Abstract
The aim of this study was to assess the effects of hydrogen sulfide on high glucose-induced mouse podocyte (MPC) injury and the underlying mechanisms. Mouse podocytes were randomly divided into 4 groups, including high glucose (HG), normal glucose (NG), normal glucose + DL-propargylglycine (PPG), and high glucose + NaHS (HG + NaHS) groups for treatment. Then, ZO-2, nephrin, β-catenin, and cystathionine γ-lyase (CSE) protein expression levels were determined by western blot. We found that high glucose significantly reduced nephrin, ZO-2, and CSE expression levels (P<0.05), and overtly elevated β-catenin amounts (P<0.05), in a time-dependent manner. Likewise, PPG at different concentrations in normal glucose resulted in significantly lower CSE, ZO-2, and nephrin levels (P<0.05), and increased β-catenin amounts (P<0.05). Interestingly, significantly increased ZO-2 and nephrin levels, and overtly reduced β-catenin amounts were observed in the HG + NaHS group compared with HG treated cells (P<0.01). Compared with NG treated cells, decreased ZO-2 and nephrin levels and higher β-catenin amounts were obtained in the HG + NaHS group. In conclusion,CSE downregulation contributes to hyperglycemia induced podocyte injury, which is alleviated by exogenous H2S possibly through ZO-2 upregulation and the subsequent suppression of Wnt/β-catenin pathway.
Keywords: Diabetic nephropathy, hydrogen sulfide, podocytes, CSE, ZO-2, β-catenin
Introduction
Diabetic nephropathy (DN), one of the common microvascular complications of diabetes, is an important cause of diabetic death and disability. In recent years, podocyte damage has been shown to play a key role in diabetic nephropathy, especially proteinuria [1]. Wnt/β-catenin signaling is a highly conserved intracellular pathway involved in embryonic kidney development. In adult kidney diseases such as acute kidney injury, renal disease, and diabetic nephropathy, this pathway is abnormally activated and involved in the pathological process of podocyte injury [2]. Zona occludens-2 (ZO-2) belongs to the membrane associated guanylate kinase (MAGUK) protein family. As a skeleton protein, it widely exists in the tight junctions of epithelial cells and within the nucleus. It participates in a variety of signal transduction pathways. Studies have shown that ZO-2 in glomerular podocytes may be involved in the regulation of Wnt/β-catenin signaling [3], and its expression may be affected by oxidative stress.
Hydrogen sulfide (H2S) is a newly discovered gaseous signaling molecule beside carbon monoxide (CO) and nitric oxide (NO). In mammals, H2S is generated with L-cysteine as a substrate by activating 5’-pyridoxal phosphate-dependent enzymes, including cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) [4]. CBS and CSE are extensively distributed in mammalian tissues and cells [5]. CBS primarily exists in the nervous system, while CSE is mainly found in the peripheral system, especially liver, kidney and the vascular system [6]. Recent studies have found that H2S has a protective effect on renal lesions in diabetic rats, which may be related to the inhibition of Renin Angiotensin System (RAS) system activation [7]. However, whether H2S has a protective effect on podocyte injury caused by high glucose is unknown. This study aimed to assess the effects of H2S on high glucose-induced glomerular podocyte injury and identify the underlying molecular mechanisms.
Materials and methods
Materials
Mouse podocytes (MPCs) were a kind gift from Professor YiFan (Medical College of Shandong University). Other reagents included: RPMI 1640 and newborn fetal bovine serum (Hyclone, USA); sugar-free 1640 (Gibco, USA); D-glucose (Dingguochangsheng biotechnology company, China); DL-propargylglycine (PPG) and NaHS (Sigma, USA); rabbit anti-ZO-2 (CST, US); rabbit anti-CSE (Proteintech, USA); mouse anti-β-catenin (Santa Cruz, USA); rabbit anti-nephrin (Abcam, USA); anti-β-actin, and horseradish peroxidase-labeled goat anti-rabbit and goat anti-mouse anti-secondary antibodies (Beijing Zhongshan Golden Bridge company, China); ECL luminescent kit (Merck Millipore, German); BCA kit (Jiangsu Biyuntian biotechnology Institute, China); film (Kodak, Japan).
Cell culture
MPCs were maintained in RPMI 1640 supplemented with 5.5 mM glucose, 10 U/mL γ-interferon, 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C in a humid environment containing 5% CO2. Before the experiment, cells were cultured in six-well plates to sub-confluency (80%), and serum-free culture media were added for 24 h for cell synchronization. Synchronized cells were divided into several groups: Group A, High glucose (HG group, 30 mM glucose); Group B, a. Normal glucose group: 5.5 mM glucose +24.5 mM mannitol (NG group), b. Normal glucose (5.5 mM glucose +24.5 mM mannitol) + PPG (0.1 mM), c. Normal glucose +1 mM PPG, d. Normal glucose +10 mM PPG; Group C, High glucose (30 mM glucose) + NaHS (100 μM). Cells were collected and analyzed by western blot at various time points.
Western blotting
Cells were harvested, washed with PBS, and lysed on ice in a lysis buffer (20 mM Tris-HCl, 0.1 mM Na3VO4, 25 mM NaF, 25 mM β-glycerophosphate, 2 mM EDTA, 2 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin, pH 7.5). Cell lysates were centrifuged at 4°C for 15 min (14000 rpm). Total protein in supernatants was quantified with the BCA protein assay kit. Equal amounts of protein (20 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. After blocking with 5% nonfat dry milk at 4°C for 1 h, the membranes were consecutively incubated with primary antibodies (12 h at 4°C) and horseradish peroxidase-labeled secondary antibodies (1:2000 dilution, room temperature, 1 h). Finally, membranes were washed in TBST and detected with the ECL kit. Quantitative results were obtained with the NIH ImageJ 1.42 software.
Statistical analysis
Results were presented as mean ± SE of at least 3 independent experiments. Comparisons were performed by one-way analysis of variance (ANOVA), followed by the Neman-Keuls test using SPSS 17.0 software package. A value of P<0.05 was considered statistically significant.
Results
Effects of high glucose on nephrin, ZO-2 and β-catenin expression in podocytes
After treatment with high glucose (30 mM) for 12 h, nephrin and ZO-2 protein expression levels were significantly reduced, with the minimum obtained at 48 h. In contrast, β-catenin protein levels were significantly increased from 12 h (P<0.05), peaking at 48 h (P<0.05) (Figure 1).
Figure 1.
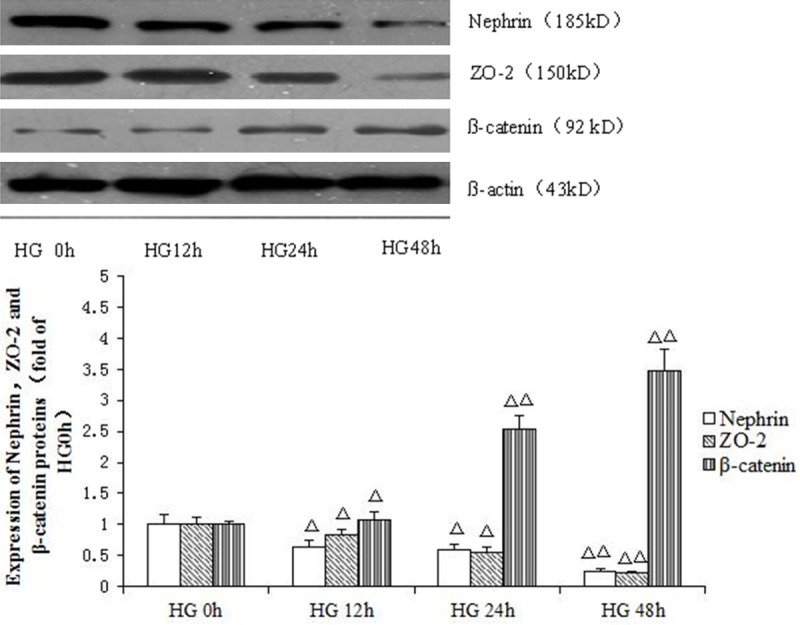
Effects of high glucose (30 mmol/L) on nephrin, ZO-2 and β-catenin proteins expression at different time points. Mean ± SD. n=3. ΔP<0.05 vs. HG 0h group; ΔΔP<0.01 vs. HG 0h group.
Effect of high glucose on CSE expression
Compared with the NG group, CSE expression levels in HG treated cells were significantly decreased (P<0.05), reaching a minimum at 48 h (P<0.01) (Figure 2).
Figure 2.
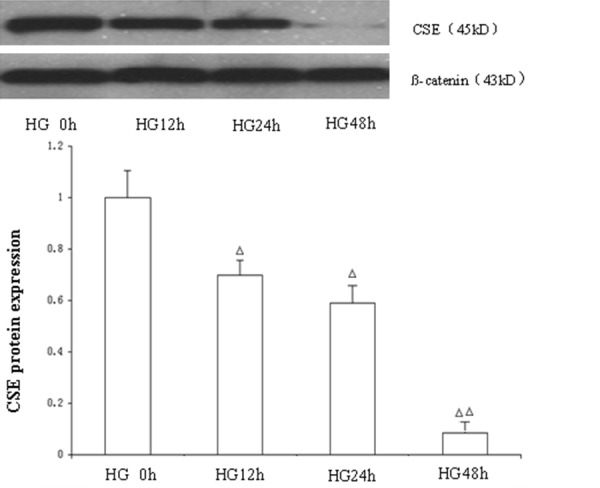
The effect of high glucose (30 mmol/L) on expression of CSE at 0 h, 12 h, 24 h and 48 h. Mean ± SD. n=3. ΔP<0.05 vs. HG 0h group; ΔΔP<0.01 vs. HG 0 h group.
Effect of PPG on nephrin, ZO-2 and β-catenin expression in MPCs
Podocytes cultured with normal glucose amounts were treated with PPG at different concentrations for 48 h. As shown in Figure 3, PPG significantly inhibited nephrin and ZO-2 expression (P<0.05) in a concentration-dependent manner. In contrast, β-catenin levels were significantly increased (P<0.05).
Figure 3.
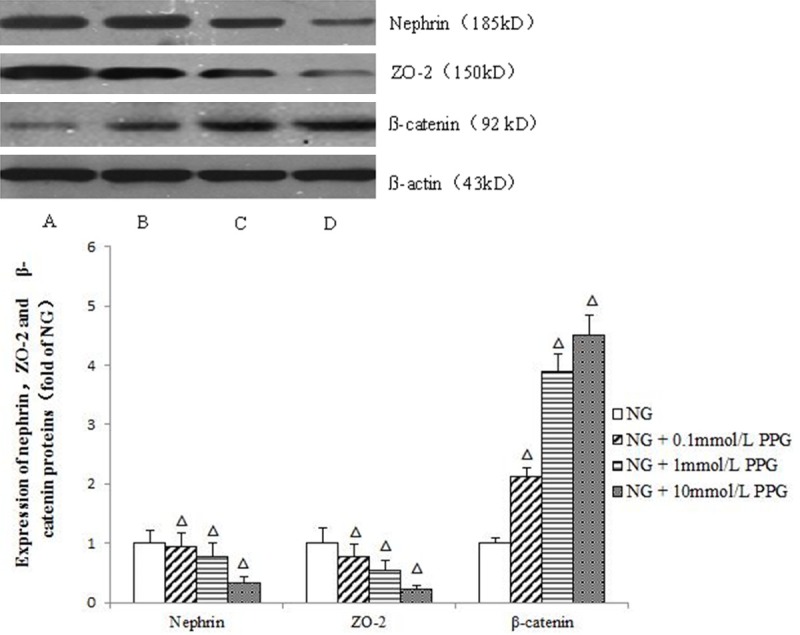
Effects of the different concentration of PPG on nephrin, ZO-2 and β-catenin at 48 h. A: NG group; B: NG +0.1 mmol/L PPG group; C: NG +1 mmol/L PPG group; D: NG +10 mmol/L PPG group. Mean ± SD. n=3. ΔP<0.05 vs. NG group; ΔΔP<0.01 vs. NG group.
Effects of NaHS on nephrin, ZO-2 and β-catenin expression in MPCs
Compared with the NG group, nephrin and ZO-2 levels in the HG group at 48 h were significantly reduced (P<0.01); meanwhile, β-catenin expression was significantsly increased (P<0.01). In the HG + NaHS group, nephrin and ZO-2 protein expression levels were significantly enhanced compared with the HG group (P<0.01), but still lower than the values obtained after NG treatment (P<0.01). In contrast, β-catenin expression was significantly decreased in the HG + NaHS group compared with the HG group (P<0.01), but still higher compared with NG treated cells (P<0.01) (Figure 4).
Figure 4.
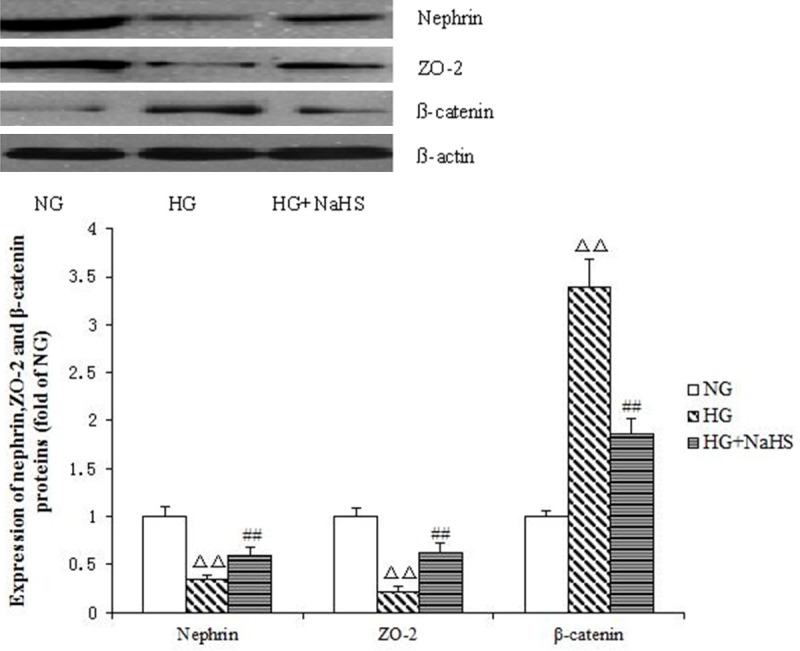
The effect of hydrogen sulfide on protein levels of nephrin, ZO-2 and β-catenin at 48 h. Mean ± SD. n=3. ΔΔP<0.01 vs. NG group; ##P<0.01 vs. HG group.
Discussion
Proteinuria is an important clinical characteristic of diabetic nephropathy and a significant indicator of clinical diagnosis. It can lead to progressive deterioration of the renal function, from microalbuminuria in the early phase to macroalbuminuria in the late period. Abnormal glucose metabolism is the initiating factor of diabetic nephropathy podocyte injury. Indeed, high blood glucose increases the permeability of the glomerular filtration membrane [8]. Podocytes are the last bulwark of glomerular filtration membranes with the ability of size and charge selectivity. Therefore, their injury causes macromolecules, such as plasma proteins, to leak out from the glomerular system. A pronounced protein leakage will increase podocyte injury and glomerulosclerosis. Podocyte loss and apoptosis are significantly involved in the generation of proteinuria, suggesting that after high glucose stimulation, podocyte injury triggers proteinuria generation at the molecular level. Nephrin is a marker protein of the podocytes’ hiatus membrane and closely related to albumin filtration. It is therefore an important marker of podocyte injury, and its down-regulation may lead to further deterioration of the renal function and glomerular sclerosis [9]. Reports have shown that nephrin reduction may be associated with abnormal activation of the Wnt/β-catenin pathway [10].
The Wnt signaling pathway plays a critical role in podocyte injury and proteinuria. The transcriptional activator adhering protein β-catenin (APC) and GSK3β enzyme form a complex in the cytoplasm in absence of Wnt. Then, the GSK3β enzyme phosphorylates and degrades β-catenin to maintain low intracellular expression. When the Wnt pathway is activated, the extracellular ligand Wnt, cell surface receptors Frizzled, and co-receptor LRP all combine. In this case, GSK3β is phosphorylated and inactivated, preventing β-catenin ubiquitination and degradation. Then, cytoplasmic β-catenin enters the nucleus and activates the transcription of target genes together with the LEF/TCF transcription factors. The expression of Wnt family proteins increases, e.g. Wnt1 in podocytes, inhibiting β-catenin phosphorylation. Cytoplasmic accumulation of β-catenin activates the critical transcription factor snail, thereby inhibiting nephrin expression and leading to podocyte dysfunction [11,12]. Recent studies have shown that ZO-2 may be involved in the regulation of the Wnt pathway [3,13].
Zona occludense-2 (ZO-2), a cytoskeletal protein, is widely expressed in epithelial tight junctions and the nucleus. ZO-2 has many protein binding sites with multiple membrane-associated guanylate kinase characteristics, including three PDZ domains, an SH3 domain, and a GuK domain [3]. ZO-2 plays several roles, including connection of occludin and claudins to cytoskeleton, and combining α-catenin, connexins 36 and 43 together with other tight junction proteins like ZO-1 and cingulin. In the kidney, ZO-2 is found in glomerular podocytes and tubular epithelial cells. In glomerular cells, ZO-2 and nestin co-localize on the podocyte foot, unlike ZO-1. ZO-2 is associated with cell proliferation, apoptosis, tumorigenesis and oxidative stress [13,14]. In addition, ZO-2 promotes the cytoplasmic accumulation of β-catenin by inhibiting GSK3β phosphorylation, and prevents the loss of nephrin to avoid large-scale podocyte integration or loss [3,13]. It may be an important mechanism leading to podocytes dysfunction and proteinuria formation.
Endogenous kidney H2S has powerful antioxidant, anti-apoptotic and anti-inflammatory effects and is catalytically synthesized by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3MST) from cysteine [15]. Yuan et al. [16] demonstrated that glomerular mesangial cell damage caused by high glucose induced reactive oxygen species (ROS) can be ameliorated by H2S. High environmental glucose levels decrease the activity of NAPDH oxidases and promote ROS production. Furthermore, high environmental glucose levels break the redox balance and decrease H2S production. However, whether podocytes contain enzymes that synthesize endogenous H2S remains unclear as well as the mechanism of these potential enzymes.
We found significantly reduced expression of nephrin in podocytes cultured with high glucose, suggesting that high glucose causes podocyte injury. Then, we assessed the changes in ZO-2 and β-catenin expression levels. As shown above, high glucose overtly reduced ZO-2 expression and activated the Wnt/β-catenin pathway. CSE is necessary for H2S production in vivo and highly expressed in the kidney. Indeed, CSE is found in mesangial cells. However, its presence in podocytes has not been demonstrated. Our results revealed that podocytes express CSE. In addition, we showed that high glucose reduces nephrin and CSE expression levels. In order to clarify whether high glucose-induced down-regulation of CSE is related to podocyte injury, we further evaluated the effects of CSE specific inhibitor on nephrin expression. As shown above, the CSE inhibitor significantly reduced nephrin expression in MPCs under normal conditions of glucose in a dose-dependent manner. These results suggest that high glucose-induced down-regulation of CSE might be a mechanism underlying podocyte injury. In addition, cells treated with NaHS produced H2S. Indeed, NaHS had a significant inhibitory effect on high glucose-induced nephrin down-regulation, suggesting that exogenous H2S plays a preventive role in MPC injury under high glucose.
In order to investigate the mechanism of H2S, we assessed the effect of the CSE specific inhibitor on ZO-2 and the Wnt/β-catenin pathway. Interestingly, the CSE inhibitor significantly decreased ZO-2 expression and enhanced β-catenin protein levels. In contrast, exogenous H2S significantly inhibited high glucose-induced down-regulation of ZO-2 and β-catenin up-regulation, indicating that the protective effect of H2S on high glucose-induced podocyte injury is possibly generated by ZO-2 regulation; however, the detailed mechanism should be further explored.
In conclusion, our findings suggest the down-regulation of CSE expression by high glucose in podocytes to be an important mechanism for podocyte injury reduction. In addition, exogenous H2S has a protective effect on high glucose-induced podocyte injury, which might be associated with increased ZO-2 levels and inhibition of the Wnt/β-catenin pathway. This study provides new insights for the prevention and treatment of diabetic nephropathy. Nevertheless, these results need to be confirmed by in vivo experiments.
Acknowledgements
This study was supported by Nature Science Foundation of Shandong Province (No. Y2006C36, No. ZR2012HL50).
Disclosure of conflict of interest
None.
References
- 1.Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007:S36–42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Mariscal L, Bautista P, Lechuga S, Quiros M. ZO-2, a tight junction scaffold protein involved in the regulation of cell proliferation and apoptosis. Ann N Y Acad Sci. 2012;1257:133–141. doi: 10.1111/j.1749-6632.2012.06537.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore PK, Bhatia M, MoochhaIa S. Hydrogen sulfide: from the smell of the Past to the mediator of the future. Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 6.Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine gammalyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Bioehem J. 2004;381:l13–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue H, Yuan P, Ni J, Li C, Shao D, Liu J, Shen Y, Wang Z, Zhou L, Zhang W, Huang Y, Yu C, Wang R, Lu L. H2S Inhibits Hyperglycemia-Induced Intrarenal Renin-Angiotensin System Activation via Attenuation of Reactive Oxygen Species Generation. PLoS One. 2013;8:e74366. doi: 10.1371/journal.pone.0074366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sward P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) 2012;204:294–307. doi: 10.1111/j.1748-1716.2011.02343.x. [DOI] [PubMed] [Google Scholar]
- 9.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Dai C, Li YJ, Zeng G, Monga SP, Liu Y. Wnt/β-Catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 13.Bautista-García P, Reyes JL, Martín D, Namorado MC, Chavez-Munguía B, Soria-Castro E, Huber O, González-Mariscal L. Zona occludens-2 protects against podocyte dysfunction induced by ADR in mice. Am J Physiol Renal Physiol. 2013;304:F77–87. doi: 10.1152/ajprenal.00089.2012. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Mariscal L, Quiros M, Diaz-Coranguez M. ZO proteins and Redox dependent processes. Antioxid Redox Signal. 2011;15:1235–1253. doi: 10.1089/ars.2011.3913. [DOI] [PubMed] [Google Scholar]
- 15.Łowicka E, Bełtowski J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 16.Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, Ni J, Yu C, Yao T, Huang Y, Wang R, Lu L. Rescue of mesangial cells from high glucose-induced over-proliferation and extrace-llular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant. 2011;26:2119–2126. doi: 10.1093/ndt/gfq749. [DOI] [PubMed] [Google Scholar]


