Abstract
Atrial fibrosis contributes to development and recurrence of atrial fibrillation (AF). TGF-β and periostin have been reported to be involved in fibrogenesis. Here we investigated the role of TGF-β and periostin in atrial fibrosis of AF and in the recurrence of AF after surgery ablation. Western blot, Masson staining, immunohistochemistry and colorimetry were performed to detect the degree of atrial fibrosis and the expression of TGF-β, periostin and collagens in 70 biopsies of right atrial appendage (RAA) obtained in this study. Then the patients who received surgical ablation were followed up for about one year. The results showed an increasing gradient of atrial expression of TGF-β, periostin and collagens paralleled by a higher level of atrial fibrosis in control, SR and AF groups. The expression of TGF-β and periostin was significantly correlated with fibrotic markers. In addition, LAD and the expression of TGF-β were larger or higher in recurrence group than that in nonrecurrence group after surgery ablation. The results suggest that upregulated expression of TGF-β and periostin in RAAs is correlated with the degree of atrial fibrosis in patients with AF.
Keywords: Periostin, transforming growth factor-β, atrial fibrillation, fibrosis, recurrence
Introduction
Atrial fibrillation (AF), the most common persistent arrhythmia encountered in clinical practice, is characterized by rapid and irregular activation of the atrial myocardium. It is a major cause of morbidity and mortality because of its association with a 5-fold increased risk of ischemic stroke [1]. It is generally accepted that atrial structural remodeling can promote development and chronicity of AF. Atrial fibrosis, a hallmarker of arrhythmogenic constructional remodeling, is considered to lead to dissociated atrial conduction and thereby contribute to an increased susceptibility and maintenance of AF [2-4]. Though heavily studied, the underlying mechanisms of fibrosis in AF have not been fully understood.
As a matricellular protein, periostin has been proved to play an important role in fibrogenesis [5-7]. TGF-β, a profibrotic growth factor, has been recognized as a main mediator of atrial fibrosis in AF [8-10]. Several studies have showed that TGF-β was an inducer of periostin [5,11], conversely, periostin itself could promote TGF-β activation and fibrosis [12,13]. Therefore, we hypothesized that TGF-β/periostin pathway was involved in fibrogensis of AF.
From the therapy standpoint, non-pharmacological treatments, such as catheter ablation, pulmonary vein isolation, maze operation et al, have increasingly become a focus of attention because of their curative effects [14,15]. In patients with valvular AF, surgical ablation during valve replacement operation has been recommended for treatment of AF, but recurrence of AF after surgery ablation remains a major problem. Recently, growing clinical evidence suggested that atrial fibrosis has been proven as a predictor for AF recurrence [16,17]. Then, we postulated that TGF-β/periostin involved in fibrosis may be correlated with AF recurrence after surgery ablation.
In the present study, we investigated the relationship between TGF-β/periostin and fibrosis in RAAs of patients with AF and determined the role of TGF-β/periostin in recurrence of AF after surgery ablation.
Methods
Patients and tissue specimens
A total of 60 patients with valvular heart diseases (VHDs) undergoing valve replacement surgery were enrolled in this study at the Drum Tower Hospital of Nanjing University Medical School. The patients were divided into three groups: sinus rhythm group (SR, n=20), paroxysmal atrial fibrillation group (PaAF, n=15, AF lasting <7 days) and persistent atrial fibrillation group (PeAF, n=25, AF lasting >7 days). Every patient had routine preoperative 2-dimensional color echocardiography. Patients with hyperthyreosis, sick sinus syndrome and renal disease were excluded from our study. Additionally, 10 healthy heart donors were studied in control group (con, n=10). The operations and the modified Cox maze III procedure were as previously described [18].
Right atrial appendages were obtained as specimens prior to the establishment of extracorporeal circulation. One part of the tissue was fixed in 4% formalin for immunohistochemistry and Masson staining, and the remaining tissue was frozen in liquid nitrogen and stored at -80°C for western blot and other analyses. Human tissue collection and analyses strictly abided by the principals outlined in the World Medical Association of Helsinki. All procedures involving human tissue were approved by the Drum Tower Hospital affiliated to Nanjing University Medical College Ethics Committee. All patients recruited in the current study gave written informed consent.
Follow-up
Any patient undergoing surgical ablation was followed up for about 12 months. All the patients underwent routine follow-up at 1 month, 3 months and then every 1-3 months after ablation, and the 24 hour Holter recordings were obtained for diagnosing recurrence of AF.
Western blot
Tissue samples washed by phosphate buffered saline (PBS) were homogenized in RIPA solubilization buffer containing a 1:100 dilution of protease inhibitor (Sigma). Protein concentrations were determined by BCA protein assay (pierce). After equal protein mixtures were separated on SDS-PAGE, gels were blotted noto polyvinylidene difluoride membranes and the membranes were incubated in Tris-buffered saline containing 0.1% Tween 20 (TBST) with 5% milk for 1 h at room temperature. Then, the membranes were incubated overnight with primary antibody including anti-TGF-β (1:200, Santa Cruz), anti-periostin (1:500, Santa Cruz), anti-col I (1:400, Abcam), and anti-col III (1:500, Abcam). Anti-GAPDH (1:5000, Bioword) was used as an internal control. After washed by TBST, the blots were performed with horseradish peroxidase-conjugated secondary antibodies (1:10000, Bioword). The reactions were developed with enhanced chemiluminescence’s regents (Millipore), and images were obtained by exposure to films. The bands were scanned and quantified using BioRad Quantity One imaging software. All the quantifications of bands were normalized by the corresponding value of GAPDH, and the values of other three groups normalized by the control group.
Immunohistochemistry
Paraffin-embedded tissue was cut into 4 μm thick sections, washed with PBS for three times and blocked with 1% bovine serum albumin (BSA) in PBS for 30 min. Then, every section was incubated with anti-TGF-β (1:100, Santa Cruz) and anti-periostin (1:100, Santa Cruz) overnight at 4°C. After washed three times with PBS, the preparations were incubated with second antibody for 20 min and then rinsed with water, counterstained with hematoxylin and mounted with glycerol gelatin.
Masson staining and fibrosis quantification
After fixation with 4% paraformaldehyde in phosphate-buffered saline for 24 h, the tissues were subjected to alcoholic dehydration and embedded in paraffin. 4 μm serial sections were sliced and subjected to Masson’s trichrome staining to highlight collagen fibers. Collagen volume fraction (CVF) was determined by Image Pro Plus software using five random images from each slide, and the mean values of CVF were obtained by one investigator blinded to the groups.
Measurement of hydroxyproline
Hydroxyproline levels were quantified using commercial assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
Baseline characteristics were expressed as mean ± S.D. for continuous variables and frequencies for categorical variables. Difference between two groups was analyzed by student’s t test (normally distributed) or Mann-Whitney (non-normally distributed); one-way analysis of variance (normally distributed) or Kruskal-Wallis test (non-normally distributed) was used between more than two groups. Categorical variables were compared using chi-square analysis. Correlation analyses were performed using Pearson/spearman correlations. Values of P<0.05 were considered significant. Analyses were performed with SPSS21.0.
Results
Patient characteristics
In this study, we recruited 60 patients with valvular heart diseases (VHDs), which were divided into three groups: sinus rhythm group (SR, n=20), paroxysmal atrial fibrillation group (PaAF, n=15), and persistent atrial fibrillation (PeAF, n=25). Ten RAAs obtained from healthy heart donors were used as the control. The clinical data of the patients with VHDs were shown in Table 1. There was no significant difference in Echocardiographic parameters among the three groups except left atrial diameter (LAD) and pulmonary artery systolic pressure (PASP). The left atrial diameter in the two AF groups was larger than the SR group (both P<0.01), and it was larger in PeAF group compared with the PaAF group (P<0.05). The pulmonary artery systolic pressures were largest in PeAF group, followed by the PaAF group and the SR group, and it reached a statistical significance between the SR group and the PeAF group (P<0.01). Table 1 showed that there were no statistically significant differences among the three groups with respect to basic data, cause of mitral valve disease, type of operations and preoperative drugs.
Table 1.
Clinical characteristics of study population
| Parameters | SR | PaAF | PeAF | P value |
|---|---|---|---|---|
| Basic data | ||||
| Sex, M/F (n) | 11/9 | 7/8 | 13/12 | 0.887 |
| Age (years) | 51.7±10.0 | 50.9±11.7 | 53.3±8.6 | 0.735 |
| BMI (kg/m2) | 22.4±2.6 | 22.8±3.0 | 22.8±3.3 | 0.905 |
| NYHA class I/II/III/IV (n) | 2/6/11/1 | 1/5/9/0 | 1/7/13/4 | 0.545 |
| Echocardiographic parameters | ||||
| LVDd (cm) | 5.4±0.7 | 5.6±0.9 | 5.7±1.1 | 0.583 |
| LVDs (cm) | 3.9±0.6 | 4.2±0.7 | 4.3±1.0 | 0.162 |
| EF (%) | 55.1±3.8 | 52.6±5.3 | 52.2±5.2 | 0.129 |
| LAD (cm) | 4.4±0.5 | 5.3±0.9** | 6.0±0.9**,# | <0.001 |
| PASP (mmHg) | 27.8±9.0 | 40.4±17.8 | 47.2±18.4** | 0.001 |
| Left atrial thrombus (n) | 0 | 1 | 3 | 0.157 |
| Type of mitral disease | ||||
| PMS/PMR/MS+MR | 6/6/8 | 6/4/5 | 9/4/12 | 0.766 |
| Combined TAP | 5 | 3 | 6 | 0.936 |
| Cause of mitral valve disease | ||||
| Degenerative/rheumatic (n) | 8/12 | 5/10 | 6/19 | 0.509 |
| Preoperative drugs (n) | ||||
| Digitalis (n) | 12 | 10 | 21 | 0.170 |
| ACEI or ARB (n) | 8 | 7 | 8 | 0.641 |
| Beta blocker (n) | 4 | 5 | 10 | 0.340 |
| CCB (n) | 10 | 10 | 13 | 0.572 |
| Digoxin (n) | 5 | 6 | 13 | 0.177 |
SR, sinus rhythm; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; BMI, Body mass index; NYHA, New York Association; LVDd, left ventricular diastolic diameter; LVDs, left ventricular end-systolic dimension; LAD, left atrial diameter; EF, ejection fraction; PASP, pulmonary artery systolic pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker. PMS, pure mitral stenosis; PMR, pure mitral regurgitation; MS+MR, mitral stenosis and mitral regurgitation. TAP, tricuspid valve repair.
P<0.01 vs. SR group.
P<0.05 vs. PaAF group.
Upregulation of TGF-β and periostin in AF
In this study, western blot revealed a significant upregulation of periostin in both PaAF group and PeAF group compared with the control group (P=0.023 and P<0.01 respectively) (Figure 1A), this was accompanied by an increased in the expression of TGF-β (P=0.013 and P<0.01 respectively) (Figure 1B). Compared with the SR group, the protein levels of periostin and TGF-β were significant higher in the PeAF group (both P<0.01). The expression of periostin as well as TGF-β was increased in the PaAF group compared to the SR group (Figure 1A, 1B), while it did not reach statistical significant with respect to TGF-β (P=0.288) (Figure 1B). In the two AF groups, both of periostin and TGF-β showed a trend to be higher in the PeAF group as compared to the PaAF group (1.88 ± 0.76 vs. 1.68 ± 0.66 and 2.01 ± 0.78 vs. 1.69 ± 0.69 respectively) (Figure 1A, 1B). To further confirm the alerted levels of periostin and TGF-β, we analyzed their expression using immunohistochemical staining. In line with the results of Western bolt, the results of immunohistochemical staining showed that expression of periostin a TGF-β were higher in AF groups than control group (Figure 1C).
Figure 1.
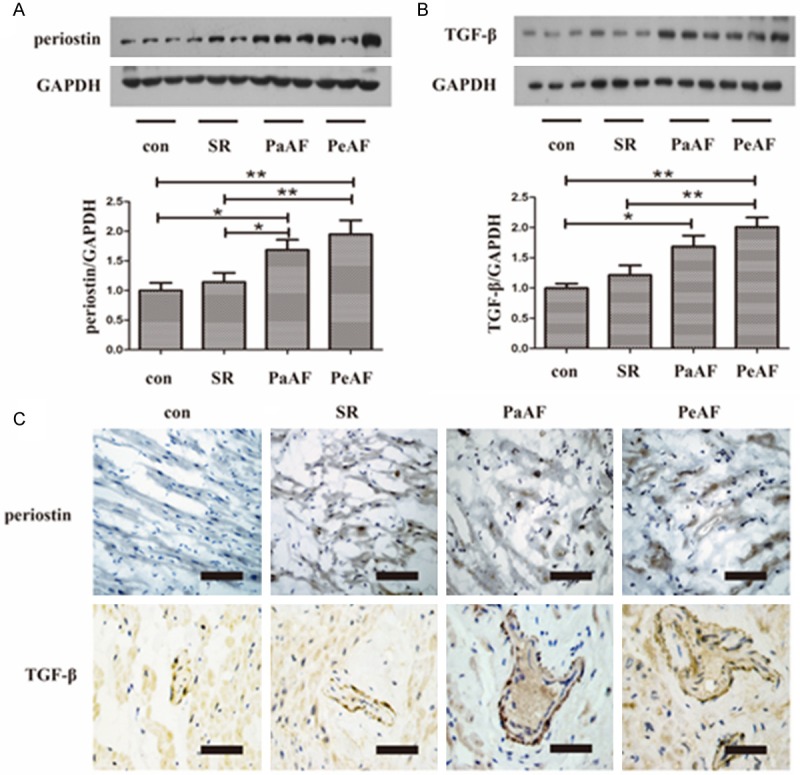
Expression of periostin and TGF-β in RAAs. A, B. Representative western blot of periostin and TGF-β in RAAs. *P<0.05 between groups, **P<0.01 between groups. C. Representative immunohistochemical staining of periostin and TGF-β in different groups. Bar =50 μm. con group, n=10; SR group, n=20; PaAF group, n=15; PeAF group, n=25. SR, sinus rhythm; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; TGF-β, transforming growth factor-β; RAAs, right atrial appendages.
Correlation between protein expression of TGF-β/periostin and the clinical parameters
As reported recently, the expression of periostin was upregulated in failing myocardium, therefore, we hypothesized that the increased expression of periostin in RAAs is associated with cardiac function. To address this issue, we evaluated the periostin level in different NYHA classes. As shown in Figure 2A, periostin expression in the PeAF group was higher than in the PaAF group, SR group and control group (P=0.033, P=0.006 and P=0.009, respectively, Figure 2A). EF value, a hallmarker of cardiac function, was negatively correlated with periostin expression (r=-0.44, P<0.001, Figure 2D). Since LAD and PASP were higher in the AF groups as well as the expression of periostin, the relationship between them was also tested here. As illustrated in Figure 2C, 2D, periostin level was positively correlated with LAD and PASP (P<0.001 and P=0.039 respectively). The previously described role of TGF-β in regulating periostin expression prompted us to evaluate the relationship between them. We found that periostin level showed a strong correlation with TGF-β level (Figure 2B).
Figure 2.
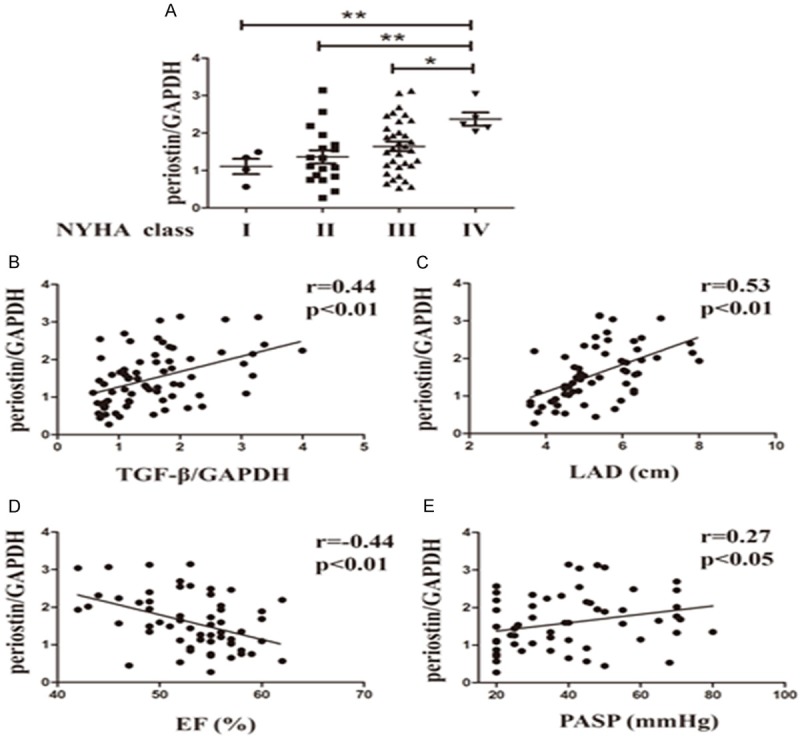
Correlation between TGF-β/periostin and other parameters. A. Protein level of periostin in different NYHA classes. *P<0.05 between groups, **P<0.01 between groups, n=60. B. Relationship between the protein expression of periostin and TGF-β. r=0.44, P<0.01, n=70. C. Relationship between the protein expression of periostin and LAD. r=0.53, P<0.01, n=60. D. Relationship between the protein expression of periostin and EF value. r=-0.44, P<0.01, n=60. E. Relationship between the protein expression of periostin and PASP. r=0.27, P<0.05, n=60. SR, sinus rhythm; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; NYHA, New York Association; LAD, left atrial diameter; EF, ejection fraction; PASP, pulmonary artery systolic pressure; TGF-β, transforming growth factor-β.
Upregulated fibrosis level in RAAs of AF
In this investigation, we evaluated fibrosis level in right atrial tissue of each group through three different aspects. Western blot analysis suggested that col I as well as col III was lowest in the con group, followed by the SR, PaAF and PeAF group (Figure 3A), although it did not reach significant difference between the former two groups for col I and col III (P=0.174 and P=0.921 respectively, Figure 3B, 3C) and between the latter two groups for col III (P=0.110, Figure 3C). In addition, col I/col III increased gradually significant in the con, SR, PaAF and PeAF group, although no statistical difference was observed between the con and SR groups (P=0.061, Figure 3D). Then, as depicted in Figure 3E, the percentage of interstitial fibrosis detected by Masson staining was highest in the PeAF group, followed by the PaAF group, the SR group and the con group. A significant difference was detected between any two groups (Figure 3F). Consistent with CVF, collagen deposition measured by Hyp content in RAAs was significantly ascended gradually in the con, SR, PaAF and PeAF groups, and it reached significant difference between any two groups (Figure 3G).
Figure 3.
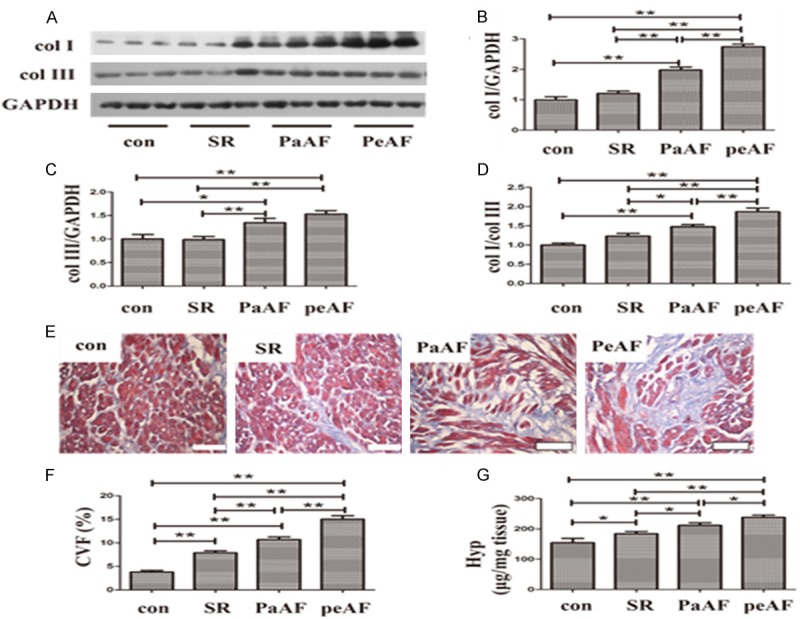
Increased fibrosis in AF. A. Representative western blot of col I and col III. In each photo, three different tissue samples were used in each group. B-D. Expression of col I, col III and their ratio. E. Representative Masson staining in RRAs of different groups. F. CVF of fibrosis in each group. G. Hydroxyproline level in each group. SR, sinus rhythm; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; col I, collagen I; col III, collagen III; CVF, collagen volume fraction; Hyp, hydroxyproline.*P<0.05 between groups, **P<0.01 between groups. con group, n=10; SR group, n=20; PaAF group, n=15; PeAF group, n=25. Bar =100 μm.
Correlations between col I, col III, col I/col III, Hyp, CVF levels and expression of TGF-β/periostin pathway in RAAs
An accumulating body of evidence suggested that TGF-β could exert its profibrosis effect via periostin in different tissues, however, there was little information regarding the role of periostin in AF. Therefore, we assessed the relationship between the expression of periostin and markers of fibrosis, such as col I, col III, col I/col III, Hyp and CVF. We verified that the protein level of periostin was strongly correlated with the markers of fibrosis except col III (P=0.06). However, TGF-β, a key mediator of fibrosis, was positively correlated with all the markers of fibrosis (Table 2).
Table 2.
Correlations between col I, col III, col I/col III, Hyp, CVF levels and expression of TGF-β/periostin axis in RAAs
| col I | col III | col I/col III | Hyp | CVF | |
|---|---|---|---|---|---|
| TGF-β | 0.499** | 0.306** | 0.419** | 0.354** | 0.513** |
| periostin | 0.422** | 0.226 | 0.358** | 0.465** | 0.478** |
col I, collagen I; col III, collagen III; Hyp, hydroxyproline; CVF, collagen volume fraction; TGF-β, transforming growth factor-β.
P<0.01, n=70.
Comparison of patients with recurrence of AF and nonrecurrence of AF after radiofrequency ablation
Given the relationship between the fibrosis and recurrence of AF, we determined whether the expressions of TGF-β or periostin in RAAs were correlated with an unsuccessful radiofrequency ablation in patient with valvular AF. In 40 patients with AF, 33 patients who underwent radiofrequency ablation during surgery operation were classified into two groups: those who had successfully restored SR postoperatively and maintained within 12 months (n=23, Nonrecurrence group), and those without restored SR within 12 months (n=10, Recurrence group). As shown in Table 3, patients in the recurrence groups had a larger LAD compared with those in the nonrecurrence group (P=0.034). There were no significant differences for the other clinical parameters between the two groups, though the latter group tended to have larger ages, BMI, LVDd and LVDs.
Table 3.
Parameters of patients followed up for one year
| Parameters | Nonrecurrence | Recurrence | p-value |
|---|---|---|---|
| Basic data | |||
| Sex, M/F (n) | 12/11 | 5/5 | 1.000 |
| Age (years) | 50.7±8.9 | 56.5±6.5 | 0.072 |
| BMI (kg/m2) | 22.5±2.8 | 24.8±3.7 | 0.06 |
| NYHA class I/II/III/IV (n) | 2/8/12/1 | 0/1/7/2 | 0.203 |
| Echocardiographic parameters | |||
| LVDd (cm) | 5.4±0.9 | 6.0±1.0 | 0.125 |
| LVDs (cm) | 4.0±0.9 | 4.5±0.9 | 0.188 |
| EF (%) | 53.1±4.6 | 50.7±5.8 | 0.214 |
| LAD (cm) | 5.6±0.6 | 6.6±1.2* | 0.034 |
| PASP (mmHg) | 44.0±18.7 | 45.1±15.0 | 0.871 |
| Postoperative drugs (n) | |||
| Digitalis (n) | 22 | 10 | 1.000 |
| ACEI or ARB (n) | 9 | 2 | 0.430 |
| Beta blocker (n) | 3 | 1 | 1.000 |
| CCB (n) | 8 | 2 | 0.682 |
| relative expression of TGF-β/periostin | |||
| TGF-β | 1.6±0.5 | 2.4±0.8* | 0.023 |
| periostin | 1.7±0.7 | 1.9±0.7 | 0.295 |
BMI, Body mass index; NYHA, New York Association; LVDd, left ventricular diastolic diameter; LVDs, left ventricular end-systolic dimension; LAD, left atrial diameter; EF, ejection fraction; PASP, pulmonary artery systolic pressure; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; TGF-β, transforming growth factor-β.
P<0.05 vs. the nonrecurrence group.
Additionally, the protein expression of TGF-β and periostin were analyzed between the two groups. Similar to LAD, TGF-β protein levels were higher in the recurrence compared with patients who restored SR after radiofrequency (P=0.023). Conversely, the difference of periostin levels between the two groups were negligible (P=0.295), though it showed a tendency to be higher in the recurrence group (Table 3).
Discussion
In the present study, we examined the expression of TGF-β/periostin and fibrosis levels in the RAAs of patients with AF. Here, three recently proposed idea may be especially important. First is the suggestion that upregulation of periostin correlate with TGF-β expression in the RAAs of patients with AF. Second, it has proposed that the expression of TGF-β/periostin was correlated with fibrotic indexes in the RAAs of patients with AF. Third, it has demonstrated that a larger LAD and the expression of TGF-β were associated with recurrence of AF within 12 months after radiofrequency ablation. Taken together, these results demonstrate that TGF-β/periostin pathway may contribute to the fibrotic process of AF.
A variety of factors are known to contribute to an increased susceptibility and maintenance of AF, but of particular importance is the interstitial fibrosis of the atrial myocardium, a hallmarker of arrhythmogenic structural remodeling in clinical AF. In the past 10 years, a great gain of the relationship between the atrial fibrosis and persistence of AF has been made [2]. Development of fibrosis results in electrical uncoupling of adjacent muscle bundles, leading to disturbed electrical continuity, which contributes to AF persistence [19]. Conversely, AF can also induce atrial fibrosis as an arrhythmia-related remodeling process, as animal models subjected to atrial tachypacing exhibits enhanced atrial fibrosis [4,20].
In the present study, although a causal relationship could not be studied, the results of Masson staining suggested an increasing gradient of atrial fibrosis in the con, SR, PaAF and PeAF groups, it supported the findings by Cao [21]. Fibrosis in atrial tissue represents excessive deposition of extracellular matrix (ECM) proteins particularly col I and col III [22]. In addition, the ratio of col I/III protein expression can affect myocardial stiffness and can induce atrial fibrosis [21,23], therefore, we detected the expression of col I, col III and their ratio in this investigation. In accordance to other studies [21], upregulation of col I, col III and col I/col III in the AF groups was found here, which also represented increased fibrosis in AF. Furthermore, the Hyp content, a typical amino acid of collagen protein, was also measured in the RAAs. The changes of Hyp levels were similar with that of CVF and collagens, which verified the fibrosis process in AF once again.
Periostin, also termed osteoblast-specific factor-2, has defined functions in inflammation, tissue remodeling and angiogenesis in different organs [5,24-27]. Several lines of evidence have indicated that periostin was highly expressed in ventricular of heart failure [6,27-29], and that overexpression of periostin gene led to cardiac dysfunction [28]. Similar to that expressed in ventricular, here, we for the first time demonstrated that the expression of periostin was increased in RAAs of heart failure with high NYHA class, and it was negatively correlated with EF value. Meanwhile, a positive correlation of periostin protein levels with LAD and PASP was verified in this study. These data promoted us to hypothesize that periostin may be an important mediator of atrial remodeling in AF, since heart failure and increased LAD were implicated with tissue remodeling.
There are a few studies looking specially at the effect of periostin on fibrosis process of various diseases [5,7,11,30]. Therefore, it is rational to presume that periostin may mediate the process of atrial fibrosis and promote atrial structural remodeling, which is a hallmarker of AF. As illustrated in the current investigation, we found that periostin was upregulated in the AF groups compared to the control and SR groups. Furthermore, a strong correlation between periostin expression and fibrotic markers was confirmed, though the exact molecular mechanisms were not fully elucidated here.
TGF-β, a profibrotic factor, is known to play an important role in atrial fibrosis, which is considered a key element of atrial remodeling of AF [14]. Consistent with the findings of others [8], the present data provided evidence of increased TGF-β in RAAs of AF, more importantly, its expression was statistically correlated with the fibrosis level.
To date, an accumulating body of evidence investigated the interaction between TGF-β and periostin. Li and coworkers demonstrated that angiotensin II increased periostin expression in cardiac fibroblasts, whereas inhibition of TGF-β normalized the elevated periostin expression [31]. In other research, addition of TGF-β resulted in induction of periostin in mesangial cells [11], renal epithelial cells [5] and human BM hTERT stromal cells [32]. These data suggested that TGF-β was a key inducer of periostin expression in a variety of tissue or cells and is accompanied with increased fibrosis [5,7,11]. While several studies showed that periostin in turn could also induce TGF-β synthesis and activate TGF-β signaling [12,13]. Although the causal relationship between TGF-β and periostin was not explored in vitro here, it showed a strong correlation with TGF-β protein level and the expression of periostin in RAAs of AF. As mentioned above, both TGF-β and periostin were significantly correlated with the markers of fibrosis, indicating that TGF-β/periostin was involved in the fibrosis process of AF.
In most patients with valvular AF, surgical ablation was usually performed during cardiac surgery. Recurrence of AF following surgical ablation may occur despite advances in surgical treatment for AF. Therefore, it is very significant to search the candidate predictors of AF recurrence after surgical ablation. In the past few years, much attention has been devoted to assessing the role of atrial fibrosis and LAD in predicting the recurrence of AF [33-35]. Recently, this point was highlighted by several clinical studies showing that atrial tissue fibrosis identified by delayed enhancement magnetic resonance could predict recurrence of AF after pulmonary vein isolation or catheter ablation [16,17]. So the clinical parameters and the expression of TGF-β and periostin were analyzed to search some markers associated with AF recurrence. Among the clinical parameters, only LAD was significantly larger in the recurrence group compared to the nonrecurrence group. These results also support previous observation that LAD was a strong independent predictor of recurrent AF post-catheter ablation [34,36].
As well as LAD, plasma TGF-β or protein levels of TGF-β in LAAs was also considered as a predictor of recurrence of AF after catheter ablation or surgery treatment [36-38]. While to our knowledge, the role of the protein expression of TGF-β in RAAs in predicting the unsuccessful surgery ablation has not been reported to date. In the investigation, it would be expected that TGF-β was upregulated in the AF recurrence group. Surprisingly, periostin, another molecule involved in the fibrosis process of AF in this study was similar between the recurrence group and the nonrecurrence group. Though the result was not in accordance with expectations, it is important to note that further studies may be mandatory to elucidate the result due to small sample circumstances. Taken together, our results showed that TGF-β but not periostin link to recurrence of AF, but it was confirmed that upregulation of TGF-β as well as periostin in RAAs was correlated with fibrosis in AF for the first time.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (81070195, 81200148, 81270281), Jiangsu Key Laboratory for Molecular Medicine of Nanjing University, Jiangsu Provincial Special Program of Medical Science (BL2012014), State Key Laboratory of Pharmaceutical Biotechnology (KF-GN-200901), the Peak of Six Personnel in Jiangsu Province (2013-WSN-008), Funds for Distinguished Young Scientists in Nanjing (Xie Jun), and Natural Science Foundation of Jiangsu Province (BK2010107).
Disclosure of conflict of interest
None.
References
- 1.Syed TM, Halperin JL. Left atrial appendage closure for stroke prevention in atrial fibrillation: state of the art and current challenges. Nat Clin Pract Cardiovasc Med. 2007;4:428–435. doi: 10.1038/ncpcardio0933. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 3.Knackstedt C, Gramley F, Schimpf T, Mischke K, Zarse M, Plisiene J, Schmid M, Lorenzen J, Frechen D, Neef P, Hanrath P, Kelm M, Schauerte P. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovasc Pathol. 2008;17:318–324. doi: 10.1016/j.carpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Nakatani Y, Nishida K, Sakabe M, Kataoka N, Sakamoto T, Yamaguchi Y, Iwamoto J, Mizumaki K, Fujiki A, Inoue H. Tranilast prevents atrial remodeling and development of atrial fibrillation in a canine model of atrial tachycardia and left ventricular dysfunction. J Am Coll Cardiol. 2013;61:582–588. doi: 10.1016/j.jacc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Mael-Ainin M, Abed A, Conway SJ, Dussaule JC, Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol. 2014;25:1724–1736. doi: 10.1681/ASN.2013060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Zi M, Tsui H, Chowdhury SK, Zeef L, Meng QJ, Travis M, Prehar S, Berry A, Hanley NA, Neyses L, Xiao RP, Oceandy D, Ke Y, Solaro RJ, Cartwright EJ, Lei M, Wang X. A novel immunomodulator, FTY-720 reverses existing cardiac hypertrophy and fibrosis from pressure overload by targeting NFAT (nuclear factor of activated T-cells) signaling and periostin. Circ Heart Fail. 2013;6:833–844. doi: 10.1161/CIRCHEARTFAILURE.112.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-beta pathway. Proc Natl Acad Sci U S A. 2012;109:10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao H, Lei H, Qin S, Ma K, Wang X. TGF-beta1 expression and atrial myocardium fibrosis increase in atrial fibrillation secondary to rheumatic heart disease. Clin Cardiol. 2010;33:149–156. doi: 10.1002/clc.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gramley F, Lorenzen J, Koellensperger E, Kettering K, Weiss C, Munzel T. Atrial fibrosis and atrial fibrillation: the role of the TGF-beta1 signaling pathway. Int J Cardiol. 2010;143:405–413. doi: 10.1016/j.ijcard.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 10.Rahmutula D, Marcus GM, Wilson EE, Ding CH, Xiao YY, Paquet AC, Barbeau R, Barczak AJ, Erle DJ, Olgin JE. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-1. Cardiovasc Res. 2013;99:769–779. doi: 10.1093/cvr/cvt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol. 2011;179:1756–1767. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23:71–84. doi: 10.1016/j.carpath.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Corradi D, Callegari S, Gelsomino S, Lorusso R, Macchi E. Morphology and pathophysiology of target anatomical sites for ablation procedures in patients with atrial fibrillation: part II: pulmonary veins, caval veins, ganglionated plexi, and ligament of Marshall. Int J Cardiol. 2013;168:1769–1778. doi: 10.1016/j.ijcard.2013.06.141. [DOI] [PubMed] [Google Scholar]
- 16.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 17.Neilan TG, Mongeon FP, Shah RV, Coelho-Filho O, Abbasi SA, Dodson JA, McMullan CJ, Heydari B, Michaud GF, John RM, Blankstein R, Jerosch-Herold M, Kwong RY. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saint LL, Damiano RJ Jr. Surgical treatment of atrial fibrillation. Mo Med. 2012;109:281–287. [PMC free article] [PubMed] [Google Scholar]
- 19.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kaab S, Nattel S. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–1475. 1475e1–28. doi: 10.1161/CIRCULATIONAHA.112.001207. [DOI] [PubMed] [Google Scholar]
- 21.Cao H, Li Q, Li M, Od R, Wu Z, Zhou Q, Cao B, Chen B, Chen Y, Wang D. Osteoprotegerin/RANK/RANKL axis and atrial remodeling in mitral valvular patients with atrial fibrillation. Int J Cardiol. 2013;166:702–708. doi: 10.1016/j.ijcard.2011.11.099. [DOI] [PubMed] [Google Scholar]
- 22.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 23.Grammer JB, Bohm J, Dufour A, Benz M, Lange R, Bauernschmitt R. Atrial fibrosis in heart surgery patients Decreased collagen III/I ratio in postoperative atrial fibrillation. Basic Res Cardiol. 2005;100:288–294. doi: 10.1007/s00395-005-0515-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SS, Jackson-Boeters L, Darling MR, Rieder MJ, Hamilton DW. Nifedipine Induces Periostin Expression in Gingival Fibroblasts through TGF-beta. J Dent Res. 2013;92:1022–1028. doi: 10.1177/0022034513503659. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita O, Yoshimura K, Nagasawa A, Ueda K, Morikage N, Ikeda Y, Hamano K. Periostin links mechanical strain to inflammation in abdominal aortic aneurysm. PLoS One. 2013;8:e79753. doi: 10.1371/journal.pone.0079753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers. 2008;23:182–186. doi: 10.1177/172460080802300308. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J. Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J Cardiol. 2014;63:373–378. doi: 10.1016/j.jjcc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–1813. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 29.Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. Ann Thorac Surg. 2009;88:1916–1921. doi: 10.1016/j.athoracsur.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohjolainen V, Rysa J, Napankangas J, Koobi P, Eraranta A, Ilves M, Serpi R, Porsti I, Ruskoaho H. Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency. Nephrol Dial Transplant. 2012;27:115–122. doi: 10.1093/ndt/gfr279. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 32.Oku E, Kanaji T, Takata Y, Oshima K, Seki R, Morishige S, Imamura R, Ohtsubo K, Hashiguchi M, Osaki K, Yakushiji K, Yoshimoto K, Ogata H, Hamada H, Izuhara K, Sata M, Okamura T. Periostin and bone marrow fibrosis. Int J Hematol. 2008;88:57–63. doi: 10.1007/s12185-008-0095-2. [DOI] [PubMed] [Google Scholar]
- 33.Akkaya M, Higuchi K, Koopmann M, Burgon N, Erdogan E, Damal K, Kholmovski E, McGann C, Marrouche NF. Relationship between left atrial tissue structural remodelling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace. 2013;15:1725–1732. doi: 10.1093/europace/eut147. [DOI] [PubMed] [Google Scholar]
- 34.den Uijl DW, Delgado V, Bertini M, Tops LF, Trines SA, van de Veire NR, Zeppenfeld K, Schalij MJ, Bax JJ. Impact of left atrial fibrosis and left atrial size on the outcome of catheter ablation for atrial fibrillation. Heart. 2011;97:1847–1851. doi: 10.1136/hrt.2010.215335. [DOI] [PubMed] [Google Scholar]
- 35.Saito T, Tamura K, Uchida D, Togashi M, Nitta T, Sugisaki Y. Histopathological features of the resected left atrial appendage as predictors of recurrence after surgery for atrial fibrillation in valvular heart disease. Circ J. 2007;71:70–78. doi: 10.1253/circj.71.70. [DOI] [PubMed] [Google Scholar]
- 36.Wu CH, Hu YF, Chou CY, Lin YJ, Chang SL, Lo LW, Tuan TC, Li CH, Chao TF, Chung FP, Liao JN, Chen SA. Transforming growth factor-beta1 level and outcome after catheter ablation for nonparoxysmal atrial fibrillation. Heart Rhythm. 2013;10:10–15. doi: 10.1016/j.hrthm.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Liu L, Li Y, Hu SS, Song YH, Wang X. Does the expression of transforming growth factor beta-1: affect the outcome of the radiofrequency modified maze procedure in patients with rheumatic atrial fibrillation? Tex Heart Inst J. 2012;39:17–23. [PMC free article] [PubMed] [Google Scholar]
- 38.On YK, Jeon ES, Lee SY, Shin DH, Choi JO, Sung J, Kim JS, Sung K, Park P. Plasma transforming growth factor beta1 as a biochemical marker to predict the persistence of atrial fibrillation after the surgical maze procedure. J Thorac Cardiovasc Surg. 2009;137:1515–1520. doi: 10.1016/j.jtcvs.2008.10.022. [DOI] [PubMed] [Google Scholar]


