Abstract
Given the various clinical and pathologic responses to neoadjuvant chemotherapy (NACT) in gastric cancer (GC), potential biomarkers that reflecting the efficacy of NACT on GC should be investigated. The aim of this study was to investigate the 15-PGDH expression response to NACT in GC patients and its relationship with prognosis of GC. Immunohistochemical method was used to assess the level of 15-PGDH expression in 56 GC patients who received NACT before surgery and 46 patients who underwent surgical treatment without NACT as well as their corresponding adjacent non-neoplastic tissues. We found that there was no correlation of 15-PGDH expression between non-cancerous gastric tissues and GC tissues (P=0.519), while 15-PGDH expression level in NACT group was higher than that in nNACT group (P=0.015). In patients with NACT, the higher level of 15-PGDH expression was significantly associated with well-moderately differentiated grade (P=0.023), I/II stage (P=0.014) and with no lymph node metastasis (P=0.016). Moreover, statistically significant differences in overall survival (OS) were found among 15-PGDH expression (log-rank test, P<0.001) and TNM stage (log-rank test, P=0.032). Most importantly, expression of 15-PGDH was found to be an independent predictive factor by multivariate analysis (Hazard ratio (HR) 0.315 [0.120-0.827], P=0.019). These findings indicated that NACT could increase 15-PGDH expression in advanced GC patients, and 15-PGDH may serve as a candidate prognostic biomarker of advanced GC response to NACT.
Keywords: Gastric cancer, 15-hydroprostaglandin dehydrogenase (15-PGDH), neoadjuvant chemotherapy (NACT), immunohistochemistry (IHC), biomarker
Introduction
Gastric cancer (GC) is one of the most common malignant tumor and remains the second leading cause of cancer-related mortality worldwide [1]. Although great progress has been made in understanding the biology of GC and in the generation of novel therapeutic agents, the five-year overall survival (OS) for patients with GC remains poor because of invasion, metastasis or recurrence [2]. This indicates that novel effective diagnosis and treatment options are required, and identification of molecular factors involved in gastric carcinogenesis may lead to improvement in the management of the disease.
Surgery still is admitted as the only curative therapy for GC; however, unfortunately most cases of GC were diagnosed in a locally advanced tumor stage. Thereby the concept of neoadjuvant treatment developed [3]. Neoadjuvant chemotherapy (NACT) has been increasingly used to improve potential tumor resectability, decrease the risk of metastatic disease, and prolong the survival time of patients with advanced GC over the past decades [4]. Despite some studies have reported that NACT could improve outcome of GC patients, it is still difficult to evaluate the efficacy of NACT because the clinical and histopathologic responses predicting the prognostic significance of NACT varies and results in divergent outcomes [5,6]. Hence, it’s pivotal to search potential biomarkers that reflecting the clinical and pathologic response to NACT.
Inflammatory responses are thought to be one of the multistep process and many factors that proposed to be involved in GC development [7,8], among which the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway was first identified as a key player in tumorigenesis [9]. PGE2, a major prostanoid in gastric tissue, is up-regulated in GC tissues and plays an important role in GC tumorigenesis including promotion of proliferation and angiogenesis, inhibition of apoptosis, immunosuppression, invasion and metastasis [10]. COX-2 is the rate-limiting enzyme in prostaglandin (PG) biosynthesis and could be found overexpressed in GC during gastric carcinogenesis [11]. It is of great importance for tumor therapy to degrade COX-2-derived PGs, which are accumulated in GC progression.
15-Hydroprostaglandin dehydrogenase (15-PGDH) is the key enzyme in PG degradation and is found to act as a natural antagonist against the function of COX-2 [12]. Accumulation of reports had investigated the role of 15-PGDH in GC. Some studies indicated that 15-PGDH expression in GC was decreased or even absent and it could be acted as a tumor suppressor in GC [13,14], while the other study showed that the expression of 15-PGDH was not either altered in gastric carcinoma or associated with clinicopathologic parameters or prognosis [15,16]. Hence, it is crucial to clarify the role of 15-PGDH expression in GC. In addition to its role in gastric tumorigenesis, little is known about the effect of NACT on 15-PGDH expression in GC. Expanding our knowledge of the complex role of 15-PGDH expression in GC and figuring out the effect of NACT on 15-PGDH expression in GC could ultimately find a potential factor that affect clinical and histopathologic response to NACT in advanced GC.
According to the above understanding, we investigated the expression of 15-PGDH in adjacent non-cancerous tissues and GC tissues with or without NACT. Then we compared the correlation of 15-PGDH expression with clinicopathologic features of GC with NACT, and their correlation with overall survival (OS) of GC patients was also evaluated.
Materials and methods
Patients and follow-up
The records of 102 advanced GC patients who underwent surgery in curative intent at the Department of Surgical Oncology in Zhongnan Hospital of Wuhan University (Wuhan China) between 2008 and 2013 were retrospectively reviewed. The demographic and clinicopathologic data were available. 56 patients received NACT before surgery (considered as NACT group), while the other 46 patients didn’t receive NACT (considered as nNACT group), and their corresponding adjacent non-neoplastic tissues were obtained (considered as normal group). The TNM staging of GC was determined according to the 7th American Joint Committee on Cancer (AJCC) TNM system [17]. By the most recent follow-up on December 31, 2013, the median follow-up duration was 19 months (range: 1-53 months). A total number of 59 (64.1%) patients died. Overall survival (OS) was defined as the interval from the diagnosis of GC to the GC-related death or the final visit. The study was approved by the scientific research ethics committee of Zhongnan Hospital of Wuhan University (The ethics committee approval was conducted in August 1st, 2013. The review opinion was approved. The relevant reference number was: Scientific research ethics [2013002]) and the written informed consent was obtained from all patients for the tissue ex vivo experimentation.
Neoadjuvant chemotherapy protocol
The detailed FOLFOX6 chemotherapy protocol was as follows: Patients received oxaliplatin at 85 mg/m2 (intravenous injection, iv >2 hours), calcium folinate at 400 mg/m2 (iv >2 hours), and 5-fluorouracil 500 mg, (iv by continuous infusion for 44 hours). Patients received two cycles of chemotherapy, with each cycle of 3 weeks. All patients also received adjuvant chemotherapy after the surgical resection of tumors.
Immunohistochemiscal staining
Immunohistochemical (IHC) staining for 15-PGDH was performed using Ultra-Sensitive S-P Kit (Maixin-Bio, Fuzhou, China) in this study. Paraffin-embedded tumor tissue blocks were cut into species of 4 μm thickness, tissue sections were dried, deparaffinized, and dehydrated. Tissue sections were treated with 1% hydrogen peroxide for 10 min to block endogenous tissue peroxidase, followed by treatment with bovine serum for 30 min to reduce nonspecific binding. Then antigen retrieval using citrate buffer (pH 6.0) was accomplished as follows: high heat microwave processing for 5 min followed by low heat microwave processing for 20 min. All the slides were incubated with rabbit polyclonal anti-15-PGDH antibody (bs-6051R, 1:200 dilution, Beijing Biosynthesis Biotechnology co., LTD., Beijing, China) for an hour at 37°C or 4°C overnight, followed by a 30-min incubation with peroxidase-labeled polymer conjugated with goat anti-mouse or anti-rabbit immunoglobulins. All slides were rinsed with phosphate-buffered saline, color developed using 3, 3’-diaminobenzidine substrate kit, and then counterstained with haematoxylin.
Evaluation of 15-PGDH positive staining
The slides were examined under Olympus BX53 microscope by two senior pathologists who were blinded to the clinicopathologic data. A consensus was achieved using a multi-headed microscope in case of discrepancy. Cytoplasmic staining with 15-PGDH antibody in tumour cells was defined as positive. IHC staining of 15-PGDH protein was assessed in terms of staining intensity and percentage of positive cells as follows: 0 (negative), 1+ (weak staining, ≤10% of cells staining positive), 2+ (moderate staining, 10-90% of cells staining positive), and 3+ (strong staining, >90% of cells staining positive) [18]. The final score for each slide was represented by the average of three representative high-power fields (hpf, ×400). Scores ≤1+ was defined as low expression and scores ≥2+ were defined as high expression.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. The differences of 15-PGDH expression level in different groups and relationship between 15-PGDH expression and clinicopathologic parameters in NACT group were compared by chi-square test or Fisher’s exact test. Kaplan-Meier method was used for survival analysis and significance among variables was calculated by log-rank test. The Cox proportional hazards regression method was used to perform multivariate analysis. All two-sided P-values <0.05 were considered statistically significant.
Results
Patient characteristics and IHC features
Among all the patients, 77 were males and 25 were females, with a median age of 57 years (range: 32-78 years) at surgery. The median follow-up duration was 19 months (range: 1-53 months). The patients were divided into three groups: Patients received NACT before surgery (NACT group, n=56), patients without NACT treatment before surgery (nNACT group, n=46) and corresponding adjacent non-neoplastic tissues (Normal group, n=46). The patient’s demographics and clinicopathologic characteristics are shown in Table 1.
Table 1.
Demographics and clinicopathologic characteristics of patients
| NACT group | nNACT group | |
|---|---|---|
| No. of patients | 56 | 46 |
| Gender, n (%) | ||
| Male | 44 (78.6) | 34 (73.9) |
| Female | 12 (21.4) | 12 (26.1) |
| Age, n (%) | ||
| <55 | 26 (46.4) | 22 (47.8) |
| ≥55 | 30 (53.6) | 24 (52.2) |
| Differentiation, n (%) | ||
| Well/moderately differentiated | 20 (35.7) | 10 (21.7) |
| Poorly differentiated | 36 (64.3) | 36 (78.3) |
| Invasion depth, n (%) | ||
| T2 | 9 (16.1) | 6 (13.0) |
| T3/T4 | 47 (83.9) | 40 (87.0) |
| TNM Stage, n (%) | ||
| I+II | 22 (39.3) | 10 (21.7) |
| III+IV | 34 (60.7) | 36 (78.3) |
| Lymph node metastasis, n (%) | ||
| No (N0) | 21 (37.5) | 16 (34.8) |
| Yes (N1, N2, N3) | 35 (62.5) | 30 (65.2) |
| Clinical Status at follow-up, n (%) | ||
| Dead or died with recurrence | 33 (58.9) | 26 (56.5) |
| Live and without recurrence | 23 (41.1) | 20 (43.5) |
| 15-PGDH expression | ||
| Low, n (%) | 8 (14.3) | 30 (65.2) |
| High, n (%) | 48 (85.7) | 16 (34.8) |
T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; well-moderately: well-moderately differentiated carcinoma; Poorly: poorly differentiated carcinoma.
IHC was performed in all specimens and the result was obtained for image-based digital analysis. 15-PGDH staining was mainly in the cytoplasm of noncancerous gastric gland epithelial cells (Figure 1A-C) or gastric cancer cells (Figure 1D-F). In corresponding adjacent non-neoplastic tissues, 19 (41.3%) tissues showed no or low-level of 15-PGDH expression and 27 (58.7%) tissues showed high-level of 15-PGDH expression; While in GC patients without NACT before surgery, 30 (65.2%) showed no or low-level 15-PGDH expression and 16 (34.8%) patients showed high-level 15-PGDH expression. In NACT group, only 8 (14.3%) patients showed no or low-level 15-PGDH expression, the other 48 (85.7%) patients showed high-level 15-PGDH expression.
Figure 1.
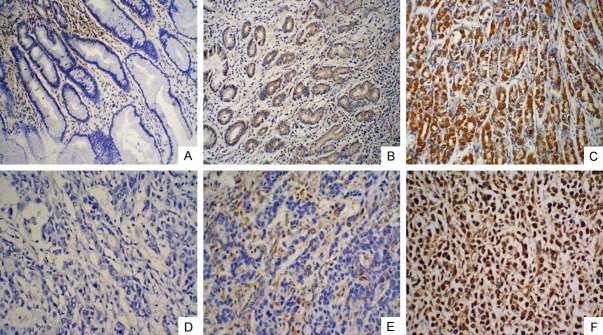
Immunoreactivity for 15-PGDH protein in adjacent noncancerous gastric tissues and GC tissues. 15-PGDH protein in adjacent noncancerous gastric tissues was mainly observed in cytoplasm of epithelial cells: A negative; B low expression; C high expression (original magnification, ×200). 15-PGDH protein in GC tissues was observed in cytoplasm of cancer cells: D negative; E low expression; F high expression (original magnification, ×200).
Expression of 15-PGDH in adjacent non-cancerous tissues and GC tissues
The level of 15-PGDH expression in adjacent non-cancerous tissues and GC tissues was compared by chi-square test or Fisher’s exact test. There was no correlation of 15-PGDH expression between non-cancerous gastric tissues and GC tissues (P=0.519), while 15-PGDH expression level in patients with NACT group was higher than that in patients without NACT (P=0.015) (Table 2).
Table 2.
Relationship of 15-PGDH expression between normal group, nNACT group and NACT group
| n | Low-15-PGDH expression | High-15-PGDH expression | P-value | |
|---|---|---|---|---|
| Normal group | 46 | 19 (41.3%) | 27 (58.7%) | 0.519* |
| nNACT group | 46 | 16 (34.8%) | 30 (65.2%) | |
| 0.015# | ||||
| NACT group | 56 | 8 (14.3%) | 48 (85.7%) |
P Normal group vs. nNACT group;
P nNACT group vs. NACT group.
Relationship between 15-PGDH expression and clinicopathologic features in GC patients with NACT
The correlation between 15-PGDH expression and clinicopathologic features of GC patients was shown in Table 3. It was found that the level of 15-PGDH expression in patients of poorly differentiated grade was significantly lowered (P=0.023) (Figure 2); the level of 15-PGDH expression was significantly lowered in patients of III/IV stage (P=0.014) and with lymph node metastasis (P=0.016); whereas there was no correlation with gender, age or invasion depth (P=0.111, P=0.827 and P=0.181, respectively).
Table 3.
Relationship of 15-PGDH expression in NACT group and clinicopathologic parameters
| Low-15-PGDH expression n=8 | High-15-PGDH expression n=48 | P-value | |
|---|---|---|---|
| Gender | |||
| Male | 8 (18.2%) | 36 (81.8%) | 0.111 |
| Female | 0 (0.0%) | 12 (100.0%) | |
| Age | |||
| <55 | 4 (15.4%) | 22 (84.6%) | 0.827 |
| ≥55 | 4 (13.3%) | 26 (86.7%) | |
| Differentiation | |||
| well-moderately | 0 (0.0%) | 20 (100.0%) | 0.023 |
| Poorly | 8 (22.2%) | 28 (77.8%) | |
| Invasion depth | |||
| T2 | 0 (0.0%) | 9 (100.0%) | 0.181 |
| T3/T4 | 8 (17.0%) | 39 (83.0%) | |
| TNM stage | |||
| I+II | 0 (0.0%) | 22 (100.0%) | 0.014 |
| III+IV | 8 (23.5%) | 26 (76.5%) | |
| Lymph node metastasis | |||
| No | 1 (4.8%) | 20 (95.2%) | 0.016 |
| Yes | 7 (20.0%) | 28 (80.0%) |
T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; well-moderately: well-moderately differentiated carcinoma; Poorly: poorly differentiated carcinoma.
Figure 2.
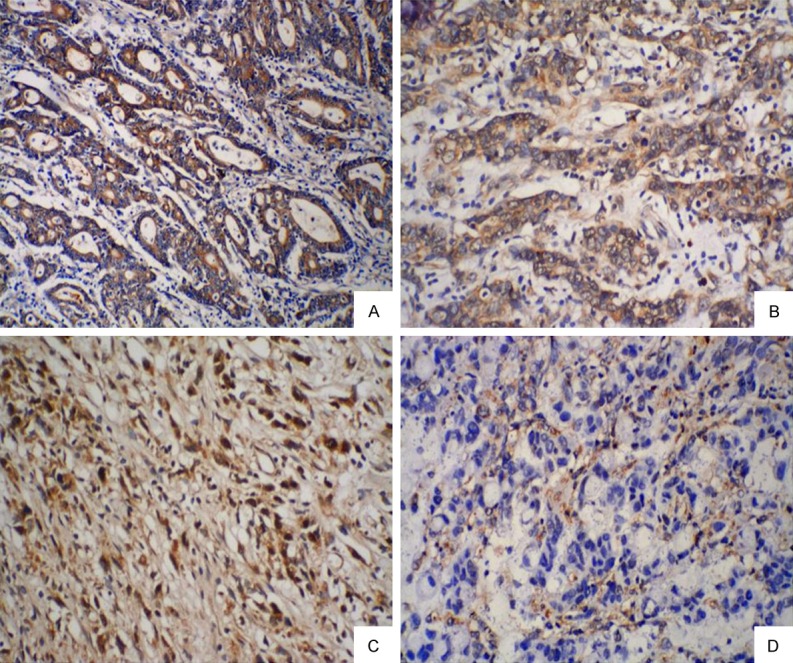
Immunohistochemical (IHC) staining of 15-PGDH in gastric cancer tissues with NACT: 15-PGDH expression in patients of well-moderately differentiated grade (A, B) was significantly higher than poorly differentiated grade (C, D) (original magnification, ×200).
Univariate and multivariate analyses of GC patients
The median survival time of patients in NACT group was 23 months (range: 6-53 months) and patients in nNACT was 18 months (range: 1-52 months). On univariate analysis, there was no significant difference of OS between patients with and without NACT (log-rank test, P=0.551) (Figure 3A). In NACT group, 15-PGDH immunoreactivity (log-rank test, P<0.001) (Figure 3B) and TNM stage (log-rank test, P=0.032) (Figure 3C) were prognostic factors significantly influencing OS. While in nNACT group, no significant difference in 15-PGDH expression (log-rank test, P=0.513) (Figure 3D) and any clinicopathologic parameters (data not shown) were found on univariate analysis.
Figure 3.
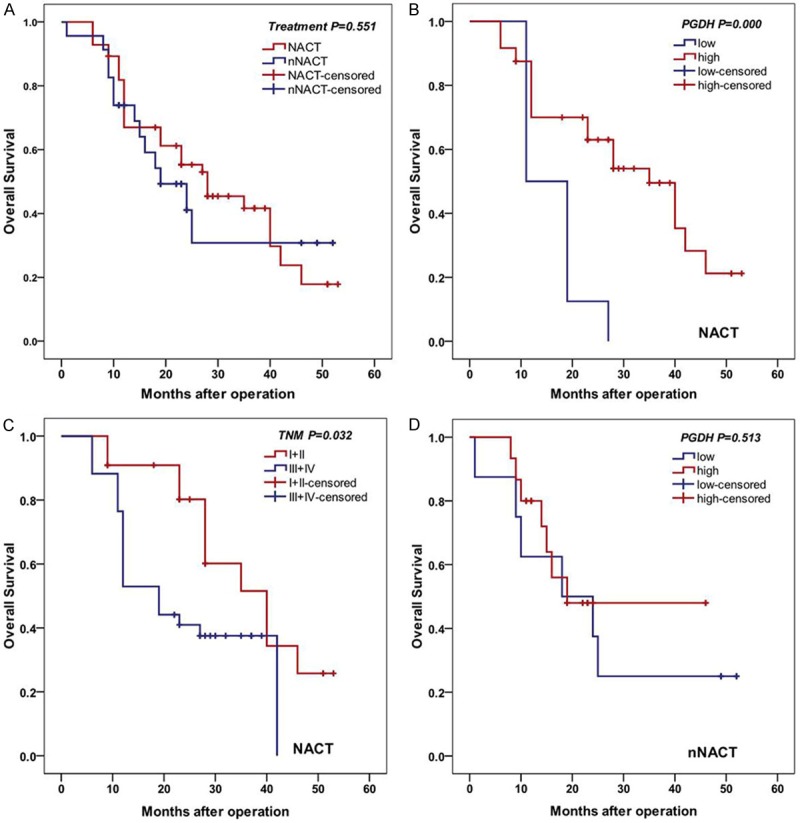
Kaplan-Meier analysis of OS. A. There was no significant difference of OS between patients’ treatment with and without NACT (log-rank test, P=0.551). B. Patients with NACT with higher 15-PGDH expression had longer OS (log-rank test, P<0.001). C. Patients with I/II stage had longer OS (log-rank test, P=0.032). D. No significant difference was seen between expression level of 15-PGDH in patients without NACT (log-rank test, P=0.513).
On multivariate analysis in patients with NACT using Cox proportional hazards model, expression of 15-PGDH was an independent predictive factor for long-term survival (Hazard ratio (HR) 0.315 [0.120-0.827], P=0.019) (Table 4).
Table 4.
Predictive factors for long-term survival by univariate and multivariate analysis in NACT group
| n | P-value univariate | P-value multivariate | Hazard ratio (HR), 95% CI | |
|---|---|---|---|---|
| Age | ||||
| <55 | 26 | 0.740 | 0.284 | 1.601 (0.676-3.793) |
| ≥55 | 30 | |||
| Differentiation | ||||
| well-moderately | 20 | 0.263 | 0.787 | 0.874 (0.329-2.322) |
| Poorly | 36 | |||
| Invasion depth | ||||
| T2 | 9 | 0.400 | 0.682 | 1.292 (0.379-4.405) |
| T3/T4 | 47 | |||
| TNM stage | ||||
| I+II | 22 | 0.032 | 0.096 | 2.564 (0.847-7.763) |
| III+IV | 34 | |||
| 15-PGDH expression | ||||
| High | 48 | 0.000 | 0.019 | 0.315 (0.120-0.827) |
| Low | 8 |
T2, tumour invasion of the muscularis propria or subserosa; T3, tumour invasion extends to or beyond the serosa; T4, tumour invasion of adjacent structures; well-moderately: well-moderately differentiated carcinoma; Poorly: poorly differentiated carcinoma.
Discussion
With large number of trails showed benefits to improve the curative resectability, decrease the risk of metastasis and prolong the survival time of patients over the past decades, NACT, that is neoadjuvant chemotherapy or preoperative chemotherapy has been used as an accepted and national guideline recommended therapeutic approach of GC treatment in most European countries. In our previous study, we have proposed that NACT could improve host anti-tumor immunity of GC patients by changing immune cells infiltration or co-inhibitory molecule expression in tumor microenvironment, thus indirectly improved prognosis of GC patients [19,20]. However, due to the variety of clinical and histopathologic responses that predicting the prognostic significance, its efficacy is difficult to clarify. Therefore, searching potential biomarkers that reflect the clinical and pathologic response to NACT would pave the way to better understand the effect of NACT in GC treatment.
The relationship between GC and expression of 15-PGDH has been reported in the literature but still remains controversial. Jang et al. [12] reported that 15-PGDH was overexpressed in normal tissue compared with gastric carcinoma tissue. Tatsuwaki et al. [14] demonstrated that reduction of 15-PGDH expression in GC associating with differentiation and stage could be an independent predictor of poor survival. Liu et al. [21] revealed that 15-PGDH expression was associated with differentiation, TNM stage, and lymph node metastasis of GC. While two conflicting results have been reported concerning the relationship between 15-PGDH expression and GC. Yoo et al. [15] reported that 15-PGDH expression was not altered in gastric carcinoma. Additionally, Alexandra et al. [16] reported that 15-PGDH expression in gastric adenocarcinoma was low-level, but not associated with clinicopathologic parameters. We found in our current study that expression level of 15-PGDH was no significant difference between GC and normal mucosa. Nonetheless, 15-PGDH expression level in patients with NACT was higher than that in patients without NACT, which matched with our assumption that NACT had an effect on 15-PGDH expression.
PG, especially PGE2, a bioactive eicosaniod synthesized from arachidonic acid by the sequential actions of the cyclooxygenases (COXs) and terminal synthases (PGES), plays a central role in tumorigenesis [22]. 15-PGDH, the key enzyme in PG degradation, mediates the inactivation of PGs by catalyzing the oxidation of 15(S)-hydroxyl group of PGs to the formation of 15-keto metabolites [23]. Therefore, 15-PGDH was logically presumed to be up-regulated to degrade the redundant PGs when abnormally elevated amount of PGs were detected, such as in the development of cancer. Accordingly, 15-PGDH should be acted as a tumor suppressor, protecting against carcinogenesis and rendering the cancerous cells susceptible to apoptosis by counteracting the action of PGE2. However, in contrast, 15-PGDH expression was decreased or even absent in many cancers, such as bladder, breast, colorectal and lung cancer.
In GC, expression of 15-PGDH and its role was controversial, and impact of NACT on 15-PGDH expression has not been reported yet. In the present study, we demonstrated that expression of 15-PGDH in advanced GC patients with NACT was higher when compared with patients without NACT, indicating that NACT could increase 15-PGDH expression in advanced GC. Furthermore, we analyzed the relationship between 15-PGDH expression and clinicopathologic parameters of advanced GC patients with NACT. The increased level of 15-PGDH expression in GC patients with NACT was significantly associated with well-moderately differentiated grade, I/II stage and without lymph node metastasis. This result suggested that 15-PGDH expression could predict the clinical and pathologic response to NACT. In this study, we further investigated OS of GC patients with NACT. 15-PGDH immunoreactivity and TNM stage were prognostic factors significantly influencing OS of patients. On multivariate analysis, expression of 15-PGDH was an independent predictive factor for long-term survival. Hereby, 15-PGDH may serve as a candidate prognostic biomarker of advanced GC response to NACT.
Previously, overexpression of 15-PGDH has been reported to lead to suppression of angiogenesis and invasion by modulating PGE2 production, vascular endothelial growth factor expression et al, and decreased proliferation and promoted apoptosis of lung and colon cancer cells [24,25]. In the current study, it was found that in NACT group, increased 15-PGDH expression was associated with better survival. Moreover, the delivery of 15-PGDH gene into tumors is likely to confer susceptibility to chemotherapeutic agents by decreasing excess accumulation of oncogenic PGE2. 15-PGDH has therefore been considered as one of the target molecules of a wide variety of chemopreventive agents with anti-inflammatory properties, such as non-steroidal anti-inflammatory drugs (NSAIDs), histone deacetylase (HDAC) inhibitors, and contributed to the suppression of growth of cancerous cells [26]. Similarly, in our present study, NACT increased 15-PGDH expression resulted in longer survival time and better outcome of GC patients, demonstrating that NACT inhibited GC progression by elevating 15-PGDH expression, which could in turn decreasing PGE2 associated inflammation in gastric cancerous environment and suppressing tumor growth.
Although the 15-PGDH expression investigated herein could be a candidate biomarker to predict the clinical and pathologic response to NACT in GC, the promising result was based on retrospective analysis of a small proportion of patients, which was the limitation of the present study. In addition, the 5-year survival rates of most of the patients were not able to follow up; hence, the OS analyses should be repeated after 5 years of follow-up. Third, the heterogeneity of patients to NACT was not considered in our present study, which could affect the clinical outcomes.
In conclusion, 15-PGDH expression was not altered in GC patients, but was increased in patients with NACT and resulted in long-term OS. Increasing 15-PGDH expression could be an independent prognostic factor for GC. Therefore, 15-PGDH may represent a candidate biomarker for predicting the prognosis of gastric cancer response to NACT. However, the mechanism of increasing of 15-PGDH expression by NACT and thus promoting prognosis remains to be further investigated.
Acknowledgements
This work was supported by Natural Foundation of Hubei Province (No. 2013CFB267) and Wuhan Science and Technology Key Project (No. 2013060602010248).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Cao L, Yu Y. Prognostic prediction in gastric cancer patients without serosal invasion: comparative study between UICC 7(th) edition and JCGS 13(th) edition N-classification systems. Chin J Cancer Res. 2014;26:596–601. doi: 10.3978/j.issn.1000-9604.2014.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol. 2015;7:39–48. doi: 10.1177/1758834014558839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An JY, Kim HI, Cheong JH, Hyung WJ, Kim CB, Noh SH. Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J. 2013;54:888–894. doi: 10.3349/ymj.2013.54.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzen S, Blank S, Lordick F, Siewert JR, Ott K. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol. 2012;19:2119–2127. doi: 10.1245/s10434-012-2254-1. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Yang ZL, Peng JS, Xiang J, Wang JP. Neoadjuvant chemotherapy for gastric cancer: a meta-analysis of randomized, controlled trials. J Gastroenterol Hepatol. 2013;28:777–782. doi: 10.1111/jgh.12152. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima H, Oshima M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol. 2013;35:139–150. doi: 10.1007/s00281-012-0353-5. [DOI] [PubMed] [Google Scholar]
- 11.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer letters. 2010;295:7–16. doi: 10.1016/j.canlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Jang TJ, Ji YS, Jung KH. Decreased expression of 15-hydroxyprostaglandin dehydrogenase in gastric carcinomas. Yonsei Med J. 2008;49:917–922. doi: 10.3349/ymj.2008.49.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song HJ, Myung SJ, Kim IW, Jeong JY, Park YS, Lee SM, Nam WH, Ryu YM, Fink SP, Yang DH, Jung HY, Kim JH. 15-hydroxyprostaglandin dehydrogenase is downregulated and exhibits tumor suppressor activity in gastric cancer. Cancer Invest. 2011;29:257–265. doi: 10.3109/07357907.2011.568562. [DOI] [PubMed] [Google Scholar]
- 14.Tatsuwaki H, Tanigawa T, Watanabe T, Machida H, Okazaki H, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Muguruma K, Sawada T, Hirakawa K, Higuchi K, Arakawa T. Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci. 2010;101:550–558. doi: 10.1111/j.1349-7006.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007;39:174–175. doi: 10.1080/00313020601123946. [DOI] [PubMed] [Google Scholar]
- 16.Thiel A, Ganesan A, Mrena J, Junnila S, Nykänen A, Hemmes A, Tai HH, Monni O, Kokkola A, Haglund C, Petrova TV, Ristimäki A. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin Cancer Res. 2009;15:4572–4580. doi: 10.1158/1078-0432.CCR-08-2518. [DOI] [PubMed] [Google Scholar]
- 17.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 18.Kang JH, Kang SH, Seo SH, Shin JH, An MS, Ha TK, Bae KB, Kim TH, Choi CS, Oh SH, Kang MS, Kim KH. Relationship between 15-hydroxyprostaglandin dehydrogenase and gastric adenocarcinoma. Ann Surg Treat Res. 2014;86:302–308. doi: 10.4174/astr.2014.86.6.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M, Li K, Maskey N, Xu Z, Peng C, Wang B, Li Y, Yang G. Decreased intratumoral Foxp3 Tregs and increased dendritic cell density by neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. 2014;7:4685–4694. [PMC free article] [PubMed] [Google Scholar]
- 20.Maskey N, Li K, Hu M, Xu Z, Peng C, Yu F, Cao H, Chen J, Li Y, Yang G. Impact of neoadjuvant chemotherapy on lymphocytes and co-inhibitory B7-H4 molecule in gastric cancer: low B7-H4 expression associates with favorable prognosis. Tumour Biol. 2014;35:11837–11843. doi: 10.1007/s13277-014-2410-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Wang X, Lu Y, Han S, Zhang F, Zhai H, Lei T, Liang J, Wang J, Wu K, Fan D. Expression of 15-PGDH is downregulated by COX-2 in gastric cancer. Carcinogenesis. 2008;29:1219–1227. doi: 10.1093/carcin/bgm297. [DOI] [PubMed] [Google Scholar]
- 22.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 23.Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr Pharm Des. 2006;12:955–962. doi: 10.2174/138161206776055958. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Xie J, Cheng L, Chang B, Wang Y, Lan X, Zhang D, Yin Y, Cheng N. Suppression of invasive properties of colorectal carcinoma SW480 cells by 15-hydroxyprostaglandin dehydrogenase gene. Cancer Invest. 2008;26:905–912. doi: 10.1080/07357900802146154. [DOI] [PubMed] [Google Scholar]
- 25.Huang G, Eisenberg R, Yan M, Monti S, Lawrence E, Fu P, Walbroehl J, Löwenberg E, Golub T, Merchan J, Tenen DG, Markowitz SD, Halmos B. 15-Hydroxyprostaglandin dehydrogenase is a target of hepatocyte nuclear factor 3beta and a tumor suppressor in lung cancer. Cancer Res. 2008;68:5040–5048. doi: 10.1158/0008-5472.CAN-07-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Na HK, Park JM, Lee HG, Lee HN, Myung SJ, Surh YJ. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem Pharmacol. 2011;82:1352–1360. doi: 10.1016/j.bcp.2011.08.005. [DOI] [PubMed] [Google Scholar]


