Abstract
Aberrant expression of receptor tyrosine kinase EphA1 in malignant tissues has been reported. However, the expression profile of EphA1 in renal cell carcinoma (RCC) and its association with clinicopathological parameters remain unknown. The aim of this study was to determine the cancerous value of the EphA1 protein expression in patients with renal cell carcinomas. This study included 144 patients with clear cell RCC (ccRCC), 18 patients with chromophobe RCC and 6 patients with papillary RCC. The EphA1 protein was detected in RCC tissue samples by an immunohistochemical staining with a specific polycolonal antibody. The correlation of the expression of the EphA1 protein with clinicopathological parameters was evaluated. High level of the expression of EphA1 was observed in all normal renal tubes. The EphA1 protein was negatively or weakly expressed in 93 out of 144 ccRCC (64.6%) and positively expressed in 51 out of 144 ccRCC (35.4%). The high level expression of the EphA1 protein was significantly associated with younger patients (P<0.001), sex (P=0.016) and lower nuclear grade (P<0.001). No significant relation between the expression of EphA1 and tumor diameter was found (P=0.316). Positive expression of EphA1 was observed in all samples of chromophobe RCC and papillary RCC. Our data indicated that the EphA1 protein may be a new marker for the prognosis of ccRCC.
Keywords: EphA1, receptor tyrosine kinase, clear cell renal cell carcinoma, Fuhrman nuclear grade
Introduction
Renal cell carcinoma (RCC) is the most common neoplasm in the kidney, with an estimated 5-year survival rate of 50-60%. RCC has the highest mortality rate of the genitourinary cancers and the incidence of RCC has risen steadily. RCC is the most common form of adult kidney cancer and accounts for 2-3% of all adult malignancies globally. In 2014, RCC estimated new cases was 63920 and estimated deaths was 13860 in the United States, account for 2-3% of all malignant diseases in adults [1]. RCC is heterogeneous and comprises several histological subtypes according to the differences in genetics, biology and behavior. RCC is consisted of clear cell RCC, papillary RCC, chromophobe RCC, collecting duct RCC, renal medullary carcinoma, Xp11 translocation RCC. Papillary (10-15%), Chromophobe (5%) and other more rare forms such as collecting duct carcinoma (<1%) comprise the remainder. The major histological subtype of RCC is clear cell RCC also called conventional RCC, which accounted for 75% in RCC. RCC is thought to arise from a variety of specialized cells located along the length of the nephron [2]. Both clear cell and papillary RCC are thought to arise from the epithelium of the proximal tubule. Chromophobe RCC is believed to arise from the distal nephron, probably from the epithelium of the collecting tubule. Each type has differences in genetics, biology and behavior. Clear cell RCC can be sporadic or familial. Chromosome 3p deletion and inactivation of the von Hippel-Lindau (VHL) suppressor gene is the most common genetic alteration [3-5]. Almost all familiar clear cell RCC arise from an inherited mutation in VHL tumor suppressor gene. The second allele of VHL has been shown to be inactivated by deletion and by promoter hypermethylation or rearrangement in the RCC. Nearly 20-40% localized ccRCC relapse even after curative nephrectomy, usually leading to incurable disease. Metastatic RCC, characterized by high resistance to radiotherapy and chemotherapy has a poor prognosis.
Receptor tyrosine kinases of the Eph family and Ephrin ligands play important roles in vascular development, tissue-border formation, cell migration, axon guidance, and angiogenesis. Abnormal expression of Eph receptor tyrosine kinases in cancers is related to malignant transformation, tumor metastasis, tumor differentiation, and outcome.
EphA1 is located on chromosome 7q34, and is the first member of the Eph family that was discovered from an erythropoietin-producing hepatoma cell line. The number of known Eph receptor tyrosine kinases (RTK) has increased to 16, making them the largest subfamily of RTKs. The Eph receptor tyrosine kinase is divided into two groups, designated EphA and EphB, according to sequence homology and ligand (ephrin) binding specificity. In addition to their functions in normal tissue, the abnormal expression of some members of the Eph family, including EphA1, has been implicated in carcinogenesis. Eph receptors have important functions in the development of cancer. However, the expression of EphA1 in RCC has not been well investigated. In this study, we investigated the expression levels of EphA1 protein in a set of clear cell RCC, papillary RCC, and chromophobe RCC samples, and determined if its expression is associated with clinicopathological parameters.
Materials and methods
Tissue samples
The RCC tissue samples in our study were collected from 168 patients (111 males, 57 females, average age = 57.7 years; range = 33-82 years old at the time of resection) with RCC as part of a study approved by the Research Ethics Board of Nantong Tumor Hospital. All patients were treated by radical or partial nephrectomy and rendered disease-free. Of the 168 RCC tumors evaluated, 144 were diagnosed as conventional clear cell RCC, 6 as papillary RCC, and 16 as chromophobe RCC. Formalin-fixed and paraffin-embedded tissues were sectioned into slices 4 μm thick and stained with hematoxylin and eosin for pathological identification.
Immunohistochemical staining
Sections from surgical specimens fixed in 10% formalin and embedded in paraffin were used for immunohistochemical staining according to the standard method. Briefly, each 4-m tissue section was deparaffinized and rehydrated. After rehydration through a graded ethanol series, the sections were autoclaved in 10 mM citrate buffer (pH 6.0) at 120°C for 2 min for antigen retrieval, then cooled to 30°C and washed with phosphate-buffered saline (PBS, pH 7.3). After non-specific sites had been blocked in 10% normal calf serum in PBS for 10 min, the sections were incubated at 4°C overnight with an anti-EphA1 polyclonal antibody (Abgent, San Diego, CA, USA) at a 1:500 dilution in antibody diluent solution (Zymed, Invitrogen), and then washed with PBS. The specificity of EphA1 antibody was previously investigated in colorectal cancer sections using blocking peptide [6,7]. Here, we used colorectal cancer and normal mucosa tissues that showed negative and positive expression of EphA1 as controls. Next, the sections were incubated with secondary antibody (Dako REAL EnVision Detection System, Dako, UK) for 30 min at room temperature. Color development was performed with 3, 3’-diaminobenzidine (DAB). Nuclei were lightly counterstained with hematoxylin. Two pathologists independently assessed the immunostained slides. Any difference in immunohistochemical scores was resolved by a consensus. Immunohistochemical staining of both normal and cancer cells was assessed according to the intensity of stained cells. Staining intensity was evaluated as: 0 = negative, 1 = weak, 2 = moderate, 3 = strong.
Statistical analysis
The statistical significance of intergroup differences was evaluated by a chi-square test. All statistical analyses were performed using SPSS software (SPSS 11.5, Chicago, IL). A two-sided P value less than 0.05 was considered statistically significant.
Results
Expression of EphA1 protein in normal renal tubes and ccRCC
The EphA1 protein was positively expressed in normal renal tubes, but not expressed in renal glomerulus (Figure 1). The subcellular location of EphA1 protein was in cytoplasm.
Figure 1.
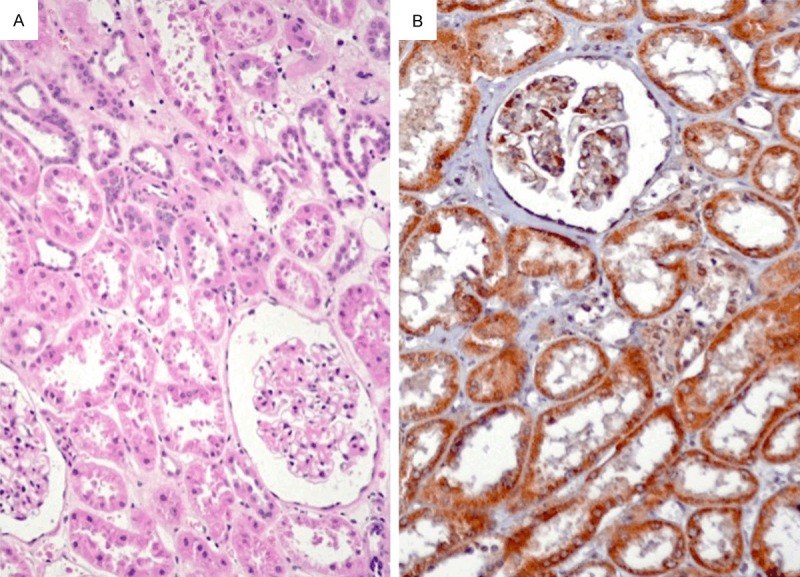
The EphA1 protein was positively expressed in normal renal tubes, but not expressed in renal glomerulus. The subcellular location of EphA1 protein was in cytoplasm. A: H&E staining (×200). B: Immunohistochemical staining (×200).
The relationship between the expression of EphA1 protein in RCC and clinicopathologic parameters
Expression levels of EphA1 protein between cancer cells and adjacent normal cells were compared (Figures 2 and 3). The EphA1 protein was negatively or weakly expressed in 93 out of 144 ccRCC (64.6%) and positively expressed in 51 out of 144 ccRCC (35.4%). The high level expression of the EphA1 protein was significantly associated with younger patients (P<0.001), sex (P=0.016) and lower nuclear grade (P<0.001) (Table 1). Fuhrman (nuclear) grade criterion used in present study is as follows. Grade I: Small, round, uniform nuclei (10 microns), inconspicuous nucleoli, look like lymphocytes (very rare). Grade II: Slightly irregular nuclei, see nucleoli at 40× only, nuclear diameter 15 microns, open chromatin (40% of tumors). Grade III: See nucleoli at 10×, nuclei very irregular, diameter 20 microns, open chromatin (30-40% of tumors). Grade IV: Mitoses; bizarre, multilobated, pleomorphic cells plus grade 3 features, macronucleoli (15% of tumors).
Figure 2.
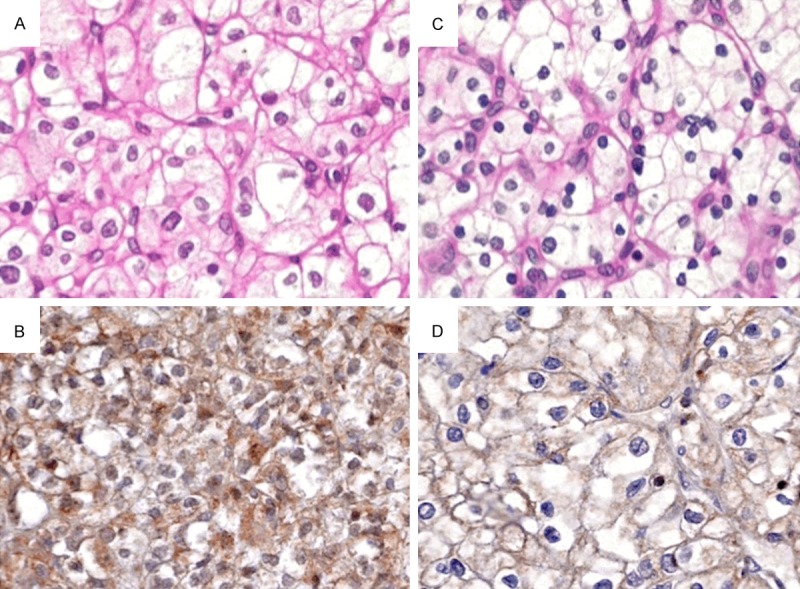
The EphA1 protein was strongly expressed in grade I ccRCC (A: H&E, B: IHC, ×200) and moderately expressed in grade II ccRCC (C: H&E, D: IHC, ×200).
Figure 3.
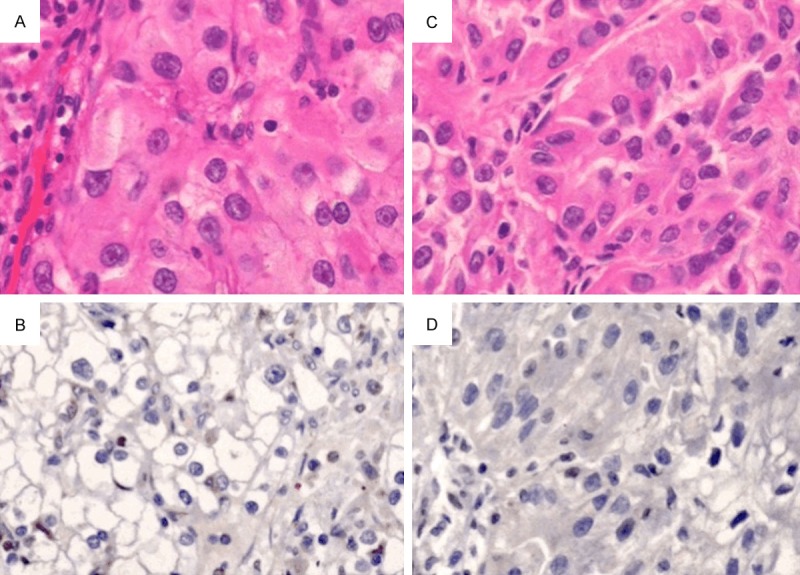
The EphA1 protein was negatively expressed in grade III ccRCC (A: H&E, B: IHC, ×200) and in grade VI ccRCC (C: H&E, D: IHC, ×200).
Table 1.
Expression of EphA1 protein in ccRCC and the association to clinicopathologic parameters
| No. | EphA1 protein | P value | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0, 1+ | 2+ | 3+ | ||||
| Sex | ||||||
| Male | 102 | 18 | 54 | 30 | 0.016 | |
| Female | 42 | 9 | 12 | 21 | ||
| Age (years) | ||||||
| <50 | 39 | 3 | 9 | 27 | <0.001 | |
| 50-70 | 87 | 18 | 45 | 24 | ||
| >70 | 18 | 3 | 12 | 3 | ||
| Tumor diameter (cm) | ||||||
| <7 | 6 | 3 | 0 | 3 | 0.316 | |
| 7-10 | 42 | 9 | 15 | 18 | ||
| >10 | 96 | 15 | 51 | 30 | ||
| Nuclear grade | ||||||
| I | 60 | 6 | 12 | 42 | <0.001 | |
| II | 69 | 12 | 49 | 8 | ||
| III+IV | 15 | 9 | 5 | 1 | ||
No significant relation between the expression of EphA1 and tumor diameter was found (P=0.316) (Table 1).
Expression of EphA1 in chromophobe RCC and papillary RCC
The EphA1 protein was positively expressed in all samples of chromophobe RCC and papillary RCC (Figure 4).
Figure 4.
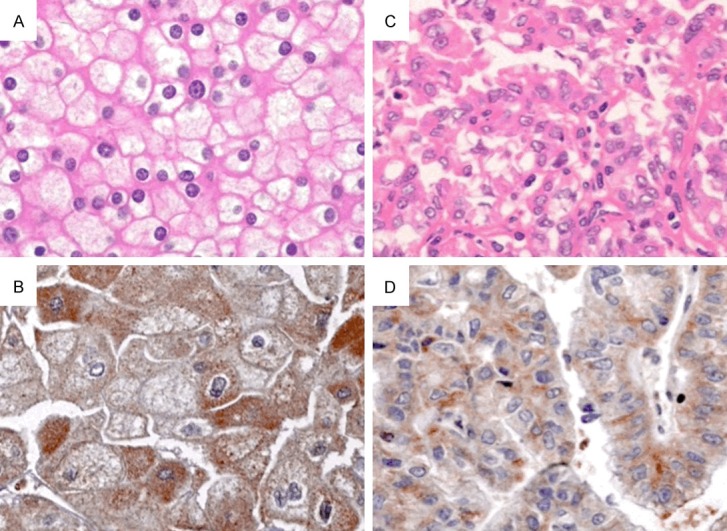
The EphA1 protein was strongly expressed in chromophobe RCC (A: H&E, B: IHC, ×200) and papillary RCC (C: H&E, D: IHC, ×200).
Discussion
Receptor tyrosine kinases of Eph family and the ligands of ephrin play important roles in the vascular development, tissue-border formation, cell migration, axon guidance and angiogenesis. Abnormal expression of Eph receptor tyrosine kinases in cancers is related to malignant transformation, tumor metastasis, tumor differentiation and prognosis. EphA1 is widely expressed in normal tissues including lung, small intestinal, kidney, bladder, thymus, and colon [8]. The expression level of EphA1 in human cancers is variable. Over-expression of EphA1 was observed in certain types of tumors including ovarian carcinoma [9], and head and neck squamous cell carcinoma [10]. Reduced expression of EphA1 was detected in prostate cancer cell lines [11], breast carcinoma cell lines [12], and basal cell carcinomas and squamous cell carcinoma specimens of the skin [13]. However, the role of EphA1 in the carcinogenesis of renal cell carcinoma is unknown. Wang et al previously reported that down-regulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis, and reduced EphA1 expression is associated with a poor overall survival [7]. Other group demonstrated that epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival [14]. Wang et al also found that increased expression of EphA1 protein in prostate cancers correlated with high Gleason score [15], and high expression of EphA1 in esophageal squamous cell carcinoma is associated with lymph node metastasis and advanced disease [16].
The reason for complex and paradoxical signals of Eph receptors and ephrins mainly attributed to Eph-ephrin bidirectional signaling [17,18]. A distinctive feature of Eph-ephrin complexes is their ability to generate bidirectional signals that affect both the receptor-expressing and ephrin-expressing cells. Eph receptors and ephrins are often upregulated in cancer cells. In many cases this may be due to oncogenic signaling pathways. For example, the Wnt/catenin/Tcf pathway promotes EphB expression in colorectal cancer cells and the Ras-MAP kinae pathway promotes EphA2 expression in breast cancer cells. Tumor suppressor activities have been reported for Eph signaling in colorectal, breast, prostate and skin cancer cells.
In this study, we evaluated the expression of EphA1 in a set of renal cell carcinomas and analyzed its association with clinicopathologic parameters. Our data showed that the high level expression of the EphA1 protein was significantly associated with younger patients, sex and lower nuclear grade. The prognostic value of nuclear grade has been validated in numerous studies. The grade affects the prognosis, but doesn’t currently affect treatment. The treatment is the same for a given stage regardless of the grade. Grade also correlates with stage in that larger tumors tend to be higher grade. The most widely used and most predictive grading system for renal cell cancer is the “Fuhrman Nuclear Grade” [19]. Fuhrman grade is on a scale of I-IV, where grade I carries the best prognosis and grade IV the worst. Nuclear grade means that the system is based on just the appearance of the nuclei of the cancer cells, rather than the appearance or structure of the cells as a whole. Nuclear characteristics used in the Fuhrman Grade particularly indicate how actively the cells are making protein.
Down-regulation of EphA1 in colorectal cancer was reported and the mechanism of hypermethylation of CpG island in promoter region has been demonstrated [7,14]. In this study, we found that EphA1 protein was positively expressed in all normal renal tubes and only expressed in about 35% ccRCC (IHC score 3+). The EphA1 protein was negatively or weakly expressed in 65% of ccRCC (IHC score 0, 1+, and 2+). The mechanism for loss of EphA1 protein in ccRCC should be confirmed in the next study program.
In conclusion, the EphA1 protein was positively expressed in normal renal tubes and parts of ccRCC. Expression of EphA1 protein is associated with nuclear grade of ccRCC, which may be a new biomarker for the prognosis of ccRCC.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81371611).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9:461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessi F, Mazzanti CM, Tomei S, Di Cristofano C, Minervini A, Menicagli M, Apollo A, Masieri L, Collecchi P, Minervini R, Carini M, Bevilacqua G. VHL and HIF-1alpha: gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med Oncol. 2014;31:840. doi: 10.1007/s12032-014-0840-8. [DOI] [PubMed] [Google Scholar]
- 4.Singh RB, Amare Kadam PS. Investigation of tumor suppressor genes apart from VHL on 3p by deletion mapping in sporadic clear cell renal cell carcinoma (cRCC) Urol Oncol. 2013;31:1333–1342. doi: 10.1016/j.urolonc.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL, Carpenter C, Liu Y, Pandite L, Signoretti S. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013;19:5218–5226. doi: 10.1158/1078-0432.CCR-13-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li G, Lu G, Sugimura H, Zhou X. Expression of EphA1 in gastric carcinomas is associated with metastasis and survival. Oncol Rep. 2010;24:1577–1584. doi: 10.3892/or_00001020. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Wang J, Sheng Z, Li G, Ma H, Wang X, Zhang R, Lu G, Hu Q, Sugimura H, Zhou X. Downregulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis. Mod Pathol. 2009;22:151–160. doi: 10.1038/modpathol.2008.188. [DOI] [PubMed] [Google Scholar]
- 8.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 9.Herath NI, Spanevello MD, Sabesan S, Newton T, Cummings M, Duffy S, Lincoln D, Boyle G, Parsons PG, Boyd AW. Over-expression of Eph and ephrin genes in advanced ovarian cancer: ephrin gene expression correlates with shortened survival. BMC Cancer. 2006;6:144. doi: 10.1186/1471-2407-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HS, Berry GJ, Fee WE Jr, Terris DJ, Sun Z. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:311–316. doi: 10.1001/archotol.130.3.311. [DOI] [PubMed] [Google Scholar]
- 11.Fox BP, Tabone CJ, Kandpal RP. Potential clinical relevance of Eph receptors and ephrin ligands expressed in prostate carcinoma cell lines. Biochem Biophys Res Commun. 2006;342:1263–1272. doi: 10.1016/j.bbrc.2006.02.099. [DOI] [PubMed] [Google Scholar]
- 12.Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882–892. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 13.Hafner C, Becker B, Landthaler M, Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol. 2006;19:1369–1377. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- 14.Herath NI, Doecke J, Spanevello MD, Leggett BA, Boyd AW. Epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival. Br J Cancer. 2009;100:1095–1102. doi: 10.1038/sj.bjc.6604970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng L, Wang H, Dong Y, Ma J, Wen J, Wu J, Wang X, Zhou X, Wang J. Increased expression of EphA1 protein in prostate cancers correlates with high Gleason score. Int J Clin Exp Pathol. 2013;6:1854–1860. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Ma J, Dong Y, Shen Z, Ma H, Wang X, Shi S, Wu J, Lu G, Peng L, Zhoud X. High expression of EphA1 in esophageal squamous cell carcinoma is associated with lymph node metastasis and advanced disease. APMIS. 2013;121:30–37. doi: 10.1111/j.1600-0463.2012.02941.x. [DOI] [PubMed] [Google Scholar]
- 17.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 18.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Paner GP, Amin MB, Alvarado-Cabrero I, Young AN, Stricker HJ, Moch H, Lyles RH. A novel tumor grading scheme for chromophobe renal cell carcinoma: prognostic utility and comparison with Fuhrman nuclear grade. Am J Surg Pathol. 2010;34:1233–1240. doi: 10.1097/PAS.0b013e3181e96f2a. [DOI] [PubMed] [Google Scholar]


