of normal saline solution being applied to each nare with a micropipette. The mice were held upright until alert after instillation.
The normal mice (n = 10) did not receive any modeling and treatment, which were considered as the normal control group. While other animals were successfully induced model, the mice were randomly and equally divided into three groups (n = 10): model group, OM-85-BV plus amoxicillin group, and isolated amoxicillin group. For the normal control group, mice were gavaged with sterile water for 3 months. The mice in the model group received modeling without any treatment. While for the OM-85-BV plus amoxicillin group, the bacterial extract OM-85 BV (OM-PHARMA, Meyrin, Geneva, Switzerland) and amoxicillin (Sigma, USA) were performed to the mice. The amounts of OM-85 BV which was given conferred to the pure bacterial extract without additives. OM-85 BV was dissolved in pyrogen-free phosphate-buffered saline. The mice were then administered OM-85 BV by using a gastrogavage for 10 consecutive days, but no treatment for the next 20 days. One month was considered as one course. The mice were treated 3 months. In addition to OM-85 BV, mice were also treated with 2 mg/kg amoxicillin. The amoxicillin was dissolved in sterile water twice daily by gavage beginning at either 5 dpi or 3 dpi and continuing for 3 months. Whereas, the mice in the isolated amoxicillin group were only subjected to amoxicillin, the amounts and time were the same as OM-85-BV plus amoxicillin group.
Histological analysis
Under lightly anesthetized by ether inhalation, limbs and head of each mouse were fixed on the rat boards. The mice were sacrificed and the nose and sinuses were harvested. The tissues were fixed in 3% paraformaldehyde, decalcified, and embedded in paraffin. Then they were cut into 4 µm sections by using microtome, and stained with standard techniques. The presence and degree of inflammation was analyzed using an Olympus BH-2 microscope (Olympus America Inc).
Quantitative real time RT-PCR
According to the manufacturer’s instructions, total RNA for RT-PCR was extracted from dissected sinonasal tissue by using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Purity of the total RNA was assessed with the A260/A280 ratio, expected values 1.8 and 2.0 was considered good. Approximately 0.25 µg of total RNA was synthesized to complementary DNA (cDNA). The products of PCR were identified by an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, Calif). The GAPDH gene was monitored as a reference. PCR amplification was conducted by the LightCycler 1.5 (Roche Diagnostics, Tokyo, Japan) using LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics, Tokyo, Japan). PCR conditions included 1 predenaturation cycle of 5 min at 95°C, 40-50 cycles of 95°C for 30 s, 58-62°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 5 min. The mRNA levels were expressed as threshold cycle (CT). Comparative CT method was offered for analysis. The amount of target was calculated by using 2−ΔΔCT method [15]. This method does not need to set up a standard curve for each assay when calculate the relative quantities of targets. Reactions were run in triplicate on three different days for each sample.
The primers sequences used were as follows: SOCS1, 5’ primer 5’-GTGGTTGTGGAGGGTGAGAT-3’ and 3’ primer 5’-CCTGAGAGGTGGGATGAGG-3’; SOCS3, 5’ primer, 5’-AGCTCCAAAAGCGAGTACCA-3’, and 3’ primer, 5’-TGACGCTCAACGTGAAGAAG-3’; TNF-α, 5’ primer 5’-GTTCATCCGTTCTCTACC-3’, and 3’ primer 5’-AGCGTCTCGTGTGTTTC-3’; and IFN-γ, 5’ primer 5’-GGTGACATGAAAATCCTGCAG-3’, 5’-CCTCAAACTTGGCAATACTCATGA-3’. The reference gene of GAPDH was: 5’ primer 5’-CGGAGTCAACGGATTTGG TC-3’ and 3’ primer 5’-AGCCTTCTCCAT GGTCGTGA-3’.
Western blotting assay for detection of SOCS1, SOCS3, TNF-α, and IFN-γ expression
For Western blotting analysis, sinus tissue was washed with 0.1% DEPC water and flashily frozen and stored at -80°C until used. Protein density was assessed by the BCA method. Protein samples (20 μL) from each group were separated by 12% standard electrophoresis sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel and transferred onto polyvinylidene difluoride (PDVF) membranes (Millipore, Bed- ford, MA). The blots were blocked with 1% defatted milk powder for 1 h at room temperature and incubated overnight at 4°C with the following antibodies: anti-SOCS1 antibody (1:1000, AnaSpec, San Jose, CA, USA), anti-SOCS3 antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), anti- TNF-α antibody (1:1000, Genzyme, Cambridge, MA), and anti- IFN-γ antibody (1:1000, Genzyme, Cambridge, MA). An anti-GAPDH antibody (Santa, Cruz Biotechnology) was considered as a loading control. After incubation with an goat anti-mouse (1:500, Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China) as the secondary antibody, the samples were conducted to enhanced chemiluminescence and densitometric analysis.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 software (SPSS, Chicago, IL, USA). The measurement data were expressed as mean ± standard deviation (SD). Multiple comparisons were conducted by one-way analysis of variance (ANOVA). There was a significantly statistical difference when P < 0.05.
Results
Histological analysis
As shown in Figure 1A-C, there were significant inflammatory and structural changes between the control and other two groups. Histologically, the animals in the control group showed obvious mast cell and eosinophilic infiltration with augmented depth of lamina propria. In addition, ciliated epithelium was lost, nonciliated eosinophilic secretory epithelial cells were present with hyperplasia, along with an undulating basement membrane zone.
Figure 1.
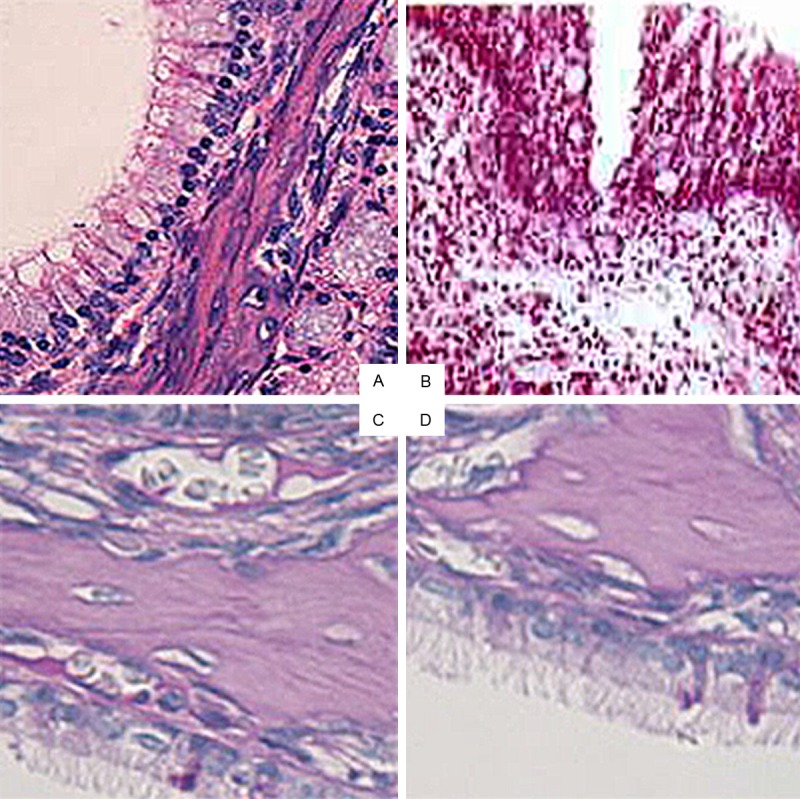
Histological analysis of CRS in the four groups (×20). A. Normal control group; B. Model group; C. OM-85-BV plus amoxicillin group; D. Isolated amoxicillin group; CRS, chronic rhinosinusitis.
SOCS1, SOCS3, TNF-α, and IFN-γ expression by quantitative real-time PCR and western blot (WB)
The mRNA and protein levels of SOCS1, SOCS3, TNF-α, and IFN-γ were showed in Figures 2, 3 and 4, respectively. The figures showed that the expression levels of SOCS1, SOCS3, TNF-α, and IFN-γ were the highest in the model group, while decreased in the OM-85-BV plus amoxicillin group and isolated amoxicillin group. Compared to the model group, the mRNA expression levels of SOCS1 (0.807 ± 0.071 vs. 0.214 ± 0.082; 0.807 ± 0.071 vs. 0.419 ± 0.052), SOCS3 (0.897 ± 0.032 vs. 0.299 ± 0.035; 0.897 ± 0.032 vs. 0.523 ± 0.036), TNF-α (0.991 ± 0.036 vs. 0.222 ± 0.026; 0.991 ± 0.036 vs. 0.423 ± 0.031), and IFN-γ (0.963 ± 0.064 vs. 0.305 ± 0.073; 0.963 ± 0.064 vs. 0.563 ± 0.055) were significantly decreased in OM-85-BV plus amoxicillin group and isolated amoxicillin group, respectively. In addition, the levels in the OM-85-BV plus amoxicillin group were significantly lower than the isolated amoxicillin group, as well as the protein levels. There were no significant differences between the OM-85-BV plus amoxicillin group and normal control group.
Figure 2.
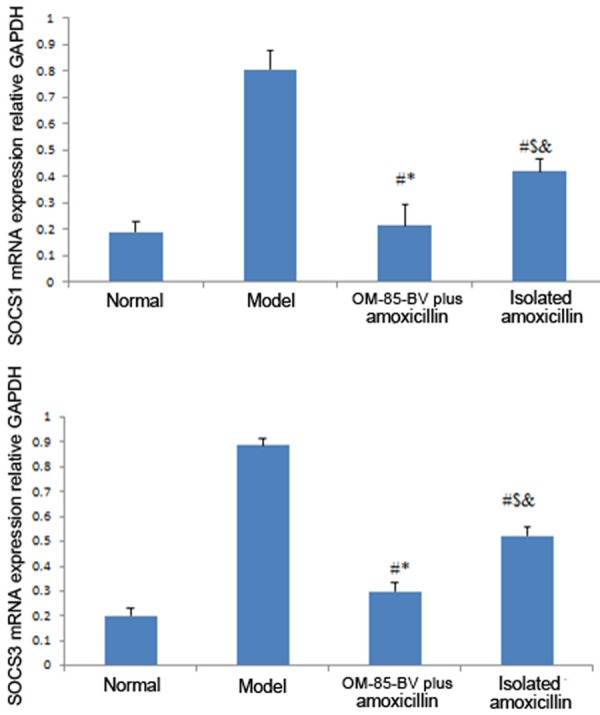
mRNA expression relative to GAPDH of SOCS1 and SOCS3. SOCS, suppressor of cytokine signaling. #P compared with the model group; *P compared with isolated amoxicillin group; $P compared with OM-85-BV plus amoxicillin group; &P compared with normal control group.
Figure 3.
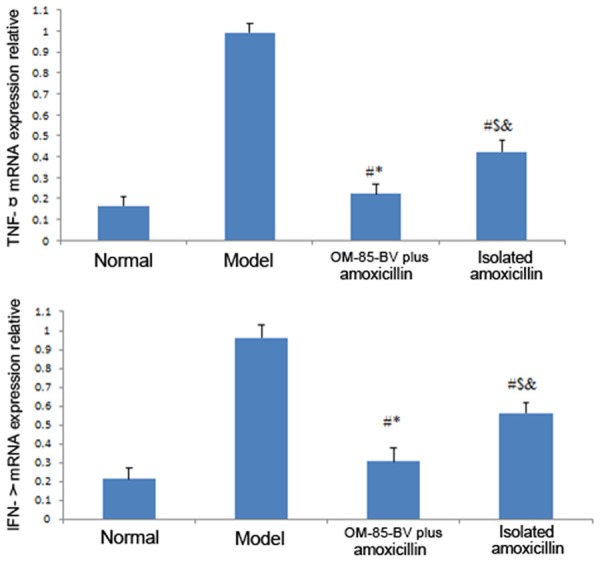
mRNA expression relative to GAPDH of TNF-α and IFN-γ. TNF, tumor necrosis factor; IFN, interferon. #P compared with the model group; *P compared with isolated amoxicillin group; $P compared with OM-85-BV plus amoxicillin group; &P compared with normal control group.
Figure 4.
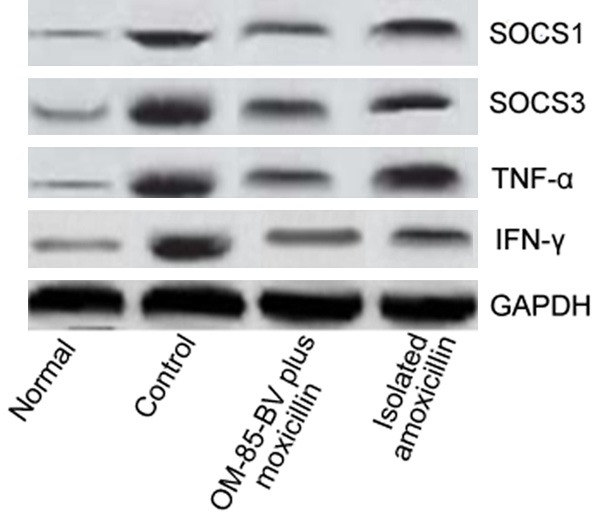
WB of CRS in the four groups. WB, western blotting; CRS, chronic rhinosinusitis.
Discussion
In the present study, the therapeutic effects of OM-85 BV as an adjunctive treatment on experimental chronic rhinosinusitis (CRS) in mice were evaluated. The results showed that the levels of SOCS1, SOCS3, TNF-α, and IFN-γ were significantly decreased by administration of OM-85-BV, indicating the bacterial extract OM-85 BV may be an alternatively adjunctive treatment for CRS by means of reducing the expression of cytokines in inflammatory sinus mucosa.
CRS, a heterogeneous group of inflammatory diseases of the nasal and paranasal cavities, impairs quality of life and may lead to the reduced workplace productivity, and serious medical treatment costs [16]. Although the high prevalence of CRS worldwide, the exact pathogenesis of the disease still remains debatable. It has been reported that bacteria, virus, and fungi serve as key infectious agents stimulating violent host inflammatory responses [17]. These triggers produce abnormal host responses, such as cytokine and chemokine signaling of nasal mucosa, which have been thought to be ultimately contributed to the persistent inflammatory process [18,19]. Previous studies have examined CRS-associated cytokines, mixed TH1/TH2 cytokine profile, suggesting that cytokines play an important pathogenic role in CRS [20]. In addition, accumulating evidence suggest that the SOCS family involves in many human chronic inflammatory diseases, such as asthma, allergic rhinitis, and atopic dermatitis [21,22]. SOCS1 and SOCS3 are the best-characterized members of the family [23]. The stimulation by IL-4, IL-13, IFN-γ, and TNF-α may result in upregulation of SOCS1 expression, while the expression of SOCS3 is induced by IL-6, IL-13, IFN-γ, and TNF-α. Moreover, Park et al. [8] found that SOCS1 and SOCS3 were increased in CRS, and suggested that the enhanced SOCS1 and SOCS3 may be a response to increased levels of various cytokines expressed in inflammatory sinus mucosa.
Pharmacotherapy is the primary treatment modality for CRS, however, despite with many therapeutic interventions (e.g. anti-inflammatory and anti-microbial intervention), therapeutic effect is often only partial and often unsuccessful. Therefore, the mechanisms of this disease are eagerly to reveal in order to further robust therapeutic strategies. Besides, novel therapies should be needed to alleviate symptoms and improve therapeutic effects. Accu-mulating studies have confirmed that the OM-85 BV, an immunostimulant, gained much attention on treatment of respiratory disease. It has been clinically indicated that administration OM-85-BV has a preventive outcome in recurrent chronic infections of the respiratory tract [24,25], besides, it can also shorten the period of convalescence when used in the acute phase of these infections [25]. Hence, we supposed it might have identical therapeutic effect on CRS.
In this study, the model of CRS was successfully induced. The levels of SOCS1, SOCS3, TNF-α, and IFN-γ was compared by RT-PCR. The results showed that there were significantly inflammatory and structural changes between the control and other treatment groups. The expression levels of SOCS1, SOCS3, TNF-α, and IFN-γ were obviously higher in the control group, while the levels were significantly reduced in treatment groups. In addition, the levels in the OM-85-BV plus amoxicillin group were lower than the isolated amoxicillin group. The results indicated that SOCS family served as critical cytokines in the development of CRS, while administration combined OM-85 BV with amoxicillin has certain therapeutic effects on treating CRS.
In conclusion, the bacterial extract OM-85 BV is considered as an effective immunomodulator on CRS inflammation in mice model using the same safe dose and time course as humans. We confirm that OM-85 BV is a low-cost alternatively adjunctive drug to treat CRS with simple oral administration, good safety, and few side effects.
Acknowledgements
This work was supported by the Otolaryngology Department of the Weifang People’s Hospital for clinical studies and laboratory parameters.
Disclosure of conflict of interest
None.
References
- 1.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–109. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 2.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;113:3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 3.Hastan D, Fokkens W, Bachert C, Newson R, Bislimovska J, Bockelbrink A, Bousquet PJ, Brozek G, Bruno A, Dahlén S. Chronic rhinosinusitis in European underestimated disease. A GA2LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Wu CS, Keller JJ, Lin HC. Chronic rhinosinusitis increased the risk of stroke: A 5-year follow-up study. Laryngoscope. 2013;123:835–840. doi: 10.1002/lary.23829. [DOI] [PubMed] [Google Scholar]
- 5.Keller JJ, Wu CS, Lin HC. Chronic rhinosinusitis increased the risk of chronic periodontitis: a population-based matched-cohort study. Laryngoscope. 2013;123:1323–1327. doi: 10.1002/lary.23720. [DOI] [PubMed] [Google Scholar]
- 6.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, Carter D, Carr T, Radtke M, Hulse KE, Seshadri S, Chandra R, Grammer LC, Harris KE, Kern R, Schleimer RP. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e310. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. SOCS, Inflammation, and Autoimmunity. Front Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, Jung JY, Hwang GH, Lee SH. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013;131:772–780. doi: 10.1016/j.jaci.2012.12.671. [DOI] [PubMed] [Google Scholar]
- 9.Bitar MA, Saade R. The role of OM-85 BV (Broncho-Vaxom) in preventing recurrent acute tonsillitis in children. Int J Pediatr Otorhinolaryngol. 2013;77:670–673. doi: 10.1016/j.ijporl.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Xuan J, Wang L, Yin H, Xuan D, Zhou Y, Hu S. The cost-effectiveness of OM-85 in managing respiratory tract infections in China. J Med Eco. 2015;18:1671–72. doi: 10.3111/13696998.2014.971159. [DOI] [PubMed] [Google Scholar]
- 11.Han L, Zheng CP, Sun YQ, Xu G, Wen W, Fu QL. A bacterial extract of OM-85 Broncho-Vaxom prevents allergic rhinitis in mice. Am J Rhinol Allergy. 2014;28:110–116. doi: 10.2500/ajra.2013.27.4021. [DOI] [PubMed] [Google Scholar]
- 12.van de Rijn M, Mehlhop PD, Judkins A, Rothenberg ME, Luster AD, Oettgen HC. A murine model of allergic rhinitis: studies on the role of IgE in pathogenesis and analysis of the eosinophil influx elicited by allergen and eotaxin. J Allergy Clin Immunol. 1998;102:65–74. doi: 10.1016/s0091-6749(98)70056-9. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay R, Slaughter T, Britton-Webb J, Mog SR, Conran R, Tadros M, Earl N, Fox D, Roberts J, Bolger WE. Development of a murine model of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:724–730. doi: 10.1016/j.otohns.2005.11.048. discussion 731-722. [DOI] [PubMed] [Google Scholar]
- 14.Khalid AN, Woodworth BA, Prince A, Quraishi SA, Antunes MB, Long FH, Bolger WE, Chiu AG, Palmer JN, Cohen NA. Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg. 2008;139:695–701. doi: 10.1016/j.otohns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Li CJ, Sun HW, Zhu FL, Chen L, Rong YY, Zhang Y, Zhang M. Local adiponectin treatment reduces atherosclerotic plaque size in rabbits. J Endocrinol. 2007;193:137–145. doi: 10.1677/JOE-06-0173. [DOI] [PubMed] [Google Scholar]
- 16.Kariyawasam HH, Scadding GK. Chronic rhinosinusitis: Therapeutic efficacy of anti-inflammatory and antibiotic approaches. Allergy Asthma Immunol Res. 2011;3:226–235. doi: 10.4168/aair.2011.3.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 18.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, Grammer LC, Schleimer RP. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan M Jr, Lane AP. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136:348–356. doi: 10.1016/j.otohns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. 1441.e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Kim K, Park SJ, Lee SH, Hwang JW, Park SH, Yum GH. Expression of SOCS1 and SOCS3 is altered in the nasal mucosa of patients with mild and moderate/severe persistent allergic rhinitis. Int Arch Allergy Immunol. 2012;158:387–396. doi: 10.1159/000333103. [DOI] [PubMed] [Google Scholar]
- 22.Cassel SL, Rothman PB. Role of SOCS in allergic and innate immune responses. Adv Immunol. 2009;103:49–76. doi: 10.1016/S0065-2776(09)03003-X. [DOI] [PubMed] [Google Scholar]
- 23.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Tarango MD, Berber A. Efficacy of a bacterial extract (OM-85 BV) in preventing recurrent respiratory tract infections in susceptible children. Clin Drug Invest. 1997;13:76–84. [Google Scholar]
- 25.Berber AC, Del-Rio-Navarro BE. Use of Broncho-Vaxom in private practice: phase IV trial in 587 children. Clin Ther. 1996;18:1068–1079. doi: 10.1016/s0149-2918(96)80062-2. [DOI] [PubMed] [Google Scholar]


