Abstract
To investigate the expression of homeobox B (Hoxb)-13 and analyze its relationship with tumor angiogenesis, epithelial-mesenchymal transition (EMT)-associated markers (E-cadherin and vimentin), clinicopathologic data and prognosis in pancreatic carcinoma. Immunohistochemistry was applied to determine the level of Hoxb-13 expression in tumor tissues and surrounding non-tumor tissues from 85 subjects with pancreatic carcinoma. Besides, vascular endothelial growth factor (VEGF), CD31, E-cadherin and vimentin were also detected in tumor tissues by immunostaining. We found that the level of Hoxb-13 expression was significantly higher in pancreatic carcinoma tissues than in paracarcinomatous tissues (P < 0.05). Hoxb-13 staining was positively correlated with VEGF (r = 0.429, P < 0.001) and microvessel density (MVD) (r = 0.454, P < 0.001). Likewise, Hoxb-13 staining was positively correlated with vimentin (r = 0.448, P < 0.001); while it was negatively correlated with E-cadherin (r = -0.405, P < 0.001). High Hoxb-13 expression was associated with aggressive clinicopathological characteristics, worse disease-free survival (DFS) (P < 0.001) and worse overall survival (OS) (P < 0.001). Multivariate analysis showed that Hoxb-13 was an independent predictor for poor DFS (P < 0.001) and OS (P = 0.002). In conclusion, our data show that overexpressed Hoxb-13 is correlated with tumor angiogenesis, aberrant expression of EMT-associated markers and aggressive clinicopathological characteristics, and serves as a promising marker for unfavourable prognosis in pancreatic carcinoma.
Keywords: Homeobox, angiogenesis, epithelial-mesenchymal transition, prognosis, pancreatic carcinoma
Introduction
Homeobox (Hox) gene family encodes a variety of homeodomain-containing transcription factors which perform important roles in embryogenesis during development via regulation of cell proliferation and differentiation [1]. 39 Hox genes of mammals have been confirmed and they consisted of 4 paralogous clusters (Hoxa, Hoxb, Hoxc and Hoxd) on autosomal chromosomes [2]. Expression of Hox genes has been observed in sorts of adult tissues of humans, where they play important roles in maintaining homeostasis [3]. Interestingly, aberrant expression of Hox gene family has been suggested in hematologic malignancies and several solid tumors, including pancreatic carcinoma [4-6]. Homeobox B (Hoxb)-13 is a crucial regulator of cellular differentiation and organogenesis during embryonic development; its gene is an important member of Hox gene family, which locates in chromosomal region 17q21.3, approximately 70 kb upstream of the Hoxb gene cluster [7]. There are reports that the Hoxb-13 gene is implicated in the risk, development and biology of tumor [8,9]. Some studies have described that Hoxb-13 is overexpressed in various malignancies such as prostate carcinoma [10-12], hepatocellular carcinoma [13], female genital organ tumor [14-18] and mammary cancer [19,20], indicating it plays a vital role in oncogenesis and progression of tumor. Nevertheless, the Hoxb-13 may act as a tumor inhibitor in colorectal neoplasms [21,22], melanocarcinoma [23], and so forth; suggesting its gene inactivation may contribute to tumorigenesis.
Pancreatic carcinoma is one of the most lethal solid malignant tumors and the fourth leading cause of cancer-related deaths in the United States [24]. Due to the aggressive features and rapid progression of the disease, the patients show a gloomy prognosis with an average 5-year survival rate of below 5% [25,26]. Operation remains the only effective means for cure. Unfortunately, the majority of patients are advanced stage at the first time of detection; any current treatment is noneffective or unfeasible due to local aggression and distant metastasis. Therefore, seek sensitive and specific biomarkers are useful for diagnosing the early stage of pancreatic carcinoma and predicting an unfavourable prognosis is of primary importance. Although some studies have described Hoxb-13 as a promising biomarker for tumor growth, invasiveness and metastasis in some types of human malignancies, there is no report about the expression of Hoxb-13 in pancreatic carcinoma and its potential clinical value.
Hence, in order to elucidate the level of Hoxb-13 expression and its relationship with tumor angiogenesis, tumor angiogenesis, epithelial-mesenchymal transition (EMT)-associated markers in pancreatic carcinoma, immunostaining of Hoxb-13, vascular endothelial growth factor (VEGF), CD31, E-cadherin and vimentin were performed; and the correlations of Hoxb-13 staining with VEGF, microvessel density (MVD), E-cadherin and vimentin were evaluated. Additionally, the relations of Hoxb-13 expression with clinicopathologic features and prognosis of the patients were also analyzed in this work.
Materials and methods
Specimens and clinicopathological data
We collected 85 patients who had undergone curative resection between 2007 and 2011 at the Affiliated Provincial Hospital of Anhui Medical University. Those patients do not receive any anticancer treatment before surgery, and the diagnosis of pancreatic carcinoma was confirmed by postoperative pathology. Tumor tissues were obtained from each patient; it is difficult to obtain normal pancreatic specimens from healthy subjects because they cannot undergo surgical operation, the corresponding paracarcinomatous tissues were thus obtained from each patient. Informed consent was obtained from all patients, and the study was carried out with the Helsinki Declaration and approval of the Human Research Ethics Committee.
The clinicopathologic data were collected by retrospective medical records, which consisted of age, gender, tumor size, tumor location, histological grade, perineural invasion, lymph node metastasis (LNM), distant metastasis, tumor-node-metastasis (TNM) stage and preoperative serum carbohydrate antigen19-9 (CA19-9) concentrations. The 85 patients included 45 males and 40 females with a mean age of 54.5 years (range 35-75 years). Tumor histological grade was defined according to the WHO classification [27]; and tumor stage was performed according to the International Union against Cancer (UICC) tumor-node-metastasis (TNM) classification [28]. Survival data including disease-free survival (DFS) and overall survival (OS) were obtained from all patients. Follow-up data were available and the mean follow-up period was 14 months (range 3-30 months).
Immunohistochemical staining
Specimens were fixed in 10% formaldehyde, embedded in paraffin wax and cut into 4-μm serial sections. Slides were deparaffinized in xylene, rehydrated in graded alcohol (100%, 95% and 75%, respectively) and washed with phosphate buffered saline (PBS). Antigen retrieval was implemented using microwave heating method in 0.01 mol/L citrate buffer (pH 6.0) for 20 minutes in afterwards. Then, endogenous peroxidase activity was quenched in 3% hydrogen peroxide. Next, sections were separately incubated with primary polyclonal rabbit antibody against Hoxb-13 (Zhongshan Jinqiao Co., Beijing, China), VEGF (Beijing Biosynthesis Biotechnology, Beijing, China), CD31 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or vimentin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The sections were incubated with biotinylated secondary antibody (mouse IgG; Zhongshan Jinqiao Co., Beijing, China) for 10 minutes at 37°C after washing. Subsequently, immunostaining was visualized with 0.6 mg/mL 3, 3-diaminobenzidine substrate. Finally, after counterstaining with hematoxylin, the sections were successively dehydrated and mounted. The negative controls were performed by omitting the primary antibodies.
Semi-quantitative assessment
Immunohistochemical results of Hoxb-13, VEGF, E-cadherin and vimentin were semi-quantitatively assessed according to the percentage of stained cells (range, 0-3; < 10% score 1, 10-50% score 2, > 50% score 3) and intensity of staining (range, 0-3; 0 absent, 1 weak, 2 moderate, 3 strong). A final total score ≤ 3 was considered to be a low expression, and > 3 was defined as a high expression. Measurement of MVD was conducted after staining for CD31. The microvessels were calculated applying 3 to 6 fields (×200); then the maximum value of microvessels was ascertained (×400) in each tissue section. All results were assessed by two well-practiced and experienced pathologists who without knowing clinicopathologic data and the results of each other. A consensus was reached by common views for all differences.
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD). The immunohistochemical results were analyzed using chi-square test. The relationships between Hoxb-13 expression status and clinicopathological parameters of the patients were assessed by the chi-square test or Fisher’s exact test. The correlations of Hoxb-13 with VEGF, MVD, E-cadherin and vimentin were assessed applying the chi-square test and Spearman’s correlation test. Kaplan-Meier survival analysis and the log-rank test were applied to evaluate the relationship between the level of Hoxb-13 expression and survival time of the patients. Multivariate analysis was performed using the Cox proportional hazards regression model to determine the independent predictors that were significant in univariate analysis. All Statistical analyses were performed applying SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA), and a value of P < 0.05 was considered statistically significant.
Results
Hoxb-13 expression in pancreatic carcinoma tissues and paracarcinomatous tissues
Immunostaining was applied to measure the expression of Hoxb-13 in pancreatic carcinoma tissues and paracarcinomatous tissues from the 85 patients. The results indicated that Hoxb-13 expression was significant higher (P = 0.027) in carcinoma specimens (58/85, 68.2%) than in paracarcinomatous specimens (19/85, 22.4%). Representative photographs for immunostaining are shown in Figure 1A, 1B.
Figure 1.
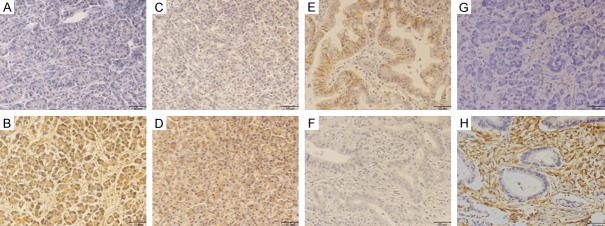
Immunohistochemical staining for Hoxb-13, VEGF, E-cadherin and vimentin. The Hoxb-13 and VEGF were principally localised in cytoplasm of tumor cells with varying staining intensity and percentage; the E-cadherin localised in cytomembrane of cells; the vimentin mainly located in cytoplasm and cytomembrane of interstitial fibroblasts. A: Negative Hoxb-13 expression. B: Positive Hoxb-13 expression. C: Negative VEGF expression. D: Positive VEGF expression. E: Positive E-cadherin expression. F: Negative E-cadherin expression. G: Negative vimentin expression. H: Positive vimentin expression. All images were taken at 200× magnification.
Relationship of Hoxb-13 with VEGF, E-cadherin and vimentin in pancreatic carcinoma tissues
High VEGF expression was found in 64 of 85 carcinoma samples (75.3%; Figure 1C, 1D). Spearman’s rank correlation test showed a significantly positive association between Hoxb-13 and VEGF expression in carcinoma samples (r = 0.429, P < 0.001; Table 1).
Table 1.
Correlations of Hoxb-13 expression with VEGF, MVD, E-cadherin and vimentin in pancreatic carcinoma tissues
| Immunoreactivity | Hoxb-13 expression | ||||
|---|---|---|---|---|---|
|
| |||||
| Low | High | r | p-value | ||
| VEGF expression | Low | 14 | 7 | 0.429 | < 0.001 |
| High | 13 | 51 | |||
| MVD value | Low | 21 | 17 | 0.454 | < 0.001 |
| High | 6 | 41 | |||
| E-cadherin expression | Low | 13 | 50 | -0.405 | < 0.001 |
| High | 14 | 8 | |||
| Vimentin expression | Low | 18 | 9 | 0.448 | < 0.001 |
| High | 12 | 46 | |||
Homeobox, Hoxb; VEGF, vascular endothelial growth factor; MVD, microvessel density. The MVD was calculated after staining for CD31.The MVD was categorized as either low or high based on the median value of 15.
In contrast, high E-cadherin expression was only detected in 22 of 85 carcinoma specimens (25.9%; Figure 1E, 1F). Spearman’s rank correlation test showed a strongly negative correlation between Hoxb-13 and E-cadherin expression in carcinoma specimens (r = -0.405, P < 0.001; Table 1).
It is similar to the VEGF in the levels of expression, high vimentin expression was found in 58 of 85 carcinoma tissues (68.2%; Figure 1G, 1H). Spearman’s rank correlation test showed a strongly positive correlation between Hoxb-13 and vimentin expression in carcinoma tissues (r = 0.448, P < 0.001; Table 1).
Association between Hoxb-13 expression and MVD in pancreatic carcinoma tissues
CD31 staining (Figure 2) was performed to calculate the MVD value and evaluate the association between Hoxb-13 and MVD in pancreatic carcinoma tissues. The mean MVD was 15.5 per field (median, 15; ranged, 3 to 37 per field). The high Hoxb-13 expression had a significantly greater MVD value (17.2±6.1 vs. 11.7±4.4; P < 0.001) than low Hoxb-13 in tumor tissues. The MVD ≥ the median value 15 was considered as high MVD, while the MVD < 15 which was regarded as low MVD. Spearman’s rank correlation test also revealed that Hoxb-13 expression was positively associated with MVD (r = 0.454, P < 0.001; Table 1).
Figure 2.
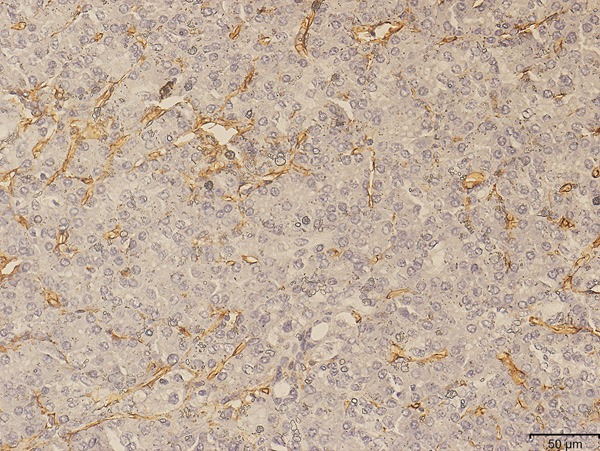
Representative section of MVD in pancreatic carcinoma tissues by CD31 staining (×200).
Correlation of Hoxb-13 expression with clinicopathological parameters
As shown in Table 2, Hoxb-13 expression was significantly correlated with histological grade (P < 0.001), perineural invasion (P < 0.001), LNM (P < 0.001), distant metastasis (P < 0.001), TNM stage (P < 0.001) and preoperative serum CA19-9 (P < 0.001). However, there was no significant association between Hoxb-13 expression and other parameters including age, gender, tumor size, tumor location.
Table 2.
Correlations between Hoxb-13 expression and clinicopathological characteristics
| Clinicopathological characteristics | Hoxb-13 expression | ||||
|---|---|---|---|---|---|
|
|
|||||
| NO. of cases | Low | High | p-value | ||
| Age (years) | < 60 | 38 | 11 | 27 | 0.616 |
| ≥ 60 | 47 | 16 | 31 | ||
| Gender | Male | 45 | 13 | 32 | 0.546 |
| Female | 40 | 14 | 26 | ||
| Tumor diameter | < 20 mm | 36 | 11 | 25 | 0.837 |
| ≥ 20 mm | 49 | 16 | 33 | ||
| Tumor location | Head | 40 | 11 | 29 | 0.426 |
| Body/tail | 45 | 16 | 29 | ||
| Histological grade | Mod-poor | 53 | 3 | 50 | < 0.001 |
| Well | 32 | 24 | 8 | ||
| Perineural invasion | Absent | 29 | 21 | 8 | < 0.001 |
| Present | 56 | 6 | 50 | ||
| LNM | Absent | 28 | 19 | 9 | < 0.001 |
| Present | 57 | 8 | 47 | ||
| Distant metastasis | Absent | 35 | 21 | 14 | < 0.001 |
| Present | 50 | 6 | 44 | ||
| TNM stage | I-II | 25 | 16 | 9 | < 0.001 |
| III-IV | 60 | 11 | 49 | ||
| Serum CA19-9 | ≤ 37 U/ml | 27 | 16 | 11 | < 0.001 |
| > 37 U/ml | 58 | 11 | 47 | ||
Homeobox, Hoxb; LNM, lymph node metastasis; TNM, tumor-node-metastasis; CA19-9, carbohydrate antigen19-9.
Survival analyses
The survival curves (Figure 3) were constructed using Kaplan-Meier method to compare the DFS and OS between high Hoxb-13 expression and low Hoxb-13 expression in the patients. The log-rank test showed that the patients with high Hoxb-13 expression (7.0 months) had a significantly decreased DFS compared to that with low Hoxb-13 expression (18.0 months; P < 0.001). Likewise, high Hoxb-13 expression (11.0 months) had a shorter OS than low Hoxb-13 expression (28.0 months; P < 0.001) in patients with pancreatic carcinoma.
Figure 3.
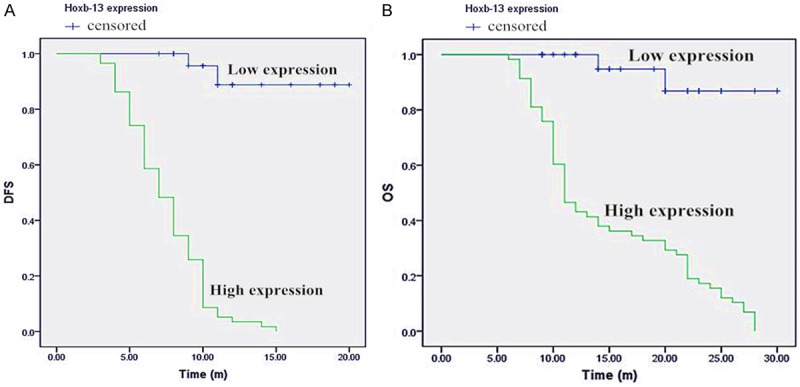
Kaplan-Meier analysis of disease-free survival (DFS) and overall survival (OS) in patients with pancreatic carcinoma according to Hoxb-13 staining. The patients with high Hoxb-13 expression had a shorter DFS (A) and OS (B) than those with low Hoxb-13 expression (n = 85; P < 0.001; log rank test).
Univariate analysis suggested that Hoxb-13 expression, histological grade, perineural invasion, LNM, distant metastasis, TNM stage and preoperative serum CA19-9 had significantly prognostic influence on DFS and OS (Table 3). Multivariate survival analysis revealed that Hoxb-13 expression as an independent predictive factor for DFS [hazard ratio (HR) 18.221; 95% confidence interval (CI) 4.269-82.543; P < 0.001] and OS (HR 9.858; 95% CI 2.248-43.225; P = 0.002). Besides, histological grade and serum CA19-9 were also independent predictive factors for prognosis (Table 4).
Table 3.
Univariate analysis of factors associated with DFS and OS
| Variables | DFS | OS | |||
|---|---|---|---|---|---|
|
|
|
||||
| Median survival time (m) | p-value | Median survival time (m) | p-value | ||
| Hoxb-13 expression | Low | 18 | < 0.001 | 28 | < 0.001 |
| High | 7 | 11 | |||
| Age (years) | < 60 | 9 | 0.462 | 11 | 0.309 |
| ≥ 60 | 10 | 20 | |||
| Gender | Male | 9 | 0.475 | 15 | 0.496 |
| Female | 9 | 20 | |||
| Tumor diameter | < 20 mm | 9 | 0.982 | 21 | 0.574 |
| ≥ 20 mm | 9 | 14 | |||
| Tumor location | Head | 9 | 0.853 | 20 | 0.528 |
| Body/tail | 9 | 15 | |||
| Histological grade | Mod-poor | 7 | < 0.001 | 11 | < 0.001 |
| Well | 16 | 27 | |||
| Perineural invasion | Absent | 15 | < 0.001 | 28 | < 0.001 |
| Present | 8 | 11 | |||
| LNM | Absent | 15 | < 0.001 | 24 | 0.005 |
| Present | 8 | 11 | |||
| Distant metastasis | Absent | 15 | < 0.001 | 25 | < 0.001 |
| Present | 7 | 11 | |||
| TNM stage | I-II | 14 | < 0.001 | 25 | 0.001 |
| III-IV | 8 | 13 | |||
| Serum CA19-9 | ≤ 37 U/ml | 11 | 0.018 | 24 | 0.050 |
| > 37 U/ml | 8 | 14 | |||
Homeobox, Hoxb; LNM, lymph node metastasis; TNM, tumor-node-metastasis; CA19-9, carbohydrate antigen19-9; DFS, disease-free survival; OS, overall survival.
Table 4.
Multivariate analysis of factors associated with DFS and OS
| Variables | DFS | OS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Hoxb-13 expression (low vs. high) | 18.221 | 4.269-82.543 | < 0.001 | 9.858 | 2.248-43.225 | 0.002 |
| Histological grade (mod-poor vs. well) | 0.226 | 0.091-0.560 | 0.001 | 0.441 | 0.201-0.968 | 0.041 |
| Serum CA19-9 (≤ 37 U/ml vs. > 37 U/ml) | 0.435 | 0.207-0.914 | 0.028 | 0.627 | 0.295-1.331 | 0.224 |
Homeobox, Hoxb; CA19-9, carbohydrate antigen19-9; DFS, disease-free survival; OS, overall survival; HR, hazard rate; CI, confidence interval.
Discussion
The present study first demonstrated the expression and clinical significances of Hoxb-13 in pancreatic carcinoma. The Hoxb-13 was overexpressed in pancreatic carcinoma tissues compared with paracarcinomatous tissues. Statistical analysis suggested that the levels of Hoxb-13 expression were dramatically higher in tumors with aggressive clinicopathological characteristics such as poor histological grade, perineural invasion, LNM, distant metastasis, advanced TNM stage and higher preoperative serum CA19-9 levels. Besides, Hoxb-13 expression was positively correlated with VEGF and MVD. Likewise, Hoxb-13 expression was positively correlated with vimentin; while it was negatively correlated with E-cadherin. Furthermore, overexpressed Hoxb-13 was correlated with worse prognosis, as an independent predictor for poor prognosis in patients with pancreatic carcinoma.
There is already mounting evidence that upregulated Hoxb-13 plays a pivotal role in tumorigenesis, infiltration, migration and apoptosis. Kim et al. [9] suggested that Hoxb-13 enhances the ability of invasion and metastasis via reducing intracellular zinc levels and increasing nuclear factor kappa B (NF-κB) signals in prostatic carcinoma. Miao et al. [16] reported that Hoxb-13 collaborates with activated ras to markedly promote ovarian carcinoma growth in vivo and confers resistance to tamoxifen-mediated apoptosis. Nevertheless, Jung et al. [21] suggested that the expression of Hoxb-13 is diminished in colorectal carcinoma and downregulated mechanism is mediated through the regulation of T-cell factor-4 (TCF4) protein stability. These inconsistent findings indicated that Hoxb-13 may serve a dual function according to the different types of tumors. For the current study, our data showed that level of Hoxb-13 expression was significantly higher in carcinoma tissues than in paracarcinomatous tissues. Moreover, we found that the levels of Hoxb-13 expression were significantly higher in tumor specimens with aggressive clinicopathologic features. These findings collectively indicate that high Hoxb-13 expression plays an important role in the development of pancreatic carcinoma.
Angiogenesis is considered to be closely associated with tumor growth, invasion, metastasis and decreased survival, including pancreatic carcinoma [29,30]. VEGF is one of the important angiogenic factors, which plays a prominent role in both physiological and pathological angiogenesis, regulates and maintains the tumor angiogenesis [31]. Certainly, the role of VEGF in tumor angiogenesis has been shown [32-34]. For instance, Mukozu et al. [32] suggested that the levels of serum VEGF might be a useful indicator of hepatocellular carcinoma in cirrhosis patients with background of hepatitis C virus, while serum VEGF levels could predict respectively the tumor type and vascular invasion in hepatocellular carcinoma. Yu et al. [33] showed that VEGF has a stimulative effect on angiogenesis of gastric cancer tissue and plays a significant part in tumor generation and metastasis. Moreover, Yao et al. [34] demonstrated that the VEGF and MVD were useful predictors for vascular invasion and metastasis of hepatocellular carcinoma. These showed that the VEGF and MVD can be used as predictive factors for tumor angiogenesis and progression. Therefore, we quantified the levels of VEGF and MVD in pancreatic carcinoma tissues to estimate whether there is an association between overexpressed Hoxb-13 and tumor angiogenesis. We found that tumors with high Hoxb-13 expression had higher MVD than those with low Hoxb-13. Spearman’s rank correlation test showed that Hoxb-13 expression was significantly and positively correlated with VEGF and MVD. These results suggested that Hoxb-13 plays a crucial role in tumor progression, and might be implicated in VEGF-mediated tumor angiogenesis in pancreatic carcinoma.
E-cadherin is a member of the epithelial cadherin family of glycoproteins which involved in Ca2+-dependent cells adhesion and assembly of different or identical cell types during tissue construction of organ and morphogenesis [35]. Decreasing E-cadherin promotes EMT event is well recognized in many solid tumors, including pancreatic carcinoma [36]. Vimentin is a major intermediate filament protein of mesenchymal cells, which plays a crucial role in the development, invasion and metastasis in tumor; and increasing expression of vimentin contributes to EMT event has also been showed in pancreatic carcinoma [37]. During EMT, tumor cells lose own epithelial traits and acquire characteristics of mesenchymal cells so that malignancy can rapidly progress [38]. Thus, EMT is regarded as a significant event in process of tumor progression to invasive, metastatic carcinoma. Excitingly, in this work, Spearman’s rank correlation test suggested a negative correlation between Hoxb-13 and E-cadherin expression. Additionally, Spearman’s rank correlation test showed a strongly positive correlation between Hoxb-13 and vimentin expression. Therefore, up-regulated Hoxb-13 is closely linked to abnormal expression of EMT-associated markers; which indirectly shows that the Hoxb-13 plays a crucial role in the invasiveness and metastasis of pancreatic carcinoma, and may be involved in E-cadherin-mediated or vimentin-mediated EMT event.
With respect to prognosis, our findings showed that patients with high Hoxb-13 expression had a significantly reduced survival time relative to patients with low Hoxb-13 expression. Furthermore, multivariate survival analysis indicated that high Hoxb-13 expression was an independent predictor of poor prognosis. The results were consistent with a previous report that the high Hoxb-13 expression showed a shorter survival than low Hoxb-13 and was an independent factor of poor prognosis in patients with hepatocellular carcinoma [13]. Therefore, these findings suggested that high Hoxb-13 expression had an adverse effect on outcome of patients with pancreatic carcinoma, and may be used for a valuable prognostic biomarker.
We have to acknowledge the limitations of the study. It is a typical single-centre study with the major shortcoming that is a relatively small sample size, and has a retrospective design with a potential selection bias. In addition, this work lacks the performance of other well-designed methods such as gene transfection, gene knock-out, cell invasion assay, cell migration assay, etc. Therefore, more randomized and multicentric prospective studies with a larger cohort of participants and more elaborate techniques are needed to support our findings.
In conclusion, our findings suggest that Hoxb-13 is overexpressed in pancreatic carcinoma tissues. Overexpressed Hoxb-13 is correlated with tumor angiogenesis, aberrant expression of EMT-associated markers and aggressive clinicopathological characteristics, and serves as an independent prognostic biomarker for poor prognosis in pancreatic carcinoma. These findings contribute to provide a new strategy for the treatment of pancreatic carcinoma and thereby further prolong survival time of the patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81272740).
Disclosure of conflict of interest
None.
References
- 1.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 2.Scott MP. A rational nomenclature for vertebrate homeobox (HOX) genes. Nucleic Acids Res. 1993;21:1687–1688. doi: 10.1093/nar/21.8.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- 4.Maroulakou IG, Spyropoulos DD. The study of HOX gene function in hematopoietic, breast and lung carcinogenesis. Anticancer Res. 2003;23:2101–2110. [PubMed] [Google Scholar]
- 5.McGonigle GJ, Lappin TR, Thompson A. Grappling with the HOX network in hematopoiesis and leukemia. Front Biosci. 2008;13:4297–4308. doi: 10.2741/3006. [DOI] [PubMed] [Google Scholar]
- 6.Gray S, Pandha HS, Michael A, Middleton G, Morgan R. HOX genes in pancreatic development and cancer. JOP. 2011;12:216–219. [PubMed] [Google Scholar]
- 7.Zeltser L, Desplan C, Heintz N. Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development. 1996;122:2475–2484. doi: 10.1242/dev.122.8.2475. [DOI] [PubMed] [Google Scholar]
- 8.Decker B, Ostrander EA. Dysregulation of the homeobox transcription factor gene HOXB13: role in prostate cancer. Pharmgenomics Pers Med. 2014;7:193–201. doi: 10.2147/PGPM.S38117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YR, Kim IJ, Kang TW, Choi C, Kim KK, Kim MS, Nam KI, Jung C. HOXB13 downregulates intracellular zinc and increases NF-κB signaling to promote prostate cancer metastasis. Oncogene. 2014;33:4558–4567. doi: 10.1038/onc.2013.404. [DOI] [PubMed] [Google Scholar]
- 10.Kim YR, Oh KJ, Park RY, Xuan NT, Kang TW, Kwon DD, Choi C, Kim MS, Nam KI, Ahn KY, Jung C. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer. 2010;9:124–137. doi: 10.1186/1476-4598-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong TO, Oh KJ, Xuan NN, Kim YR, Kim MS, Lee SD, Ryu SB, Jung C. Evaluation of HOXB13 as a molecular marker of recurrent prostate cancer. Mol Med Rep. 2012;5:901–904. doi: 10.3892/mmr.2012.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varinot J, Cussenot O, Roupret M, Conort P, Bitker MO, Chartier-Kastler E, Cheng L, Compérat E. HOXB13 is a sensitive and specific marker of prostate cells, useful in distinguishing between carcinomas of prostatic and urothelial origin. Virchows Arch. 2013;463:803–809. doi: 10.1007/s00428-013-1495-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JY, Sun QK, Wang W, Jia WD. High-level expression of HOXB13 is closely associated with tumor angiogenesis and poor prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:2925–2933. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Yamashita T, Ishikawa M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol Rep. 2005;13:721–726. [PubMed] [Google Scholar]
- 15.Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, Yokohama Y, Ishikawa M. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol. 2006;28:931–938. [PubMed] [Google Scholar]
- 16.Miao J, Wang Z, Provencher H, Muir B, Dahiya S, Carney E, Leong CO, Sgroi DC, Orsulic S. HOXB13 promotes ovarian cancer progression. Proc Natl Acad Sci U S A. 2007;104:17093–17098. doi: 10.1073/pnas.0707938104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez R, Garrido E, Pina P, Hidalgo A, Lazos M, Ochoa R, Salcedo M. HOXB homeobox gene expression in cervical carcinoma. Int J Gynecol Cancer. 2006;16:329–335. doi: 10.1111/j.1525-1438.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 18.Lopez R, Garrido E, Vazquez G, Pina P, Perez C, Alvarado I, Salcedo M. A subgroup of HOX Abd-B gene is differentially expressed in cervical cancer. Int J Gynecol Cancer. 2006;16:1289–1296. doi: 10.1111/j.1525-1438.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Cantile M, Pettinato G, Procino A, Feliciello I, Cindolo L, Cillo C. In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer. 2003;39:257–264. doi: 10.1016/s0959-8049(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Shah N, Jin K, Cruz LA, Park S, Sadik H, Cho S, Goswami CP, Nakshatri H, Gupta R, Chang HY, Zhang Z, Cimino-Mathews A, Cope L, Umbricht C, Sukumar S. HOXB13 mediates tamoxifen resistance and invasiveness in human breast cancer by suppressing ERalpha and inducing IL-6 expression. Cancer Res. 2013;73:5449–5458. doi: 10.1158/0008-5472.CAN-13-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung C, Kim RS, Zhang H, Lee SJ, Sheng H, Loehrer PJ, Gardner TA, Jeng MH, Kao C. HOXB13 is downregulated in colorectal cancer to confer TCF4-mediated transactivation. Br J Cancer. 2005;92:2233–2239. doi: 10.1038/sj.bjc.6602631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuda H, Toyota M, Ishida W, Furihata M, Tsuchiya M, Kamada M, Tokino T, Shuin T. Epigenetic inactivation of the candidate tumor suppressor gene HOXB13 in human renal cell carcinoma. Oncogene. 2006;25:1733–1742. doi: 10.1038/sj.onc.1209200. [DOI] [PubMed] [Google Scholar]
- 23.Muthusamy V, Duraisamy S, Bradbury CM, Hobbs C, Curley DP, Nelson B, Bosenberg M. Epigenetic silencing of novel tumor suppressors in malignant melanoma. Cancer Res. 2006;66:11187–11193. doi: 10.1158/0008-5472.CAN-06-1274. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 26.Gong ZH, Holly EA, Bracci PM. Survival in Population-based Pancreatic Cancer Patients: San Francisco Bay Area, 1995-1999. Am J Epidemiol. 2011;174:1373–1381. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klöppel G, Lüttges J. WHO-classification 2000: exocrine pancreatic tumors. Verh Dtsch Ges Pathol. 2001;85:219–228. [PubMed] [Google Scholar]
- 28.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Benckert C, Thelen A, Cramer T, Weichert W, Gaebelein G, Gessner R, Jonas S. Impact of microvessel density on lymph node metastasis and survival after curative resection of pancreatic cancer. Surg Today. 2012;42:169–176. doi: 10.1007/s00595-011-0045-0. [DOI] [PubMed] [Google Scholar]
- 30.Hoem D, Straume O, Immervoll H, Akslen LA, Molven A. Vascular proliferation is associated with survival in pancreatic ductal adenocarcinoma. APMIS. 2013;121:1037–1046. doi: 10.1111/apm.12057. [DOI] [PubMed] [Google Scholar]
- 31.Hoeben A, Landuyt B, Highley MS. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 32.Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013–1021. [PubMed] [Google Scholar]
- 33.Yu YF, Zhang Y, Shen N, Zhang RY, Lu XQ. Effect of VEGF, P53 and telomerase on angiogenesis of gastric carcinoma tissue. Asian Pac J Trop Med. 2014;7:293–296. doi: 10.1016/S1995-7645(14)60041-9. [DOI] [PubMed] [Google Scholar]
- 34.Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220–226. [PubMed] [Google Scholar]
- 35.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 36.Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed) 2012;17:2059–2069. doi: 10.2741/4037. [DOI] [PubMed] [Google Scholar]
- 37.Handra-Luca A, Hong SM, Walter K, Wolfgang C, Hruban R, Goggins M. Tumour epithelial vimentin expression and outcome of pancreatic ductal adenocarcinomas. Br J Cancer. 2011;104:1296–1302. doi: 10.1038/bjc.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier HJ, Wirth T, Beug H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers (Basel) 2010;2:2058–2083. doi: 10.3390/cancers2042058. [DOI] [PMC free article] [PubMed] [Google Scholar]


