Abstract
Numerous cytokines participate in the occurrence and development of inflammation and renal interstitial fibrosis. Previous studies confirmed that TGF-β1 overexpressed in diabetic nephropathy. As a downstream signal protein of TGF-β1 family, SMAD has an important role in the process of α-SMA mediated renal interstitial fibrosis. This study aimed to study astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissue of diabetic KKAy mice, to reveal its potential impact on renal interstitial fibrosis. 20 type II diabetic KKAy mice were randomly equally divided into model group and astragaloside group, while 10 male C57BL/6J mice were selected as the control. Astragaloside at 40 mg/(kg•d) was given when the KKAy mice fed with high-fat diet to 14 weeks old. The mice were killed at 24 weeks old and the kidney tissue samples were collected. Pathology morphological changes were observed. TGF-β1, SMAD2/3, and α-SMA expression levels were determined by immunohistochemistry. Compared with control, mice kidney in model group appeared obvious fibrosis and up-regulated blood glucose level, TGF-β1, SMAD2/3, and α-SMA expression (P < 0.05). Mice in astragaloside group exhibited alleviated renal interstitial fibrosis compared with the model. Its blood glucose level, TGF-β1, SMAD2/3, and α-SMA expression levels were significantly lower than the model group (P < 0.05). Astragaloside can delay the renal fibrosis process in diabetic mice by influencing the TGF-β/SMADS signaling pathway and down-regulating TGF-β1, SMAD2/3, and α-SMA expression.
Keywords: Astragaloside, diabetic mellitus, TGF-β1, α-SMA
Introduction
Diabetic nephropathy is an important complication of diabetes mellitus. Fibrosis and inflammation play important roles in diabetic nephropathy development, and its main pathological changes include renal tubular and glomerular fibrosis and inflammation. Renal tubular lesions appeared earlier than the glomerular lesions in the renal fibrosis process, which is an important factor in chronic renal failure [1,2]. Therefore, inhibiting tubular interstitial fibrosis has an important effect in delaying chronic renal failure. Many cytokines involved in the renal fibrosis process including transforming growth factor beta 1 (TGF-β1). TGF-β1 can induce epithelial mesenchymal transition in renal tubular epithelial cells, and overexpressed in the process of diabetic nephropathy [3,4]. As TGF-β family downstream signal transduction protein, SMAD protein is associated with renal fibrosis. It was found that when the renal tubular epithelial cells were stimulated by exogenous TGF-β, SMAD2 could be phosphorylated and transferred into the nucleus. It can induce renal tubular epithelial cells secreting collagen (I, III, and IV types) and α-SMA protein expression [5,6]. Astragaloside is extracted from leguminous plant astragalus with anti-oxidant, anti-inflammatory, and protecting kidney effects. It can protect kidney through multiple pathways. This study intends to study astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissue of diabetic KKAy mice, to reveal its potential impact on renal interstitial fibrosis and provide basis for astragaloside protecting kidney.
Materials and methods
Experimental animal
10 male C57BL/6J mice and 20 male KKAy mice weighted 22-24 g were provided by Sun Shandong University. They were fed in the SPF animal laboratory and KKAy mice were given high-fat diet.
Mice were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Drugs and reagents
Astragaloside is provided by Sigma. DAB kit, TGF-β1 and α-SMA polyclonal antibodies are provided by the Beijing Zhongshan Biological Technology Co., LTD. Phosphorylated SMAD2/3 polyclonal antibody is purchased from Wuhan Boster biotechnology co., LTD. PBS: 0.01 mol/L, pH 7.2, antigen repair fluid: 0.01 mol/L, pH 6.0.
Instruments
Imago-Pro Plus5.1 image analysis software and Roche glucose meter were used in this study.
Modeling
KKAy mice were fed with high-fat diet to 14 weeks old. Diabetic model was determined according to the random blood glucose level ≥ 13.9 mmol/L [7]. Mice general status was observed daily.
Grouping
KKAy mice with similar blood glucose level were randomly equally divided into model group and drug group. Astragaloside at 40 mg/(kg•d) was given to the drug group through intragastric administration. 10 C57BL/6J mice were selected as normal control with normal diet. The mice in the normal control and model group received normal saline at 40 mg/(kg•d). The mice were killed at 24 weeks old and the kidney tissue samples were collected and fixed with 4% paraformaldehyde.
Detection index
(1) Blood glucose assay: The blood was extracted from orbital venous to detect fasting blood-glucose level at 16, 20, and 24 weeks.
(2) Renal tissue morphological observation: Renal tissue was embedded by paraffin for sectioning. It received conventional HE staining and Masson staining, and observed under the light microscope.
(3) Immunohistochemistry: TGF-β1, SMAD2/3, and α-SMA expression were detected by immunohistochemistry according to the SABC kit manual. Antibody dilutions were 1:200 for TGF-β1, 1:400 for SMAD2/3, and 1:500 for α-SMA. 0.01 mol/L PBS was selected as negative control. Ten glomerular were randomly selected to detect the absorbance and calculate the average protein expression.
Statistical analysis
All statistical analyses were performed using SPSS19.0 software (SPSS Inc., USA). Numerical data were presented as means and standard deviation (X̅ ± SD). Differences between multiple groups were analyzed by t-test or one-way ANOVA and LSD test. P < 0.05 was considered as significant difference.
Results
General status comparison
Mice in the control group showed sensitive response and smooth hair. Mice in the model group mice exhibited weak mental state, slow movement, polydipsia and polyuria, insensitive response, and rough hair. The symptom became worse gradually following the age. Mice in the astragaloside group presented better mental state and response sensitivity than the model group, but worse than the control.
Astragaloside impact on KKAy diabetic mice blood glucose
14 weeks KKAy mice showed significantly higher fasting blood glucose level than the control (P < 0.05). Fasting glucose level increased obviously at 16, 20, and 24 weeks in model mice, and decreased in astragaloside group (P < 0.05) (Figure 1).
Figure 1.
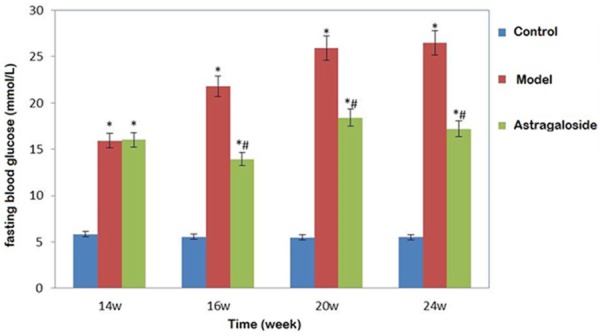
Astragaloside impact on KKAy diabetic mice blood glucose. *P < 0.05, compared with control; #P < 0.05, compared with the model group.
Astragaloside impact on KKAy diabetic mice histomorphology
24 weeks mice in the control showed clear glomerular and renal tubular structure without interstitial fibrosis. Mice in the model group exhibited increased glomerular mesangial matrix, significantly proliferated mesangial cells, renal tubular epithelial cell cytoplasm vacuoles degeneration, and increased renal interstitial inflammatory cells. Renal tubular cytoplasm vacuoles were relatively fewer in the mice from the astragaloside group with no significant fibrosis (Figure 2).
Figure 2.
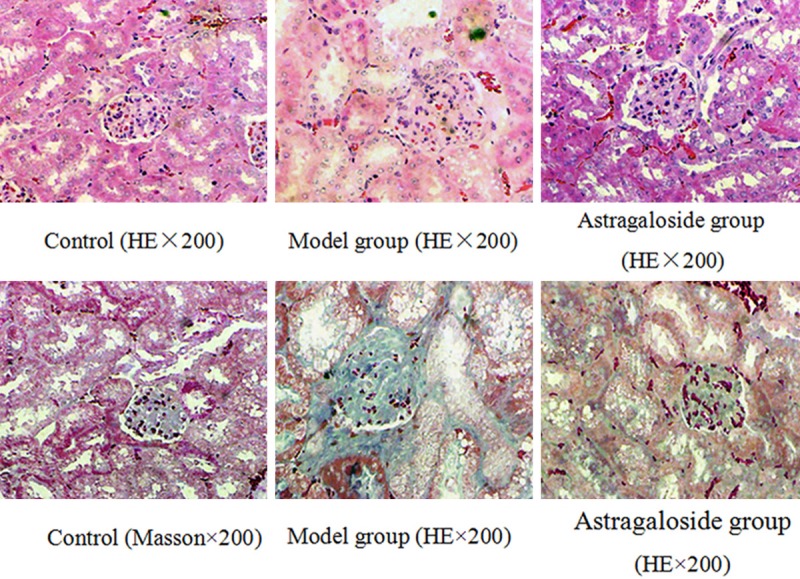
Astragaloside impact on KKAy diabetic mice histomorphology.
Astragaloside impact on TGF-β1, SMAD2/3, and α-SMA expression in the KKAy diabetic mice kidney
TGF-β1 expressed weak in the control, while it expressed strongly in the renal tubular epithelial cell cytoplasm in the model group. Compared with the model group, TGF-β1 decreased obviously in the astragaloside group. Cytoplasm α-SMA expression increased in model group and astragaloside group. Specially, it significantly reduced in astragaloside group compared with the model group (P < 0.05). There was less SMAD2/3 phosphorylation in the renal tubular and glomerular cell nucleus from the control group. It increased markedly in the model group, while it declined in the astragaloside group (P < 0.05) (Figures 3 and 4).
Figure 3.
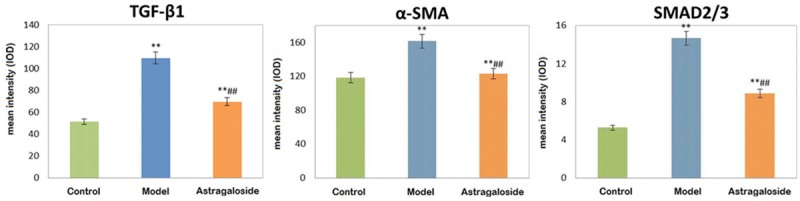
Astragaloside impact on TGF-β1, SMAD2/3, and α-SMA expression in the KKAy diabetic mice kidney. *P < 0.05, **P < 0.01, compared with control; #P < 0.05, ##P < 0.05, compared with the model group.
Figure 4.
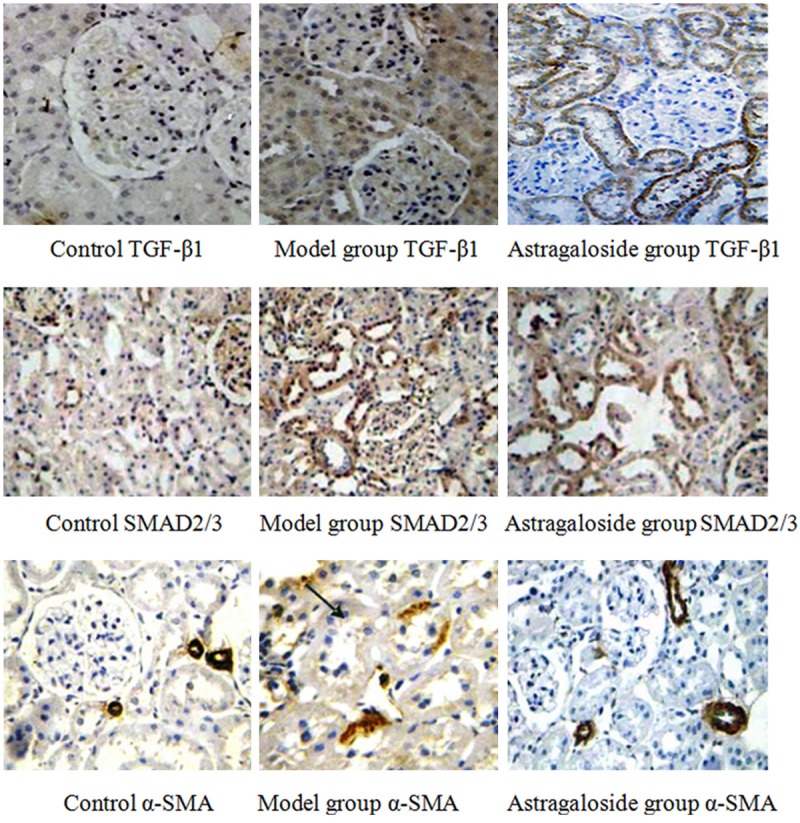
Astragaloside impact on TGF-β1, SMAD2/3, and α-SMA expression in the KKAy diabetic mice kidney (×200).
Discussion
Astragalus membranaceus benefits for detumescence. Astragaloside is the main active ingredient of leguminous plant astragalus membranaceus that has multiple pharmacological activities. Astragaloside and polysaccharide are the main monomer composition extracted from astragalus membranaceus. It has been found that astragalus membranaceus and astragaloside have numerous pharmacological effects such as anti-stress, reducing blood glucose, immune regulation, diuresis, and protecting kidney [8]. Astragalus membranaceus can delay the process of diabetic nephropathy and alleviate glomerular mesangial matrix proliferation. However, the mechanism of astragaloside protecting kidney has not been fully elucidated. Studies have shown that [9], astragaloside could protect rats podocytes under high glucose condition or STZ induced type I diabetes. It can inhibit integrin ligase expression, up-regulate α3β1 integrin protein expression in podocytes, and delay podocytes apoptosis. In addition, astragaloside could regulate Bax/Bcl-2 through Caspase-3 pathway to inhibit podocytes apoptosis [10]. Some researches confirmed that astragaloside can improve rat’s renal tubular damage by reducing inflammatory factors level in acute kidney injury through down-regulating NF-κB expression and inhibiting phosphorylation p38MAPK pathway [11,12]. Fukuda revealed that astragaloside can inhibit high glucose induced human renal tubular epithelial cells apoptosis by down-regulating TGF-β1 and inhibiting p38MAPK pathway [13,14]. Recent studies suggested that [15,16], astragaloside can inhibit NRK-52E tubule cells generating ROS and inhibit EMT through the antioxidant effect. Thus, we hypothesized that astragaloside may affect inflammation and fibrosis through TGF-β1 mediated pathways. Therefore, this study aimed to observe astragaloside impact on TGF-β1, SMAD2/3, and α-SMA expression in diabetic KKAy mice and explore its influence on renal interstitial fibrosis.
Renal interstitial fibrosis and renal tubule lesions play important roles in kidney disease and are closely related to renal hypofunction. Renal tubular epithelial cells EMT might be a key link in the process of diabetic nephropathy. An important mechanism for renal function deterioration in the process of diabetic nephropathy is renal tubule interstitial fibrosis. Myofibroblasts is a sign of renal fibrosis. It is the main source of producing extracellular matrix in the process of renal fibrosis. Proximal tubule epithelial cell could transdifferentiate to fibroblasts. Therefore, the key to delay chronic kidney disease development is to suppress tubular interstitial fibrosis [17,18]. Renal tubular epithelial cells may transform to myofibroblasts after activation, resulting in secretion and contractility increase, large amount of extracellular matrix production, and renal interstitial fibrosis. TGF-β plays an important role in the process of renal tubular epithelial cells EMT. SMAD protein is a downstream signal transduction protein of TGF-β. This research observed astragaloside impact on fasting blood glucose and renal pathological morphology through using astragaloside intervene diabetic KKAy mice. The results showed that compared with the control group, mice kidney in model group presented obvious fibrosis and significant higher blood glucose level. Compared with model group, astragaloside group exhibited alleviated renal interstitial fibrosis with mild interstitial cells hyperplasia and lower blood glucose level. It suggested that astragaloside can reduce diabetic KKAy mice blood glucose level and inhibit renal fibrosis development. TGF-β1, SMAD2/3, and α-SMA proteins expression increased significantly in model mice kidney compared with control. Their levels decreased in astragaloside group compared with the model group. α-SMA is a marker for mesenchymal cells as participating in renal tubular epithelial cells EMT process. α-SMA level can reflect the degree of renal fibrosis. Previous in vitro experimental results confirmed that SMAD2/3 expressed in human mesangial cells and involved in SMADS signaling pathway in the process of TGF-β1 inducing gene transcription type I collagen [19,20]. Renal tubule cell interstitial transformation has four steps: renal tubular epithelial cells loss adhesion; α-SMA protein expression and actin cytoskeleton rearrangement; renal tubular basement membrane destruction; renal tubular epithelial cell migration, invasion abilities increase. TGF-β1 is an important cytokine in the process. TGF-β1 can regulate cell proliferation and differentiation. Its up-regulation revealed renal interstitial fibrosis. It can inhibit matrix-degrading enzymes expression, promote renal tubule cell EMT, suppress renal tubule cell proliferation, and lead to renal fibrosis and inflammation [20,21]. In vitro studies indicated that high glucose stimulation can activate SMAD signaling pathway in glomerular mesangial cells and tubular epithelial cells, resulting in TGF-β overexpressed. In vivo study showed that TGF-β1, SMAD2/3, α-SMA expression were positive correlated in diabetic model mice kidney. TGF-β/SMAD signaling pathways activation increased α-SMA protein expression [22-24]. All of the results suggested that TGF-β1, SMAD2/3, and α-SMA up-regulation and activation are important in renal fibrosis process of diabetic nephropathy. In our study, astragaloside intervention can decrease TGF-β1, SMAD2/3, and α-SMA in diabetic KKAy mice, indicating that astragaloside can alleviate renal fibrosis in mice through down-regulating TGF-β1, SMAD2/3, and α-SMA expression to inhibit TGF-β/SMADS signaling pathway.
Astragalus membranaceus is thought to be helpful for detumescence and diuresis. Our results found that astragaloside can improve diabetic mice renal fibrosis by affecting TGF-β/SMADS signaling pathway and down-regulating TGF-β1, SMAD2/3, α-SMA expression. Above all, astragaloside has protective effect to the kidney by inhibiting renal tubular interstitial fibrosis and tubular epithelial mesenchymal transdifferentiation.
Disclosure of conflict of interest
None.
References
- 1.Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, Yang JK. miR-21 overexpression enhances TGF-beta1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392:163–172. doi: 10.1016/j.mce.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Araoka T, Abe H, Tominaga T, Mima A, Matsubara T, Murakami T, Kishi S, Nagai K, Doi T. Transcription factor 7-like 2 (TCF7L2) regulates activin receptor-like kinase 1 (ALK1)/Smad1 pathway for development of diabetic nephropathy. Mol Cells. 2010;30:209–218. doi: 10.1007/s10059-010-0109-9. [DOI] [PubMed] [Google Scholar]
- 3.Ding Z, Chen Z, Chen X, Cai M, Guo H, Chen X, Gong N. Adenovirus-mediated anti-sense ERK2 gene therapy inhibits tubular epithelial-mesenchymal transition and ameliorates renal allograft fibrosis. Transpl Immunol. 2011;25:34–41. doi: 10.1016/j.trim.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 4.He F, Yu L, Tong JR, Luo ZM, Zhu QZ, Wang Y, Zhang JL. Role of integrin-linked kinase in renal tubular epithelial-mesenchymal transition and the regulatory effect of urokinase on its expression in mice with obstructive nephropathy. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:909–913. [PubMed] [Google Scholar]
- 5.Yin Y, Qi F, Song Z, Zhang B, Teng J. Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats. Biosci Trends. 2014;8:217–226. doi: 10.5582/bst.2014.01081. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Chen Y, Luo Y, Gui D, Huang J, He D. Astragaloside IV ameliorates diabetic nephropathy involving protection of podocytes in streptozotocin induced diabetic rats. Eur J Pharmacol. 2014;736:86–94. doi: 10.1016/j.ejphar.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Muraki A, Oie M, Kanegawa N, Oda A, Sawashi Y, Kaneko K, Yoshikawa M, Goto T, Takahashi N, Kawada T, Ohinata K. Soymorphin-5, a soy-derived mu-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARalpha systems in diabetic KKAy mice. Am J Physiol Endocrinol Metab. 2012;302:E433–440. doi: 10.1152/ajpendo.00161.2011. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Yu L, She T, Gan Y, Liu F, Hu Z, Chen Y, Li S, Xia H. Astragaloside IV attenuates Toll-like receptor 4 expression via NF-kappaB pathway under high glucose condition in mesenchymal stem cells. Eur J Pharmacol. 2012;696:203–209. doi: 10.1016/j.ejphar.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Qi W, Chen X, Poronnik P, Pollock CA. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol. 2006;38:1–5. doi: 10.1016/j.biocel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–1017. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 12.Reidy K, Susztak K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis. 2009;54:590–593. doi: 10.1053/j.ajkd.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23:1351–1363. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou MS, Yu J, Nie GM, He WS, Luo LM, Xu HT. 1, 25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss. Int J Med Sci. 2010;7:290–299. doi: 10.7150/ijms.7.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv L, Wu SY, Wang GF, Zhang JJ, Pang JX, Liu ZQ, Xu W, Wu SG, Rao JJ. Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother Res. 2010;24:219–224. doi: 10.1002/ptr.2915. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Zhang Y, Ge Y, Yan M, Kuang R, Zheng X. Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro. Planta Med. 2008;74:1259–1264. doi: 10.1055/s-2008-1081290. [DOI] [PubMed] [Google Scholar]
- 17.Salmon AH, Neal CR, Harper SJ. New aspects of glomerular filtration barrier structure and function: five layers (at least) not three. Curr Opin Nephrol Hypertens. 2009;18:197–205. doi: 10.1097/MNH.0b013e328329f837. [DOI] [PubMed] [Google Scholar]
- 18.Xavier S, Niranjan T, Krick S, Zhang T, Ju W, Shaw AS, Schiffer M, Bottinger EP. TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol. 2009;20:2127–2137. doi: 10.1681/ASN.2008070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112:1661–1672. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- 20.Tan S, Wang G, Guo Y, Gui D, Wang N. Preventive Effects of a Natural Anti-Inflammatory Agent, Astragaloside IV, on Ischemic Acute Kidney Injury in Rats. Evid Based Complement Alternat Med. 2013;2013:284025. doi: 10.1155/2013/284025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng R, Deng Y, Chen Y, Fan J, Zhang M, Zhong Y, Zhu R, Wang L. Astragaloside IV attenuates complement membranous attack complex induced podocyte injury through the MAPK pathway. Phytother Res. 2012;26:892–898. doi: 10.1002/ptr.3656. [DOI] [PubMed] [Google Scholar]
- 22.Qi W, Niu J, Qin Q, Qiao Z, Gu Y. Astragaloside IV attenuates glycated albumin-induced epithelial-to-mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells. Cell Stress Chaperones. 2014;19:105–114. doi: 10.1007/s12192-013-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng LQ, Tang JW, Wang Y, Zhao JR, Shang MY, Zhang M, Liu SY, Qu L, Cai SQ, Li XM. Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br J Pharmacol. 2011;162:1805–1818. doi: 10.1111/j.1476-5381.2011.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gui D, Huang J, Guo Y, Chen J, Chen Y, Xiao W, Liu X, Wang N. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-kappaB-mediated inflammatory genes expression. Cytokine. 2013;61:970–977. doi: 10.1016/j.cyto.2013.01.008. [DOI] [PubMed] [Google Scholar]


