Abstract
The specific and available markers proteins of neonatal hypoxic-ischemic encephalopathy (HIE) injury are correlated with disease severity and the disability in childhood. Exploring the mechanism of HIE is very helpful to the targeted therapeutic approach in clinical. This study aims to explore the cell death-related proteins or biomarkers that plays roles in the HIE injury. In this study, 15 patients were included the 487 autopsies patients performed at the Department of Pathology. The lactate dehydrogenase (LDH) assay was used to detect the cell viability of NGF-differentiated PC12 cell. TUNEL assay was employed to examine the apoptotic cells in embedded slides samples. Three ER stress-related protein, including ATF6, p-Perk and IRE-1 were investigated using Western blot assay for the ER stress examination. The apoptosis associated caspase-12 and CHOP protein were detected by Western blot. The results indicated that LDH activity of living cells during hypoxia was significantly enhanced to 45% and 64% after 8 hours and 24 hours. The TUNEL results showed that plenty of the PC12 cells became the positive staining cells when treated with 0.1% O2 hypoxia. ER stress UPR pathway protein, cleaved ATF6, was increased significantly when treated with 0.1% O2 compared with the cells treated with 20% O2. Furthermore, the caspase 12 activation was triggered when the cells treated with the 0.1% O2. In conclusion, apoptosis is served as an important factor that triggers the HIE brain injury through cleaving the ATF6 and caspase-12 ER stress-related protein.
Keywords: ER stress, neonatal hypoxic-ischemic encephalopathy, apoptosis, ATF6, caspase-12
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a complex disease process in which injury severity, duration, and timing of the antenatal injury are difficult to discern [1,2]. The HIE could cause high infant mortality and long-term morbidity rates [3]. Though whole body hypothermia therapy has improved outcomes [4,5], 40% of neonates with HIE have neurological disability at 18 to 24 months of age despite therapy. However, exploration of the mechanism is very important for the HIE therapy in the birth or thereafter [6]. The specificity and availability of markers proteins of neuronal injury that correlated with disease severity could predict the neurodevelopment of disability in childhood, which would be very helpful to the targeted therapeutic approach exploration in clinical. At the biochemical level, a large cascade of events occurs during the HIE injury [7].
Many studies have illustrated that the cell death involves in the HIE. Sanders et al. [8] found that an energy failure, glutamate mediated excitotoxicity and Na/K ATPase failure lead to necrotic cell death in HIE. Pei et al. [9] discovered that the DAPK1-p53 interaction is a signaling point of convergence of necrotic and apoptotic pathways and is a desirable target for the treatment of ischemic insults. Zhou et al. [10] found that the cell death in HIE rats models was triggered by the mitochondrial death and autophagic activation pathways. Recent years, the mechanism and diagnosis of HIE is mainly depends on the clinical history, clinical examination and other investigation or image techniques [11]. Therefore, it is very urgent to identify novel markers or pathogenic proteins to provide a more accurate HIE diagnosis and therapy. This study aims to explore the cell death-related proteins or biomarkers that plays roles in the HIE injury.
Materials and methods
Subjects and samples
Fifteen patients were included in this study, which were selected from the 487 autopsies patients performed at the Department of Pathology of the Fourth Affiliated Hospital of Harbin Medical University during June 2008 to August 2013. All of these 15 patients with a pathologic HIE diagnosis and good state of brain preservation. The autopsies were performed with a 3- to 12- hours post-mortern delay. Brains were fixed for 15 days by immersion in formalin and then cut. The samples were embedded in paraffin after 4 hours of fixation in Bouin’s solution. The embedded slides were stained by the TUNEL assay. The diagnosis of HIE was confirmed according to standard histopathological criteria [12]. The clinicopathological characteristics and other information were listed in Table 1. The present was approved by the ethics committee of Harbin Medical University. All of the patients approved this study and gave their consents for this study.
Table 1.
Clinical pathological data of 15 patients diagnosed as HIE
| Characteristics | Number |
|---|---|
| Case amounts | 15 |
| Gender | |
| Male | 10 |
| Female | 5 |
| Gastational age (weeks) | 38 |
| Postnatal age (days) | 4 |
| Neuropathologic diagnosis | |
| Slight evolved HIE | 8 |
| Different stages of HIE | 4 |
| Acute HIE | 3 |
Lactate dehydrogenase (LDH) activity analysis
The hypoxic-ischemic induced cell model was established by employing the rat pheochromocytoma cell line P12, which was differentiated with nerve growth factor (NGF) (Sigma, CA, USA) for five days, and hypoxia (0.1% O2). The treated P12 cells were cultured in 24-well plates at a density of 5 × 104 cells/well. The cell viability was analyzed by the lactate dehydrogenase (LDH) assay with the cytotoxicity detection kit (Tiangen, Beijing, China) due to the protocol of kit.
Detection of apoptosis
The cell apoptosis was detected by flow cytometry analysis that monitored annexin V-FITC binding and propidium iodide (PI) uptake simultaneously according to the manufacturer’s instruction (Sigma, CA, USA). The samples were analyzed by fluorescence on a FACSan flow cytometry (Beckman, MA, USA). Potential DNA fragmentation was examined by the TUNEL apoptosis detection kit (Chemicon, CA, USA) following the manufacture’s instruction. Apoptosis and death bodies were stained as bright.
Western blot
Brain sections were isolated, snap-frozen in liquid nitrogen and immediately homogenized in ice-cold RIPA lysis buffer (Sigma, CA, USA) containing a cocktail of protease inhibitors (Roch, Switzerland). Denatured total cell lysates were run in SDS-PAGE, transferred to nitrocellulose membranes and blocked overnight at 4°C in 5% milk. The blocked membranes were incubated overnight at 4°C with the IRE-1, ATF6, p-Perk and CHOP monoclonal antibody (Santa Cruz, USA, 1:2000) and β-actin monoclonal antibody (Santa Cruz, USA, 1:2000) followed by 1 hour incubation at room temperature with an HRP-conjugated secondary antibody (Santa Cruz, USA, 1:3000). Immuno-detection was performed using the ECL reagents (Amersham Biosciences, UK). The blots were scanned and the pixel count and intensity of each band was quantified using the Scion image software (Scion, MD). Signals were normalized against β-actin and the results were expressed as a percentage of the negative control signal.
Statistics analysis
The analyses were performed with the SPSS 19.0 statistical software package (Microsoft, CA, USA). The significant differences among or between groups were determined by Student’s t test. The mean and standard deviations of the result between groups was used and P value < 0.05 was taken as statistically significant. The TUNEL positive cells were evaluated independently by three observers using uniform criteria.
Results
Neuropathological diagnosis of HIE patients
By analyzing the clinical characteristics of the 15 HIE patients, we found that the average gestational age was 38 weeks, and average postnatal age was 4 days. We also differentiated the neuropathological type, and there were 8 cases with slight evolved HIE, 4 cases with different stages of HIE and 3 cases with the acute HIE (Table 1).
Effects of hypoxia on the NGF-differentiated PC12 cell activity and apoptosis
The PC12 cells were treated with the normal concentration of 20% O2 and low oxygen concentration of 0.1% O2 for 8 hour and 24 hours, respectively. The cell activity was determined using the LDH assay. The LDH assay results indicated that the LDH activity of living cells during hypoxia was significantly enhanced to 45% and 64% after 8 hours and 24 hours (Figure 1A, P < 0.05). In order to verify whether the enhanced activity of LDH (decreased cell activity) was due to apoptotic pathway, TUNEL assay was performed after 24 hours of hypoxia. The TUNEL results showed that plenty of the PC12 cells became the positive staining cells, while event no PC12 cells with positive staining in the normal oxygen treated cells (Figure 1B).
Figure 1.
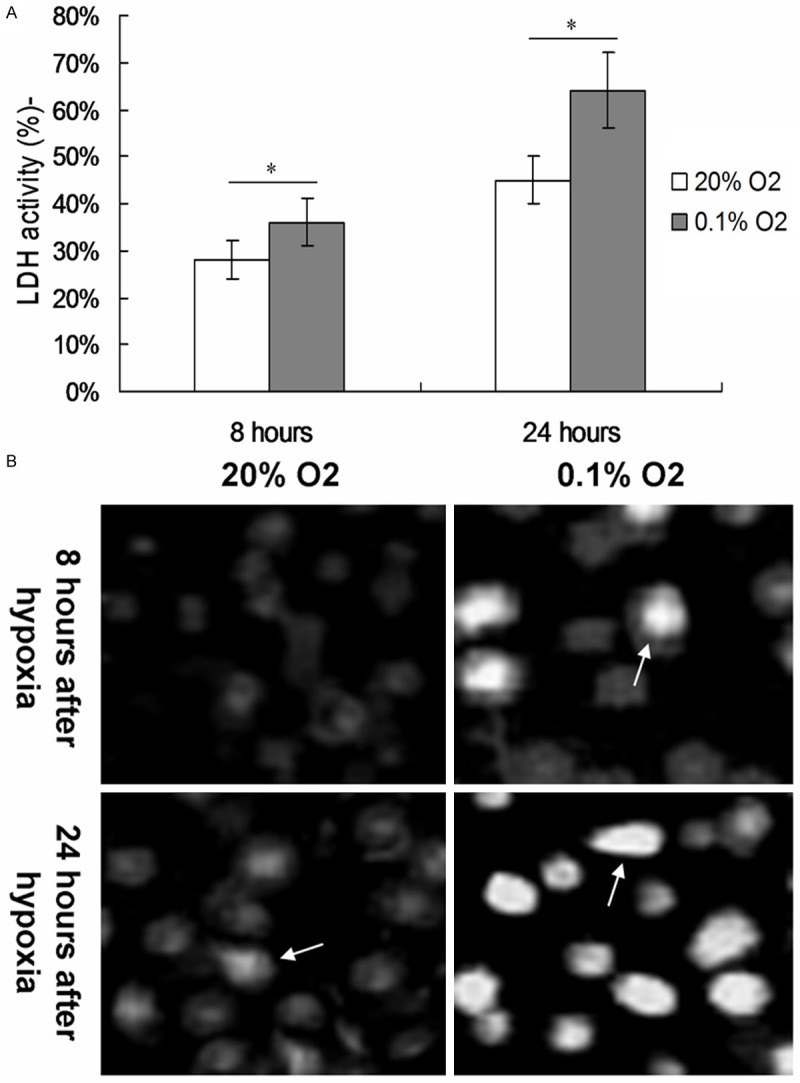
Effects of hypoxia on the cell viability and apoptosis of treated PC cells. A. Cell viability of PC 12 cells treated with hypoxia for 8 and 24 hours was detected by using LDH assay. B. Cell apoptosis was examined by TUNEL assay 8 and 24 hours after hypoxia treatment with 0.1% O2. *P < 0.05 represent the cell viability of PC 12 cells in 0.1% O2 treated cells compared to 20% O2 treated cells for 8 and 24 hours.
ATF6 involves in the hypoxia induced cell apoptosis
Fifteen cases of neonates diagnosed HIE children were selected for the detection of three mainly UPR factors of ER stress, including p-Perk, IRE-1 and cleaved ATF6, by western blot assay. The results showed that the treatment of hypoxia triggered the cleavage of ATF6 both after 8 hours and 24 hours (Figure 2A). The cleaved ATF6 expression in hypoxia treatment group was significantly higher compared to normal O2 treatment group (P < 0.05, Figure 2B). However, there were no significant difference of p-Perk and IRE-1 protein among the every four group (P > 0.05, Figure 2B).
Figure 2.
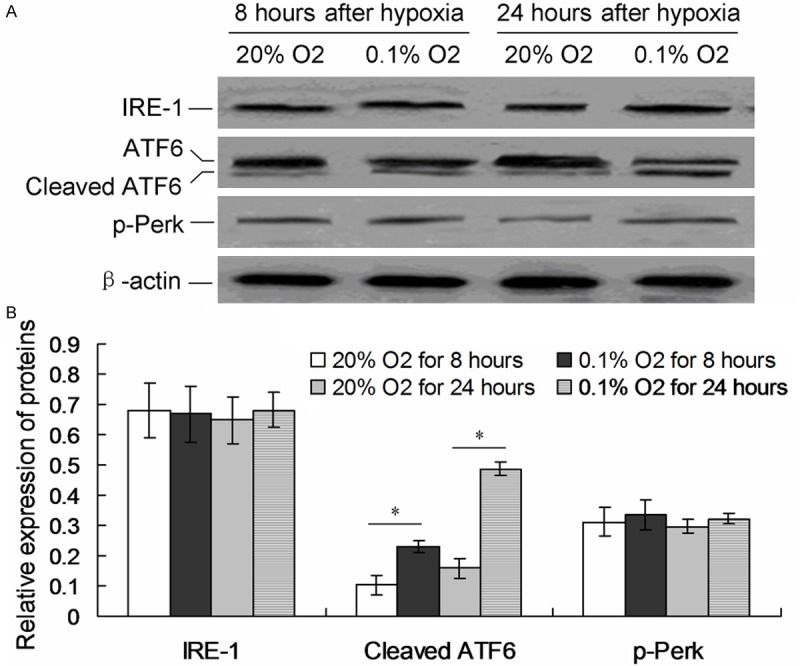
Examination of UPR pathway proteins. A. Western blot analysis of IRE1, p-Perk and cleaved ATF6 proteins. B. Statistical analysis of IRE1, cleaved ATF6 and p-PERK protein. *P < 0.05 represent the IRE1, cleaved ATF6 or p-Perk protein in 0.1% O2 treated cells compared to 20% O2 treated cells for 8 and 24 hours.
Hypoxia triggers caspase-12 induced apoptosis
To investigate the specific pathway which caused the apoptosis, the cellular levels of cleaved caspase-12 and CHOP protein were examined by individual western blot (Figure 3A). When treated with 0.1% O2 hypoxia, the levels cleaved caspase-12 were significantly higher compared to 20% O2 group both after 8 hours and 24 hours (P < 0.05, Figure 3B). But no changes of CHOP protein were discovered in both 20% O2 and 0.1% O2 hypoxia treatment (P > 0.05).
Figure 3.
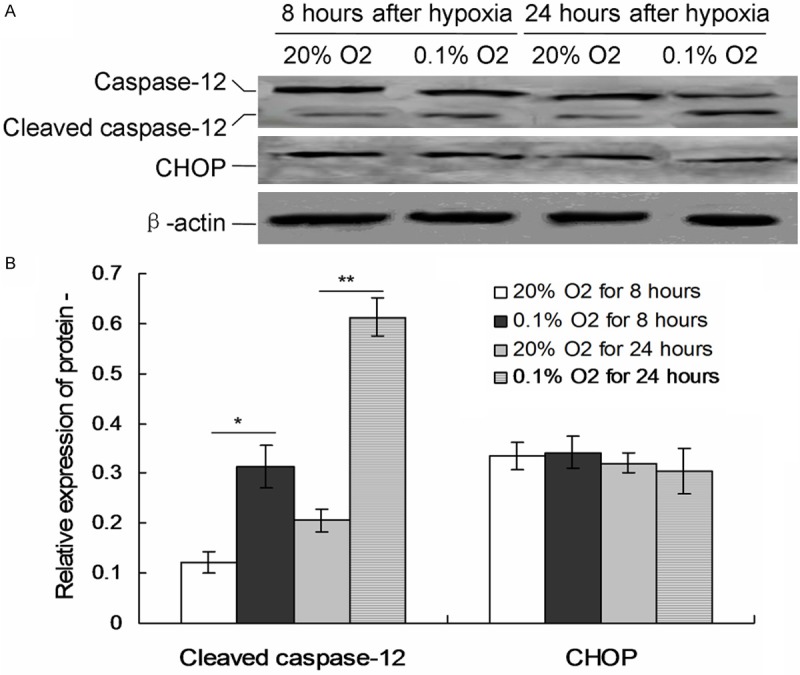
Expression of ER stress related apoptosis proteins in the cells treated with 0.1% O2. A. The levels of CHOP and caspase-12 were detected by western blots. B. Statistical analysis of CHOP and cleaved caspase-12. *P < 0.05 represent the CHOP or cleaved caspase-12 protein in 0.1% O2 treated cells compared to 20% O2 treated cells for 8 and 24 hours.
Discussion
The present study examined a series of ER stress specific apoptotic proteins as the promising biomarkers, which may involve in the excitatory-oxidative cascade of brain injury in infants with HIE. Although whole body hypothermia in neonates with HIE is associated with a reduction in death and neurologic impairment [13,14], there is also no real-time means of determining the therapeutic efficacy of hypothermia and who ultimately may benefit from adjunctive therapy. The neurologic evaluation changes over time always influenced by mediacations, and is subjected to examiner bias. Therefore, discovering an effective and specific therapeutic target in the early time of HIE is very urgent for the HIE therapy in clinical setting.
Apoptosis was selected to reflect the extent of neuronal injury because they are expressed in neurons, astrocytes and other HIE brain tissues [15,16]. Apoptosis associated proteins are the key protein in astroctyes that is functionally involved during regeneration and gliosis and whose expression is induced by brain damage [17]. Our results also indicated that the cells activity in brain tissues was decreased, and the DNA in cells appeared many fragments (TUNEL assay), all of which represent obvious apoptotic phenomenon.
To investigate the specific mechanism of the cell activity of the hypoxia treated cells, the ER stress (UPR pathway) associated proteins, including p-Perk, IRE-1 and cleaved ATF6 level were detected. The results indicated that ATF6 protein was cleaved when treated with 0.1% O2. Thus, the ATF6 pathway may involve in the hypoxia triggered apoptosis. The data strongly confirmed that the emergence of an ER stress after treating with hypoxia (0.1% O2) in brain tissue cells. What’s most important is that the treatment of hypoxia caused ER stress. So we speculated that the hypoxia may participate in the pathogenic process of HIE.
The apoptosis related factors, such as cleaved caspase 12 and CHOP protein, which are the ER stress pathway proteins. The previous study indicated that cleaved caspase-12 can trigger the apoptosis by activate caspase-3 indirectly, and CHOP can directly trigger apoptosis [18,19]. In this study, we found that there were no significant changes of CHOP protein (activated) in all of the four groups either treated with 20% O2 or 0.1% O2. However, the 0.1% hypoxia stimuli could induce the cleavage of caspase-12 significantly. According to the above results, we found that the hypoxia treatment in brain cells could indirectly trigger caspase-12 protein cleaving caused apoptosis.
For the therapeutic perspective, anti-apoptotic agents reduce neuronal loss following experimental cerebral hypoxia-ischemia, which suggests that the apoptotic process is reversible. Therefore, the synthesis and use of new specific inhibitor of ATF6 and caspase-12 can be explored as therapeutic agents when administered soon after a hypoxic-ischemic injury in HIE patients.
In conclusion, apoptosis serves as an important factor that triggers the HIE brain injury through cleaving the ATF6 and caspase-12 ER stress-related protein.
Acknowledgements
This study was granted by Science and technology offends pass item in Heilongjiang Province (GC12C306-3); Science and technology research projects of education department of Heilongjiang Province (11551211); Post-Doctoral Foundation of Heilongjiang Province (LRB2008-384).
Disclosure of conflict of interest
None.
References
- 1.Yin X, Meng F, Wang Y, Wei W, Li A, Chai Y, Feng Z. Effect of hyperbaric oxygen on neurological recovery of neonatal rats following hypoxic-ischemic brain damage and its underlying mechanism. Int J Clin Exp Pathol. 2013;6:66–75. [PMC free article] [PubMed] [Google Scholar]
- 2.Chalak LF, Sanchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164:468–474. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill CD, Jadcherla SR. Esophageal mechanosensitive mechanisms are impaired in neonates with hypoxic-ischemic encephalopathy. J Pediatr. 2013;162:976–982. doi: 10.1016/j.jpeds.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Tian F, Wang K, Hu J, Xie Y, Sun S, Zou Z, Huang S. Continuous spinal anesthesia with sufentanil in labor analgesia can induce maternal febrile response in puerperas. Int J Clin Exp Med. 2013;6:334–341. [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas-Escobar M, Weiss MD. Biomarkers of brain injury in the premature infant. Front Neurol. 2013;3:185. doi: 10.3389/fneur.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Jimenez M, Sacristan S, Morales C, Garcia-Villanueva M, Garcia-Fernandez E, Alcazar A, Gonzalez VM, Martin ME. Apoptosis-related proteins are potential markers of neonatal hypoxic-ischemic encephalopathy (HIE) injury. Neurosci Lett. 2014;558:143–148. doi: 10.1016/j.neulet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Sanders RD, Manning HJ, Robertson NJ, Ma D, Edwards AD, Hagberg H, Maze M. Preconditioning and postinsult therapies for perinatal hypoxic-ischemic injury at term. Anesthesiology. 2010;113:233–249. doi: 10.1097/ALN.0b013e3181dc1b84. [DOI] [PubMed] [Google Scholar]
- 9.Pei L, Shang Y, Jin H, Wang S, Wei N, Yan H, Wu Y, Yao C, Wang X, Zhu LQ, Lu Y. DAPK1-p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death. J Neurosci. 2014;34:6546–6556. doi: 10.1523/JNEUROSCI.5119-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Wang J, Jiang J, Stavrovskaya IG, Li M, Li W, Wu Q, Zhang X, Luo C, Zhou S, Sirianni AC, Sarkar S, Kristal BS, Friedlander RM, Wang X. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathway and autophagic activation in experimental models of ischemic injury. M Neurosci. 2014;34:2967–2978. doi: 10.1523/JNEUROSCI.1948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY, He XH. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2013;8:e66748. doi: 10.1371/journal.pone.0066748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales C. Lesiones hipoxico-isquemicas del sistema nervioso central en el periodo perinatal. Rev Esp Patol. 2002;35:5–20. [Google Scholar]
- 13.Higgins RD, Raju T, Edwards AD, Azzoparki DV, Bose CL, Clark RH, Ferriero DM, Guillet R. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice kennedy shriver NICHD workshop. J Pediatr. 2011;159:851–858. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C, Xie P, Zhao F, Zhang L, An W, Zhan Y. Mechanism of the promotion of steatoic HepG2 cell apoptosis by cholesterol. Int J Clin Exp Pathol. 2014;7:6807–6813. [PMC free article] [PubMed] [Google Scholar]
- 15.Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, Li YY. The role of TNF-alpha, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci. 2014;18:905–909. [PubMed] [Google Scholar]
- 16.Kichev A, Rousset CI, Baburamani AA, Levison SW, Wood TL, Gressens P, Thornton C, Hagberg H. Tumor necrosis factor-related apoptosis-inducing ligand signaling and cell death in the immature central nervous system after hypoxiaischemia and inflammation. J Biol Chem. 2014;289:9430–9439. doi: 10.1074/jbc.M113.512350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y, Bi L, Zhang P, Wang F, Lin F, Ni W, Wu J, Jiang L. Thioredoxin-1 inhibitor PX-12 induces human acute myeloid leukemia cell apoptosis and enhances the sensitivity of cells to arsenic trioxide. Int J Clin Exp Pathol. 2014;7:4765–4773. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Shi Q, Xu K, Gao C, Chen C, Li XL, Wang GR, Tian C, Han J, Dong XP. Familial CJD associated PrP mutants within the transmembrane region induced Ctm-PrP retention in ER and trigger apoptosis by the ER stress in SH-SY5Y cells. PLoS One. 2011;6:e14602. doi: 10.1371/journal.pone.0014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang LJ, Ma DQ, Cui H. Proteomic analysis of immature rat pups brain in response to hypoxia and ischemia challenge. Int J Clin Exp Pathol. 2014;7:4645–4660. [PMC free article] [PubMed] [Google Scholar]


