Abstract
Diabetic Retinopathy (DR) is one of the most common complications of the late phase diabetes, and also a common cause of blindness. High mobility group box 1 (HMGB-1) is considered to be an inflammatory mediator in the late phase that promotes inflammation and neovascularization in diabetes. Therefore, this paper discussed the role of HMGB-1 in diabetic retinopathy inflammation and neovascularization. 96 adult SD rats were randomly divided into control and diabetes group. The diabetic rat model was established by intraperitoneal injection of streptomycin (0.1 mol/L). Western blot was applied to determine HMGB-1 and its receptor RAGE and TLR2 protein expression in the serum. TUNEL was used to detect retinal apoptosis. Immunofluorescence was performed to test HMGB1 protein expression in retina. HBGM-1 and RAGE expression in diabetic rat retina was significantly higher than the control (P < 0.05), while TLR2 expression was lower (P < 0.05). TUNEL detection showed that diabetic rat retinal cells presented obviously higher apoptosis rate (P < 0.05). Immunofluorescence test revealed that HMGB1 largely expressed in the diabetic rat retinal cells (P < 0.05). HMGB1 may involve in the pathogenesis of diabetic retinopathy by binding with RAGE receptor to accelerate rat retinal cells apoptosis.
Keywords: Diabetes, retinal inflammation, apoptosis, HMGB-1
Introduction
In 2011, there were 366 million people suffered from diabetes worldwide. Chronic complications accounted for the main harm of diabetes, while diabetic retinopathy (DR) was a common one and the first blinding eye disease. DR prevalence in China reached 37% of the diabetic population [1]. Some reports suggested that its prevalence can increase to 54% after 10-20 years. It is very difficult to reverse the process once DR occurs. Though significant progress had been reached on DR clinical prevention and treatment in these decades, its blindness rate did not decrease significantly. Its control methods included candesartan inhibition of DR, bevacizumab effectively reduce the macular edema, and the improved vitrectomy technology [2]. This brought great pressure and challenge to our health economy and people’s quality of life. Recent reports suggested that vitreous cavity injection of vascular endothelial growth factor inhibitor can only prevent retinal inflammation and neovascularization to a certain extent [3]. It indicated that VEGF blocker cannot inhibit inflammatory reaction and neovascularization completely, and this suggested that VEGF is not the only important factor to promote angiogenesis in retinal tissue and inflammatory mediator. High mobility group box 1 protein (HMGB-1) is a kind of conservative nucleoprotein with relative molecular mass only 25000. HMGB-1 would be released from the nucleus passively under necrosis. Its expression is low normally. However, HMGB-1 can be actively secreted by a variety of cells, including monocytes, DC cells, NK cells, etc. Its major receptor is receptor for advanced glycationend (RAGE) and toll-like receptor 2 (TLR2) [4]. It could trigger inflammation occurrence and neovascularization by binding with receptors. The major pathological physiology of DR is inflammatory formation and neovascularization. Thus, it is necessary to clarify whether HMGB-1 participated in DR to provide basis for DR clinical prevention.
Materials and methods
Animals
96 SD rats weighted 200 ± 20 g were purchased from Nanchang University animal experimental center. The rats were maintained at temperature 24 ± 3°C and standard relative humidity, and fed by day and night alternation, light and shade alternation.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Main reagents and instruments
RNA extraction kit was bought from Beijing Solarbio co., LTD. Streptozotocin (STZ) was purchased from Sigma. Sodium citrate buffer and PBS were from Wuhan Boster Company. M-PER lysis was bought from THERMO. Primary antibody for HMGB-1, RAGE and TLR2 were got from R&D Company. DAPI bought from Jiangsu Beyotime Biotechnology. SW-CJ-2DF bechtop was provided by Chongqing Pharmaceutical co., LTD. Inverted fluorescence microscope was from Leica. HD3100 ultrasonic cell disruptor was bought from Chongqing Maike Science And Technology Company.
Modeling and grouping
96 rats were randomly divided into control and diabetes groups. The rats in diabetes group received 60 mg/kg 2% STZ in 0.1 mol/L sodium citrate buffer intraperitoneal vein injection [5]. Urine glucose and blood glucose were tested in modeling rats at 4 weeks after injection. Modeling was evaluated success when urine sugar values over (+++) and blood glucose concentration greater than 16.7 mmol/L [6]. Diabetes group rats kept the blood glucose level within the requirement standards. Blood glucose level was measured at 1st, 2nd, 3rd, and 4th week. The rats in two groups were fed with normal diet after successful modeling.
RT-PCR
RT-PCR was performed to test gene expression in the retina and the primers used were listed in Table 1 [7]. Retinal cells were cracked with 500 μl TPK lysis buffer and homogenated for 30 s. RNA was extracted according to the kit manual and reverse transcripted to cDNA. RT-PCR was applied on Rotor-Gene Q cycler.
Table 1.
Primers used for RNA and cDNA
| Gene | Forward | Reverse |
|---|---|---|
| β-Actin | 5’-CGT AAA GAC CTC TAT GCC AA-3’ | 5’-AGC CAT GCC AAA TGT GTC AT-3’ |
| TNF-α | 5’-CAA AAT TCG AGT GAC AAG CCT G-3’ | 5’-GAG ATC CAT GCC GTT GGC-3’ |
| VEGF | 5’-AAC CAT GAA CTT TCT GCT CTC TTG-3’ | 5’-GCC TGG CTC ACC GCCTTG GCT TGT C-3’ |
| ICAM-1 | 5’-CCC TGT CAG TCC GGA AAT AA-3’ | 5’-GAT GAC TTT TGA GGG GGA CA-3’ |
| PLA2 | 5’-CAG AAU GCU UCC AAU CGU A-3’ | 5’-CAG CAA GGA UCC UCG CUA U-3’ |
Western blot
Rat’s retina was stripped from the eye and washed by PBS. After 5 min centrifugation at 500× g, the tissue was added with 400 μl CER I reagent. Then the tissue was ultrasonicated for 5 min and added with 22 μl precooled CER II. The cytoplasm protein was extracted after 5 min centrifugation at 12000× g [8-11]. Western blot was used to detect HMGB-1, RAGE, and TLR2 expression, and ImageLab software was applied for calculation.
Immunofluorescence
Isolated retinal tissue was washed by PBS for 2 hours and fixed by methanol for 20 minutes. After 10 minutes of natural drying, the tissue was incubated with primary antibody at 4°C overnight and secondary antibody at 37°C less than 1 hour [11]. Fluorescence microscope was used for observation and photograph.
TUNEL detection
Integrate eyeball with 1-2 mm optic nerve was extracted and fixed by 10% paraformaldehyde. After ethanol dehydration step by step, xylene transparency, and paraffin embedding, the tissue was sliced at 4-5 μm thickness [12]. TUNEL was detected according to the instrument after 10 minutes dewaxing and graded ethanol hydration.
Statistical analysis
All statistical analyses were performed using SPSS18.0 software (Chicago, IL). Numerical data were presented as means and standard deviation (± SD). Differences between multiple groups were analyzed using t-test. P < 0.05 was considered as significant difference.
Results
General information
General information analysis showed that there was significant difference in weight, blood glucose, and eyeball weight between the two groups (P < 0.05) (Table 2).
Table 2.
General information analysis
| Parameters | Normal (n = 48) | STZ-induced diabetic rats (n = 48) | P |
|---|---|---|---|
| Body weight (g) | 428.4 ± 3.2 | 209 ± 7.4 | 0.005 |
| Eyeballweigt (mg/100g B.W) | 35.2 ± 3.2 | 69.4 ± 6.2 | 0.03 |
| Serum glucose (mg/dL) | 118.3 ± 5.2 | 521.6 ± 3.1 | 0.02 |
HMGB1, PLA2, TNF-α, VEGF, ICAM-1 gene expression
RT-PCR measurement showed that HMGB1, PLA2, TNF-α, VEGF, ICAM-1 gene overexpressed significantly in diabetes rats compared with control. Of which, HMGB1 and PLA2 presented the largest increase (> 4.6 fold), while TNF-α, VEGF, ICAM-1 also rise over 2.4 times (Figure 1).
Figure 1.
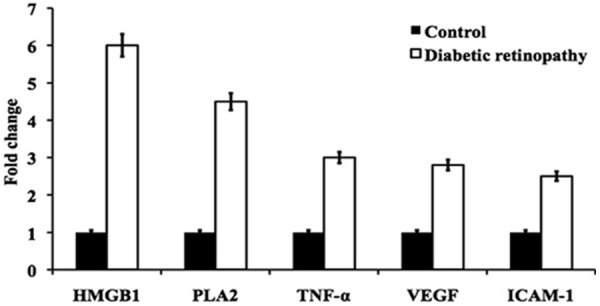
Represent the mRNA expression of HMGB1, PLA2, TNF-α, VEGF, ICAM-1 from the retinal tissue of control and diabetic retinopathy rats.
HMGB1 and its receptors protein expression
Western blot was used to detect HMGB1 and its receptors expression on retina. It was found that HMGB1 and RAGE expression increased obviously in diabetes rats (P < 0.05), while TLR2 expression reduced significantly (P < 0.05) (Figure 2).
Figure 2.
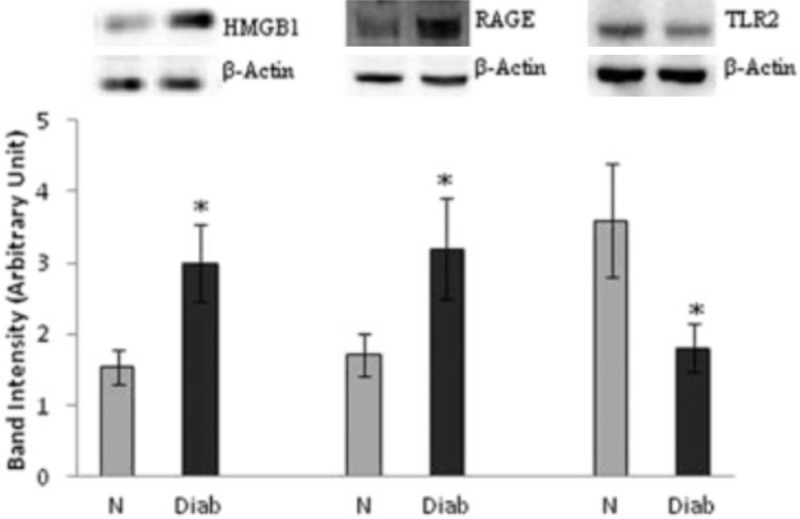
Protein expression of HMGB-1, RAGE, TLR2were measured by western blot assay. The results showed the expression of HMGB-1, RAGE, TLR2 adjusted to that of beta-actin in the same sample and each experiment was repeated 3 times with fresh samples. The value represented here as mean ± SD of 48 rats in each group of the two groups. N = Normal, Diab = diabetic rat. *P < 0.05 compared to normal.
TUNEL detect retinal cells apoptosis
TUNEL detection revealed that diabetes rats showed stronger retinal cells apoptosis than control (Figure 3).
Figure 3.
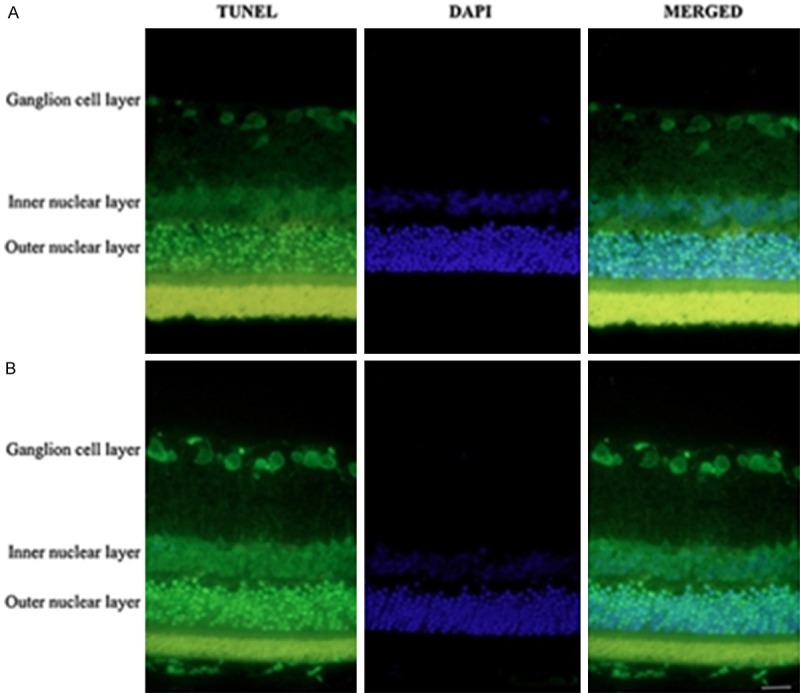
Apoptosis was detected in retinal tissues by TUNEL and DAPI staining. A. The retinal layer of control rats. B. The retinal layer of STZ-induced diabetic retinopathy rats. The apoptotic cells of retinal ganglion were detected by TUNEL (green), and the nuclei (blue) were stained with DAPI. Scale bar = 50 μm.
Immunofluorescence
As shown in Figure 4, retinal cells from diabetes rat exhibited abundant HMGB1 compared with control.
Figure 4.
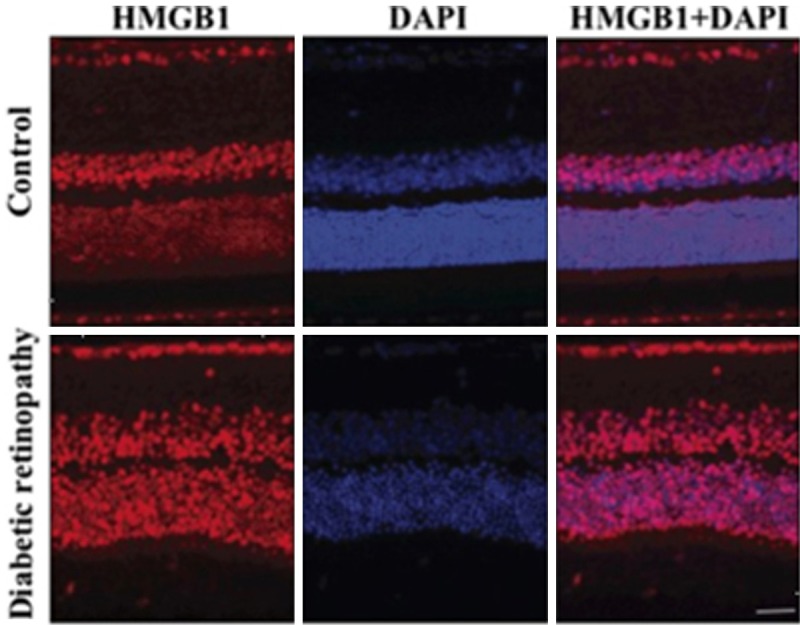
Represent the HMGB1 (red) and DAPI (blue) staining of retinal layer of control and STZ-induced diabetic retinopathy rats. The high expression of HMGB1 was noted in diabetic retinopathy. Scale bar = 50 μm.
Discussion
DR is one of the most common microvascular complications of diabetes. Its pathological change includes neovascularization and inflammation, which is a major cause of diabetic patients’ visual loss [13]. DR occurrence and development mechanism is quite complicated and has not been fully understood. Previous studies showed that VEGF, IGF-1 and TNF-α are important stimulus [14]. Following the study progression, the role of HMGB-1 protein in DR development received more attention. HMGB-1 is a kind of highly conservative nucleus protein. It was reported that multiple pathological stimulus may cause HMGB-1 released out of the cell in addition to necrosis. Related studies showed that HMGB-1 can not only promote inflammatory reaction, but also can stimulate neovascular formation [14,15].
Our study found that HMGB1, PLA2, TNF-α, VEGF, ICAM-1 gene overexpressed significantly in diabetes rats compared with control. Among them, HMGB1 and PLA2 presented the largest increase, while TNF-α, VEGF, ICAM-1 also rise over 2.4 times. RT-PCR results revealed that sustained high blood glucose may activate HMGB1, PLA2, TNF-α, VEGF, and ICAM-1 gene expression, and these genes has obvious correlation with DR. Related research showed that TNF-α can stimulate human retina barrier directly through NF-κB signaling pathway. VEGF, ICAM-1, PLA2 was thought to be associated with neovascularization in DR, as they can stimulate retinal vascular endothelial cell proliferation specifically [13-19]. This study observed that retinal cells PLA2, TNF-α, VEGF, and ICAM-1 gene expression increase participated in DR development, which is likely to be associated with sustained hyperglycemia state. Our research also found that HMGB-1 overexpressed in the retinal cells of diabetic rats (6.1 times), suggesting that long-lasting hyperglycemia can stimulate HMGB-1 gene expression and HMGB-1 may participate in DR in rats.
Following immunofluorescence assay found HMGB-1 protein highly expressed in diabetic rats, and it was significantly higher than in the control (P < 0.05). TUNEL assay showed that retinal cells apoptosis rate increased markedly (P < 0.05). Some studies suggested HMGB1 and ROS overexpressed in the retina under acute ischemia-reperfusion injury [20-23]. Volz HC found that though HMGB1 changed later in DR rats compared with other inflammatory factors such as VEGF, its sustain time was longer [16]. This suggested that HMGB1 protein may be involved in the later phase of DR.
It was thought that HMGB-1 may participate in inflammation and neovascularization in DR through binding with receptor for advanced glycationend (RAGE) and toll-like receptor 2 (TLR2) [23-25]. However, our study found that TLR2 protein reduced obviously in diabetic rats, while RAGE protein increased significantly. It suggested that HMGB-1 may participate in DR occurrence and development mainly through RAGE.
To sum up, HMGB-1 may involve in DR through binding with RAGE, and HMGB-1 content is positively correlated with retinal cells apoptosis. Detecting HMGB-1 level and HMGB-1 target therapy in clinic could help diabetes complications prediction and treatment.
Acknowledgements
Reseach supported by the National Natural Science Foundation of China (NSFC No: 81400372, 81160118, 81060063); Clinical Medicine Research Special-purpose Foundation of China (No: L2012052); Science and Technology Platform Construction Project of Jiangxi Province (No: 2013116); Jiangxi Province Voyage Project (No: 2014022); Jiangxi Province Education Department Scientific Research Foundation (No: GJJ14170); Jiangxi province Degree and Postgraduate Education Reform Project (No: 2015); Youth Science Foundation of Jiangxi Province (No: 20151BAB215016); Technology and Science Foundation of Jiangxi Province (No: 20151BBG70223); Health Development Planning Commission Science Foundation of Jiangxi Province (No: 20155154).
Disclosure of conflict of interest
None.
References
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 2.Federation ID. International Diabetes Federation: IDF Diabetes Atlas. Brussels, Belgium: 2013. [Google Scholar]
- 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 4.Dvoriantchikova G, Hernandez E, Grant J, Santos AR, Yang H, Ivanov D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Invest Ophthalmol Vis Sci. 2011;52:7187–7194. doi: 10.1167/iovs.11-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 6.Anfuso CD, Assero G, Lupo G, Nicotra A, Cannavo G, Strosznajder RP, Rapisarda P, Pluta R, Alberghina M. Amyloid beta(1-42) and its beta(25-35) fragment induce activation and membrane translocation of cytosolic phospholipase A2 in bovine retina capillary pericytes. Biochim Biophys Acta. 2004;1686:125–138. doi: 10.1016/j.bbalip.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Nicotra A, Lupo G, Giurdanella G, Anfuso CD, Ragusa N, Tirolo C, Marchetti B, Alberghina M. MAPKs mediate the activation of cytosolic phospholipase A2 by amyloid beta(25-35) peptide in bovine retina pericytes. Biochim Biophys Acta. 2005;1733:172–186. doi: 10.1016/j.bbalip.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Singh NK, Hansen DE 3rd, Kundumani-Sridharan V, Rao GN. Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCgamma-cPLA(2) activation and retinal neovascularization. Blood. 2013;121:1911–1923. doi: 10.1182/blood-2012-03-419234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudla B. Vascular endothelial growth factor (VEGF)-part 1: in physiology and pathophysiology. Endokrynol Pol. 2011;62:444–455. [PubMed] [Google Scholar]
- 10.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 11.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 12.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 13.Giurdanella G, Motta C, Muriana S, Arena V, Anfuso CD, Lupo G, Alberghina M. Cytosolic and calcium-independent phospholipase A(2) mediate glioma-enhanced proangiogenic activity of brain endothelial cells. Microvasc Res. 2011;81:1–17. doi: 10.1016/j.mvr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Lupo G, Anfuso CD, Ragusa N, Strosznajder RP, Walski M, Alberghina M. t-Butyl hydroperoxide and oxidized low density lipoprotein enhance phospholipid hydrolysis in lipopolysaccharide-stimulated retinal pericytes. Biochim Biophys Acta. 2001;1531:143–155. doi: 10.1016/s1388-1981(01)00102-0. [DOI] [PubMed] [Google Scholar]
- 15.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucolo C, Leggio GM, Drago F, Salomone S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem Pharmacol. 2012;84:88–92. doi: 10.1016/j.bcp.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T. Age-related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012;200:1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R, American Diabetes A. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 20.El-Asrar AM, Missotten L, Geboes K. Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Mol Vis. 2011;17:508–518. [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 22.Bustin M, Lehn DA, Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990;1049:231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Sun W, Gao R, Su Y, Umehara H, Dong L, Gong F. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology (Oxford) 2013;52:1739–1747. doi: 10.1093/rheumatology/ket134. [DOI] [PubMed] [Google Scholar]
- 24.Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113:163–171. doi: 10.4149/bll_2012_039. [DOI] [PubMed] [Google Scholar]
- 25.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]


