Abstract
Nasopharyngeal cancer (NPC) is a tumor of epithelial origin with complex etiology. Currently the standard treatment of NPC is radiotherapy, but therapy failure is quite common, making radioresistance an important issue. This study explores the association of specificity protein 1 (Sp1) protein expression with clinicopathological significance and disease prognosis in NPC patients receiving radiotherapy. A total of 82 NPC patients (55 males and 27 females, median age: 48 years old) were enrolled and received radiotherapy between September 2011 and March 2014. Tumor tissue and grossly adjacent normal mucosa were obtained in each patient. Sp1 expression was detected by western blot and immunohistochemical analysis, and the associations with clinicopathological status and radiotherapy response were analyzed. Our Results showed Sp1 protein expression was higher in CNE-1 and CNE-2 nasopharyngeal cancer cells than in normal nasopharyngeal mucosal NP69 cells. All 82 patients’ tissue sections were stained positive for the Sp1 protein, and 39 (47.6%) patients showed higher level than adjacent normal mucosa. Sp1-overexpression in the tumor tissue was correlated with a higher tumor stage, nodal status, clinical stage and distant metastasis (P < 0.01). Patients with higher Sp1 expression in pretreatment biopsies had a lower radiotherapy response compared to those with lower expression. In conclusion, Sp1 may play roles in radioresistance of nasopharyngeal cancer which attributes to tumor invasiveness, and serve as a novel prognostic marker of NPC radiotherapy. However, further studies are required to validate our findings in larger samples and explore more detailed mechanisms underlying radioresistance of Sp1.
Keywords: Nasopharyngeal cancer (NPC), specificity protein 1 (Sp1), Epstein-Barr virus (EBV), prognosis, radioresista
Introduction
Nasopharyngeal cancer (NPC) is one epithelial tumor originated from pharyngeal recess of the nasopharynx, with a special world distribution and complex etiology. Epidemiological studies have shown strong associations between nasopharyngeal cancer and genetic susceptibility, Epstein-Barr virus (EBV) infection, environmental factors and dietary carcinogens [1]. More than 80% of nasopharyngeal cancer patients are from Southern China and Southeast Asia, however, this cancer is rare in America, Europe and other parts of the world [2]. The standard treatment for nasopharyngeal cancer is radiotherapy [3]. Nasopharyngeal cancer is highly radiosensitive and chemosensitive in the early stages [4]. However, it is usually resistance to these treatment modalities in the more advanced stages [5]. Therefore, in locally advanced nasopharyngeal cancer, radiotherapy is usually integrated with the chemotherapeutic drug cisplatin in combination with fluorouracil [6,7]. Although the radiotherapy technology and equipment are improved continually, the prognosis of locally advanced nasopharyngeal cancer is still very poor, with a low 5 year survival rate [8]. Radioresistance still remains the reason for treatment failure of many nasopharyngeal cancer patients. Therefore, it is very important to differentiate these radioresistant patients before therapy for judging the prognosis and optimization of individual treatment [9].
In order to better understand the mechanism of therapy resistance and prediction of radiotherapy effect, a lot of studies have been focused on the specifically expressed proteins in nasopharyngeal cancer. Epstein-Barr virus gene BHRF1 can inhibit nasopharyngeal cancer cellular apoptosis caused by radiation, contributing to fast recovery of cells after the radiation [10]. Another EB virus-encoded protein latent membrane protein 1 (LMP1) also contribute to radioresistance in nasopharyngeal cancer by suppressing the p53-mediated apoptosis pathway [11]. Leukemia inhibitory factor (LIF), whose production can be enhanced by LMP1, promotes nasopharyngeal cancer progression and radioresistance [12]. The radioresistance of nasopharyngeal cancer is also related with enhanced expression of antioxidant enzyme manganese superoxide dismutase (SOD2), 14-3-3 σ and Raf kinase inhibitory protein (RKIP) [13-15].
Specificity protein 1 (Sp1) protein is one member of transcription factor family and can bind GC/GT-rich promoter elements through its C(2)H(2)-type zinc fingers at C-terminal domains. Through either activating or repressing the activity of gene promoters, Sp1 protein regulates expression of multiple genes involved in differentiation, cell cycle and oncogenesis [16]. Growing evidence indicates that Sp1 protein play a critical role in many tumor types by regulating expression the growth and metastasis genes [17]. Over-expression of Sp1 can upregulate ABCG2 expression in lung cancer cells, which is a member of the ATP binding cassette (ABC) transmembrane proteins that plays an important role in chemotherapeutic resistance of cancer cells [18]. However, there are no investigations focused on radioresistance of Sp1 in nasopharyngeal cancer.
Hence, this study aimed to evaluate the expression of Sp1 in nasopharyngeal cancer cell lines and tissue samples at protein levels by western bolt and immunohistochemistry. We also analyzed the correlation of Sp1 protein expression with clinicopathological parameters and radiotherapy response of nasopharyngeal cancer patients.
Materials and methods
Cell culture
Nasopharyngeal cancer cell lines CNE-1 and CNE-2 were routinely maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (Hyclone), 2 mM sodium pyruvate, 2 mM penicillin-streptomycin L-glutamine (Invitrogen) and 2 mM minimum essential media nonessential amino acids solution (Invitrogen). Normal nasopharyngeal mucosal NP69 cells were cultured in RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen). All cell lines were grown in a humidified atmosphere of 5% CO2 at 37°C.
Patients and specimens
This study consisted of 82 patients with nasopharyngeal cancer (55 males and 27 females), and the age of the patients ranged from 30 to 75, with a median age of 48 years old. All these patients were diagnosed with nasopharyngeal cancer by computed tomography (CT) or magnetic resonance imaging (MRI), who underwent treatment between September 2011 and March 2014, at the Affiliated Hospital of Guangxi Medical University (Nanning, China). Distant metastases were detected by a chest X-ray, liver ultrasound and bone scan. The nasopharyngeal cancer tissue and adjacent tissue were obtained from pretreatment endoscopic biopsies of patients, and formalin-fixed, paraffin-embedded tissues were cut to acquire biopsy sections at a thickness of 4 mm and mounted on 3-aminopropyl-tri-ethoxysilane (APES) slides. All 82 nasopharyngeal cancer patients were treated with radiotherapy and 67 patients received concurrent chemotherapy using the cytotoxic drugs (cisplatin and 5-fluorouracil). Complete response (CR, radiosensitive) NPC was defined as no local recurrence after radiotherapy for 2 month, with no local residual disease 6 weeks after treatment. Non-CR (radioresistant) patients was defined as lesions without complete regression of 6 weeks after treatment or longer, or with recurrence after 2 months after treatment, including partial response (PR), no change (NC) and progressive disease (PD). We acquired from all study subjects with written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the Guangxi Medical University Research Ethics Committee.
Western blot analysis
Proteins from nasopharyngeal cancer cell lines or tissues were extracted using cell lysis buffer and their concentrations were determined by bicinchoninic acid protein concentration assay kit (Beijing Biosea Biotechnology Co. Ltd., China). Proteins (50 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to PVDF membrane (Bio-Rad, Hercules, CA, USA). Then PVDF membrane was blocked with 3% BSA in Tris-buffer saline (TBS) containing 0.05% Tween 20 for 1 hour, followed by incubation with primary mouse monoclonal antibody against human Sp1 (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C overnight. After washing, the PVDF membrane was incubated with second rabbit monoclonal antibody against mouse (1:1000 dilutions) conjugated to horseradish peroxidase for 2 h at room temperature. The blots were visualized by enhanced chemiluminescence (Pierce® ECL Plus Western Blotting Substrate, Pierce Biotechnology, IL, USA). GAPDH was used as an internal control.
Immunohistochemistry analysis
Paraffin sections of nasopharyngeal cancer tissues were dewaxed in xylene and rehydrated with ascending series of ethanol. Then in order for antigen retrieval, the sections were heated by 700W microwave oven for 15 min in 10 mM citrate buffer (0.1 mM citric acid, 0.1 M sodium citrate; pH 6.0). After washing with PBSTX (0.05M PBS, 0.1% Triton-X 100), sections (5 mm in thickness) were incubated with primary antibody against human Sp1 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A) at the concentrations of 1:100 at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated IgG secondary antibody (Medical Biological Laboratory, Nagoya, Japan) at 37°C for 30 min. After incubating with complex/horseradish peroxidase (1: 200 dilution) at 37°C for 30 min, sections were visualized by immersion in diaminobenzidine solution (10 × DAB, TBS, 30% H2O2). Slides were counterstained with hematoxylin before dehydration and mounting. Slides without primary antibody incubation served as a control for the background staining. The brown positive cells in section were counted, and then converted the total counts into cell densities for quantitation.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS version 14.0). All quantitative data were expressed as mean ± SEM. Student t test (unpaired, two tailed) was performed to compare the means of 2 groups. Correlations were examined using Chi-square analysis. P < 0.05 was considered statistically different.
Results
Patient characteristics and treatment outcome
Our study included 82 patients, with median age of 48 years old (range 30~75). The patient characteristics were listed in Table 1. Pre-treatment imaging was performed using magnetic resonance imaging (MRI) (n = 42, 53%), computed tomography (CT) scan (n = 32, 41%) or both (n = 5, 6%). Radiation therapy was performed in all patients. The median follow-up time was 2.7 years (range, 1.9-3.6). At the end of the study, all patients remained alive. Fifty-eight patients acquired complete response (CR). Twenty-four patients suffered from Non-CR, including partial response (PR, n = 8), no change (NC, n = 8), or progressive disease (PD, n = 8).
Table 1.
Association between Sp1 expression and clinicopathological parameters in nasopharyngeal carcinoma patients
| Sp1 expression level | ||||
|---|---|---|---|---|
|
|
||||
| All cases | Low expression | High expression | P value | |
| Age | 0.182 | |||
| ≥ 50 yeara | 44 | 23 (53.5%) | 22 (56.4%) | |
| < 50 year | 38 | 20 (46.5%) | 17 (43.6%) | |
| Gender | 0.342 | |||
| Male | 55 | 29 (67.4%) | 23 (59.0%) | |
| Female | 27 | 14 (32.6%) | 16 (41.0%) | |
| Tumor stage | 0.001 | |||
| T1 | 12 | 9 (20.9%) | 3 (7.7%) | |
| T2 | 28 | 20 (46.5%) | 8 (20.5%) | |
| T3 | 26 | 9 (20.9%) | 17 (43.6%) | |
| T4 | 16 | 5 (11.7%) | 11 (28.2%) | |
| Nodal status | 0.008 | |||
| N0 | 22 | 16 (37.2%) | 6 (15.4%) | |
| N1 | 31 | 20 (46.5%) | 11 (28.2%) | |
| N2 | 22 | 5 (11.6%) | 17 (43.6%) | |
| N3 | 7 | 2 (4.7%) | 5 (12.8%) | |
| Clinical stage | 0.008 | |||
| I | 6 | 4 (9.3%) | 2 (5.2%) | |
| II | 24 | 19 (44.2%) | 5 (12.8%) | |
| III | 29 | 12 (27.9%) | 17 (43.6%) | |
| IV | 23 | 8 (18.6%) | 15 (38.4%) | |
| Distant metastasis | 0.008 | |||
| M0 | 63 | 38 (88.3%) | 25 (64.1%) | |
| M1 | 19 | 5 (11.7%) | 14 (35.9%) | |
Mean age;
WHO, World Health Organization; Sp1 expression was determined by immunohistochemical analysis.
Expression of Sp1 protein in vitro and in vivo by western blot analysis
In order to investigate the differential expression of Sp1 in nasopharyngeal cancer, we performed western blot analysis to determine Sp1 protein expression. CNE-1 and CNE-2 nasopharyngeal cancer cells were found to have a higher level of Sp1 protein expression as compared to normal nasopharyngeal mucosal NP69 cells (P < 0.05) (Figure 1A, 1B). We further explored the in vivo expression of Sp1 in nasopharyngeal cancer patients. Western blot was performed in tumor tissues. According to the criterion of a 1.5-fold differential expression (tumor tissue compared to the normal counterpart), there were 39 out of 82 patients (47.6%) whose tumor had Sp1 over-expression (≥ 1.5-fold), and 43 patients (52.4%) low expression (between 0.86- to 1.5-fold) (Table 1). Sp1 protein expression was determined by western blot analysis from four pairs of normal (N) and tumor (T) fresh tissues from nasopharyngeal cancer patients with Sp1 over-expression (Figure 2A, 2B).
Figure 1.
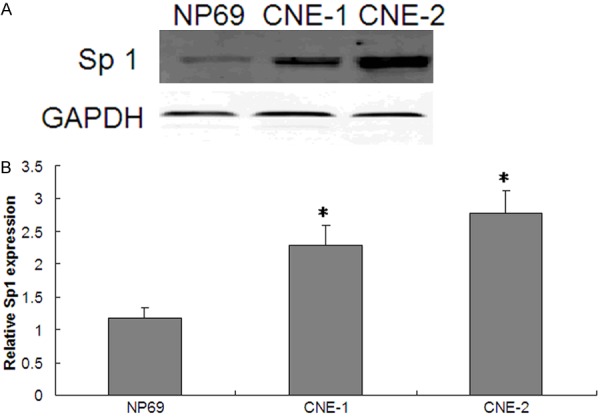
Sp1 is highly expressed in nasopharyngeal carcinoma patients than in healthy controls. A. Protein expression was determined by western blot analysis in nasopharyngeal carcinoma CNE-1 and CNE-2 cells and normal nasopharyngeal mucosal NP69 cells. GAPDH protein expression served as an internal control. Representative pictures from three independent experiments are shown. B. Relative expression of Sp1 protein. The Y ordinate indicates grey value of Sp1 normalized to that of GAPDH. Data were expressed as mean ± SD and a two-tailed, unpaired t-test was performed. Significant difference from the control group is denoted by “*” (P < 0.01). There were 30 samples in each group. The results suggest that the expression of Sp1 is upregulated in nasopharyngeal carcinoma. N = 20, *P < 0.01; NP69: normal nasopharyngeal mucosal cell; CNE-1, CNE-2: nasopharyngeal carcinoma cells.
Figure 2.
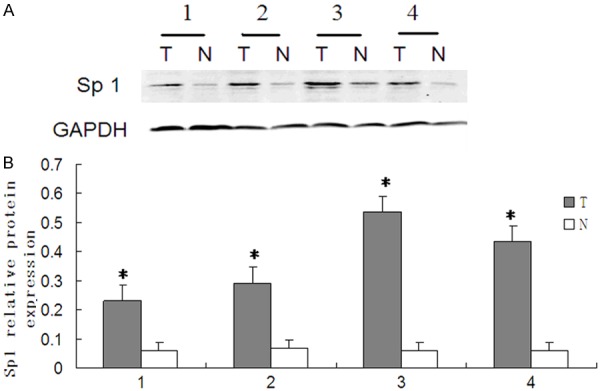
Sp1 is highly expressed in nasopharyngeal carcinoma tissue than in normal tissue adjacent to cancer. A. Protein expression was determined by western blot analysis from four nasopharyngeal carcinoma patients in cancer tissues and normal mucosal tissue adjacent to cancer, respectively. GAPDH protein expression served as an internal control. Representative pictures from three independent experiments are shown. B. Relative expression of Sp1 protein. The Y ordinate indicates grey value of Sp1 normalized to that of GAPDH. Data were expressed as mean ± SD and a two-tailed, unpaired t-test was performed. Significant difference from the control group is denoted by “*” (P < 0.01). The results suggest that the expression of Sp1 is upregulated in nasopharyngeal carcinoma tissue than in normal mucosal tissue adjacent to cancer. *P < 0.01; N, normal nasopharyngeal mucosal tissue adjacent to cancer; T, tumor tissue.
Immunohistochemical analysis of tumor samples in nasopharyngeal cancer patients
Of 82 patient tissue sections, all were immunopositive for Sp1 protein, and immunostaining was observed to be predominantly cytoplasmic and nuclear with variable staining intensity. A total of 43 (52.4%) tissues had low Sp1 expression and 39 (47.6%) with high expression (Figure 3A-C). The results of Sp1 immunohistochemical staining were in accordance with those of western blot. Therefore, we divided nasopharyngeal cancer patients into low expression group (43 cases) and high expression group (39 cases) to investigate the associations of Sp1 expression with clinicopathological parameters or radiotherapy response in nasopharyngeal cancer patients.
Figure 3.
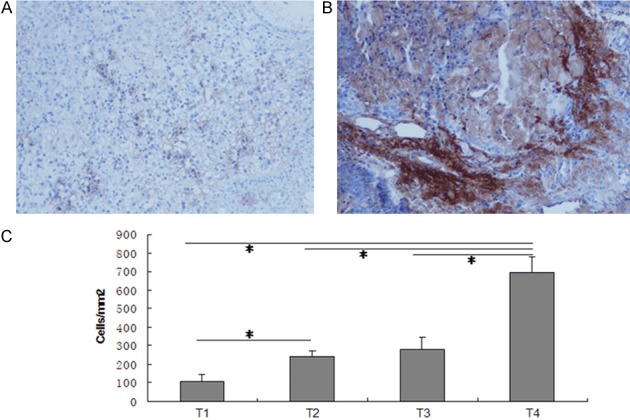
Immunohistochemical expression of Sp1 in nasopharyngeal carcinoma tissues. Various expression levels of cytoplasmic staining of Sp 1 were observed in nasopharyngeal carcinoma tissues, including low staining (A) and high staining (B) of Sp1 proteins. (C) Quantification analysis demonstrated significant difference in the quantities of Sp1 + cells in various nasopharyngeal carcinoma tissues. Data were expressed as mean ± SD. The data of each group was from three repeated experiments. A significant difference between groups is denoted by “*”. Original magnification: × 400.
Association of Sp1 immunohistochemical staining with clinicopathological parameters
The associations between Sp1 over-expression and clinicopathological parameters are summarized in Table 1. No associations were found between Sp1 over-expression and age or gender. There was significant statistical correlation between Sp1 over-expression and tumor stage, nodal status, clinical stage and distant metastasis (P < 0.05) (Table 1).
Correlation of Sp1 immunohistochemical staining with radiotherapy response
All 82 patients received radiation therapy, and fifty-eight patients acquired complete response (CR) and twenty-four patients suffered from Non-CR. Patients with higher Sp1 expression as observed in pretreatment biopsies had a lower radiotherapy response of nasopharyngeal cancer compared to those with lower expression (Table 2).
Table 2.
Association between Sp1 expression and radiation therapy response in nasopharyngeal cancer patients
| Therapy response | All cases | Sp1 low expression | Sp1 high expression | P value |
|---|---|---|---|---|
| CR | 48 | 37 (51.7%) | 11 (48.3%) | |
| Non-CR | 34 | 6 (79.2%) | 28 (20.8%) | < 0.01 |
Abbreviations: CR, complete response; Non-CR (including PR, partial response; NC, no change; PD, progressive disease.
Discussion
In this study, we used western blot method to detect the tissue sections of nasopharyngeal cancer patients, and found that the expression of Sp1 protein in cancer cell lines and tissue samples were increased compared with their normal counterparts. We further analyzed the clinicopathological significance and clinical effect of radiotherapy of Sp1 overexpression, and found that the high expression of Sp1 was related with higher tumor stage, nodal status, clinical stage and distant metastasis. Sp1 over-expression is also related with poor response of radiotherapy in nasopharyngeal cancer patients. This indicates that Sp1 protein may be one new predictive and prognostic protein for radiotherapy sensitivity in nasopharyngeal cancer.
Sp1 protein level was higher in various cancer tissues or cells than in normal tissues or cells [19-21]. Therefore, we quantitatively examined Sp1 expression levels of nasopharyngeal cancer tissues in order to determine relationship between Sp1 expression levels and various tumor stage, nodal status, clinical stage and distant metastasis of glioma tissues. In our study, higher level of Sp1 expression was related with in nasopharyngeal cancer with higher tumor stage, nodal status, clinical stage and distant metastasis (Table 1). Su et al have reported that Sp1 expression levels were elevated in metastatic NPC cell line than in non-metastatic NPC cell lines, and inhibition of Sp1 expression and activity in metastatic NPC cells resulted in reduced expression and secretion of MMP-9 and VEGF, leading to inhibition of cell migration and invasion [22]. These results suggest that MMP-9 and VEGF are target genes of Sp1 and Sp1 may promote tumor progression through MMP-9 and VEGF in the nasopharyngeal cancer patients [23]. Our study firstly investigated Sp1 expression in human nasopharyngeal cancer tissues and analyzed its expression with detailed clinicopathological parameters. Indeed, Sp1 expression plays roles in invasion, metastasis and progression of a variety of tumors, such as breast cancer and glioma [24,25].
In this study, we found the correlation between Sp1 high expression and poor radiation therapy response in nasopharyngeal cancer patients. We therefore hypothesize that Sp1 expression is strongly associated with tumor radioresistance. Nasopharyngeal cancer is initiated and promoted by EB virus [26]. Two EB virus-encoded protein genes BHRF1 and LMP1 both contribute to radioresistance in nasopharyngeal cancer [10,11]. This indicates that radioresistance phenotype may be acquired after the initiation of nasopharyngeal cancer. Indeed, radioresistance may be characteristics of cancer stem cell (CSC) and cells with radioresistance were enriched in stem cell-like population of nasopharyngeal cancer [11,27]. Over-expression of Sp1 has been found to enhance ABCG2 expression in lung cancer cells, which plays an important role in chemotherapeutic resistance of cancer cells and phenotypic characterization of cancer stem like cells of nasopharyngeal cancer [18,28]. Furthermore, silencing of Sp1 significantly inhibited clonogenicity and the stem-cell like phenotype of NPC cells, confirming the association between radioresistance and stem cell characteristics linked by Sp1 in nasopharyngeal cancer [29].
A variety of proteins have been found to be correlated with radioresistance of nasopharyngeal cancer, such as SOD2, 14-3-3 σ, RKIP, Y-Box-binding protein-1 and αV integrin [13-15,30,31]. Our finding adds a new protein to the above list. In this study, we found that Sp1-overexpression was associated with tumor progression and reduced radiosensitivity in nasopharyngeal cancer. In addition, it supports the previous report on nasopharyngeal cancer cell lines: increasing of Sp1-expression was observed in metastatic sublines. An important question for radiation oncologists is how to identify patients with locally advanced nasopharyngeal cancer who will show resistance to radiotherapy. In this regard, Sp1 may be a promising biomarker to predict radiotherapy response and assess therapeutic efficacy of nasopharyngeal cancer, thus aiding selection of individualized therapy to improve clinical outcome and reduce the risk of recurrence. The radioresistance of Sp1 may attribute to tumor invasiveness and progression in nasopharyngeal cancer patients receiving radiotherapy. Therefore, Sp1 could also be a promising target molecule for novel therapeutic strategies aimed at overcoming radioresistance of nasopharyngeal cancer. However, further studies are required to include more clinical samples to validate our findings and more detailed experiments to explore mechanisms underlying the radioresistance of Sp1 in nasopharyngeal cancer.
Disclosure of conflict of interest
None.
References
- 1.Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50:330–338. doi: 10.1016/j.oraloncology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Yu YL, Yang YY, Ji MF, Yu BH, Liang ZH, Reng X. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev. 2010;11:29–32. [PubMed] [Google Scholar]
- 3.Lee AW. Contribution of radiotherapy to function preservation and cancer outcome in primary treatment of nasopharyngeal carcinoma. World J Surg. 2003;27:838–843. doi: 10.1007/s00268-003-7109-2. [DOI] [PubMed] [Google Scholar]
- 4.Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 5.Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99:1311–1318. doi: 10.1111/j.1349-7006.2008.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AW, Yau TK, Wong DH, Chan EW, Yeung RM, Ng WT, Tong M, Soong IS, Sze WM. Treatment of stage IV(A-B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation. Int J Radiat Oncol Biol Phys. 2005;63:1331–1338. doi: 10.1016/j.ijrobp.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, Leung SF, Thephamongkhol K, Pignon JP MAC-NPC Collaborative Group. Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma. Cochrane Database Syst Rev. 2006:CD004329. doi: 10.1002/14651858.CD004329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao CN, Luo JW, Gao L, Yi JL, Huang XD, Wang K, Zhang SP, Qu Y, Li SY, Cai WM, Xiao JP, Zhang Z, Xu GZ. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for T4 nasopharyngeal carcinoma. Oral Oncol. 2013;49:175–181. doi: 10.1016/j.oraloncology.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Chen J, Guo Y, Lin H, Zhang Z, Feng G, Hao Y, Cheng J, Liang P, Chen K, Wu H, Li Y. Identification of prognostic biomarkers for response to radiotherapy by DNA microarray in nasopharyngeal carcinoma patients. Int J Oncol. 2012;40:1590–1600. doi: 10.3892/ijo.2012.1341. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Pan XH, Zhou JH, Yu L, Kong XT, Zhou SM, Li ZJ, Fu Q, Sun XY. The effect of Epstein-Barr virus gene BHRF1 expression on radioresistance of nasopharyngeal carcinoma cells. ORL J Otorhinolaryngol Relat Spec. 1998;60:329–333. doi: 10.1159/000027619. [DOI] [PubMed] [Google Scholar]
- 11.Yang CF, Peng LX, Huang TJ, Yang GD, Chu QQ, Liang YY, Cao X, Xie P, Zheng LS, Huang HB, Cai MD, Huang JL, Liu RY, Zhu ZY, Qian CN, Huang BJ. Cancer stem-like cell characteristics induced by EB virus-encoded LMP1 contribute to radioresistance in nasopharyngeal carcinoma by suppressing the p53-mediated apoptosis pathway. Cancer Lett. 2014;344:260–271. doi: 10.1016/j.canlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Liu SC, Tsang NM, Chiang WC, Chang KP, Hsueh C, Liang Y, Juang JL, Chow KP, Chang YS. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest. 2013;123:5269–5283. doi: 10.1172/JCI63428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu Y, Zhang H, Zhao S, Hong J, Tang C. The effect on radioresistance of manganese superoxide dismutase in nasopharyngeal carcinoma. Oncol Rep. 2010;23:1005–1011. doi: 10.3892/or_00000726. [DOI] [PubMed] [Google Scholar]
- 14.Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang PF, Li C, Peng F, Tang CE, Li JL, Chen ZC, Xiao ZQ. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res. 2010;70:3450–3462. doi: 10.1158/0008-5472.CAN-09-4099. [DOI] [PubMed] [Google Scholar]
- 15.Ruan L, Wang GL, Yi H, Chen Y, Tang CE, Zhang PF, Li MY, Li C, Peng F, Li JL, Chen ZC, Xiao ZQ. Raf kinase inhibitor protein correlates with sensitivity of nasopharyngeal carcinoma to radiotherapy. J Cell Biochem. 2010;110:975–981. doi: 10.1002/jcb.22611. [DOI] [PubMed] [Google Scholar]
- 16.Davie JR, He S, Li L, Sekhavat A, Espino P, Drobic B, Dunn KL, Sun JM, Chen HY, Yu J, Pritchard S, Wang X. Nuclear organization and chromatin dynamics--Sp1, Sp3 and histone deacetylases. Adv Enzyme Regul. 2008;48:189–208. doi: 10.1016/j.advenzreg.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Yang WJ, Song MJ, Park EY, Lee JJ, Park JH, Park K, Park JH, Kim HP. Transcription factors Sp1 and Sp3 regulate expression of human ABCG2 gene and chemoresistence phenotype. Mol Cells. 2013;36:368–375. doi: 10.1007/s10059-013-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang JY, Wu CH, Lai MD, Chang WC, Hung JJ. Overexpression of Sp1 leads to p53-dependent apoptosis in cancer cells. Int J Cancer. 2009;125:2066–2076. doi: 10.1002/ijc.24563. [DOI] [PubMed] [Google Scholar]
- 20.Davie JR, He S, Li L, Sekhavat A, Espino P, Drobic B, Dunn KL, Sun JM, Chen HY, Yu J, Pritchard S, Wang X. Nuclear organization and chromatin dynamics--Sp1, Sp3 and histone deacetylases. Adv Enzyme Regul. 2008;48:189–208. doi: 10.1016/j.advenzreg.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Kong LM, Liao CG, Fei F, Guo X, Xing JL, Chen ZN. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010;101:1463–1470. doi: 10.1111/j.1349-7006.2010.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su B, Xiang B, Wang L, Cao L, Xiao L, Li X, Li X, Wu M, Li G. Profiling and comparing transcription factors activated in non-metastatic and metastatic nasopharyngeal carcinoma cells. J Cell Biochem. 2010;109:173–183. doi: 10.1002/jcb.22395. [DOI] [PubMed] [Google Scholar]
- 23.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 24.Kong LM, Liao CG, Zhang Y, Xu J, Li Y, Huang W, Zhang Y, Bian H, Chen ZN. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014;74:3764–3778. doi: 10.1158/0008-5472.CAN-13-3555. [DOI] [PubMed] [Google Scholar]
- 25.Dong Q, Cai N, Tao T, Zhang R, Yan W, Li R, Zhang J, Luo H, Shi Y, Luan W, Zhang Y, You Y, Wang Y, Liu N. An axis involving SNAI1, microRNA-128 and SP1 modulates glioma progression. PLoS One. 2014;9:e98651. doi: 10.1371/journal.pone.0098651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgos JS. Involvement of the Epstein-Barr virus in the nasopharyngeal carcinoma pathogenesis. Med Oncol. 2005;22:113–121. doi: 10.1385/MO:22:2:113. [DOI] [PubMed] [Google Scholar]
- 27.Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73:1219–1231. doi: 10.1158/0008-5472.CAN-12-1408. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Zhang GH, Li SL. Isolation and Phenotypic Characterization of Cancer Stem Like Cells from Nasopharyngeal Carcinoma. Drug Res (Stuttg) 2015;65:323–6. doi: 10.1055/s-0034-1382077. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JP, Zhang H, Wang HB, Li YX, Liu GH, Xing S, Li MZ, Zeng MS. Down-regulation of Sp1 suppresses cell proliferation, clonogenicity and the expressions of stem cell markers in nasopharyngeal carcinoma. J Transl Med. 2014;12:222. doi: 10.1186/s12967-014-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tay WL, Yip GW, Tan PH, Matsumoto K, Yeo R, Ng TP, Kumar SD, Tsujimoto M, Bay BH. Y-Box-binding protein-1 is a promising predictive marker of radioresistance and chemoradioresistance in nasopharyngeal cancer. Mod Pathol. 2009;22:282–290. doi: 10.1038/modpathol.2008.181. [DOI] [PubMed] [Google Scholar]
- 31.Ou J, Luan W, Deng J, Sa R, Liang H. αV integrin induces multicellular radioresistance in human nasopharyngeal carcinoma via activating SAPK/JNK pathway. PLoS One. 2012;7:e38737. doi: 10.1371/journal.pone.0038737. [DOI] [PMC free article] [PubMed] [Google Scholar]


