Abstract
Background: Deregulation of transformer 2β (Tra2β) has been implicated in several cancers. However, the role of Tra2β expression in prostate cancer (PCa) is unclear. Therefore, this study was to investigate the expression of Tra2β in PCa and evaluated its association with clinicopathological variables and prognosis. Methods: Thirty paired fresh PCa samples were analyzed for Tra2β expression by Western blot analysis. Immunohistochemistry (IHC) assay was performed in 160 PCa samples after radical prostatectomy and adjacent non-cancerous tissues. Tra2β protein expression was divided into high expression group and low expression group by IHC. We also investigated the association of Tra2β expression with clinical and pathologic parameters. Kaplan-Meier plots and Cox proportional hazards regression model were used to analyze the association between Tra2β protein expression and prognosis of PCa patients. Our results showed that Tra2β was significantly upregulated in PCa tissues by western blot and IHC. Results: Our data indicated that high expression of Tra2β was significantly associated with lymph node metastasis (P=0.002), clinical stage (P=0.015), preoperative prostate-specific antigen (P=0.003), Gleason score (P=0.001), and biochemical recurrence (P=0.021). High Tra2β expression was a significant predictor of poor biochemical recurrence free survival and overall survival both in univariate and multivariate analysis. Conclusion: We show that Tra2β was significantly upregulated in PCa patients after radical prostatectomy, and multivariate analysis confirmed Tra2β as an independent prognostic factor.
Keywords: Prostate cancer, transformer 2β, prognosis
Introduction
As the most common malignant neoplasm in men, prostate cancer (PCa) is the second most common cause of cancer related deaths [1]. Although the prognosis of PCa patients with localized or regional disease is good, the 5-year survival rate is only 29% [2]. However, the mechanisms underlying carcinogenesis and progression of PCa have yet to be fully explored. Conventional prognostic factors such as Gleason score or preoperative PSA levels are insufficient to predict patient outcome for current therapies. They are even more limited in identifying insignificant PCa. Therefore, further efforts to find new diagnostic pathways and therapeutic options are urgently needed to optimize patient management [3,4]. Only a minority of these patients is at risk of dying of PCa, but many of these patients receive unnecessary postoperative interventions such as adjuvant radiation and often suffer treatment morbidity [5]. Current tools have limited capacity to identify patients that are most at risk of metastasis and PCa death [6]. Thus, more specific and relevant markers should be explored to the resolve the unmet need for predicting men with PCa at high risk currently.
Transformer 2β (Tra2β) is a member of the serine/arginine-rich (SR)-like protein family, is an important RNA-binding protein involved in alternative splice [7]. Tra2β protein is encoded by the TRA2B gene on human chromosome 3 which generates 5 transcripts (Tra2β1-5) by alternative splicing [8]. Tra2β has been reported to influence alternative splicing of transcripts essential for the proper functions of multiple tissues [9]. The Tra2β is increased in breast, cervical, ovarian, and colon cancer, and Tra2 β expression is associated with cancer cell survival [10]. In this study, we aimed to investigate the role of Tra2β in PCa. We show that Tra2β was significantly upregulated in PCa patients after radical prostatectomy, and multivariate analysis confirmed Tra2β as an independent prognostic factor.
Materials and methods
Study population
A total of 160 human PCa who underwent radical prostatectomy and paired adjacent noncancerous tissues were obtained from the Department of Oncology, Second Affiliated Hospital, Medical School of Xi’an Jiaotong University between 2005 and 2008. Written informed consent was obtained from all PCa patients and this study was approved by the Ethics Committee of Medical School of Xi’an Jiaotong University. This investigation conformed to the principles outlined in the Declaration of Helsinki. Demographic and clinicopathological data of PCa patients were collected from medical records. None of the PCa patients received androgen deprivation treatment, chemotherapy, or radiation therapy prior to radical prostatectomy. The tissue samples were snapfrozen in liquid nitrogen and stored at -80°C until used. The histopathology of each specimen was reviewed on the HE-stained tissue section to confirm diagnosis and tumor content at least 70% of PCa cells in the tissue samples. The following biochemical and clinicopathological parameters were recorded: biochemical relapse, preoperative serum prostate-specific antigen, clinical stage, lymph node status, angiolymphatic invasion status, Gleason score, margin status, and seminal vesicle invasion status. The time to biochemical recurrence was defined as the period between radical prostatectomy and the measurement of two successive values of serum prostate-specific antigen level ≥0.2 ng/ml.
Western blot analysis
The frozen tumor sections from 30 recently diagnosed prostate carcinoma patients and their corresponding adjacent benign prostate were lysed in cell lysis buffer (Sigma-Aldrich). Samples were homogenized and then kept at 4°C for 30 min. Cell extracts were cleared by centrifugation at 1,5000 g for 30 min at 4°C and protein concentration was determined using a BCA kit (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA). Fifty μg total proteins from each sample were heated at 95°C for 5 min after mixing with equal volume of 2x SDS loading buffer. Samples were separated on 12.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels and electro transferred to PVDF membranes (Millipore, CA, USA). The membrane was blocked in TBS-T buffer contain 5% skim milk at room temperature for 2 h. The membranes were incubated with Tra2β antibody diluted (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA, 1:2000) in TBS-T overnight at 4°C. After washing with TBS-T, the membrane was incubated in 5% skim milk in TBS-T buffer containing a secondary antibody (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA, 1:5000), for 60 min at room temperature with shaking. Proteins of interest were detected using a Chemo-luminescent Reagent Plus kit (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA) and visualized by autoradiography after various exposure times. For normalization of protein loading, the monoclonal antibody against GAPDH at 1:2000 (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA) was used.
Immunohistochemistry
Immunohistochemical analyses were performed on formalin-fixed paraffin-embedded sections of 160 PCa, their corresponding adjacent benign tissues. Sections were de-waxed, re-hydrated, and endogenous peroxidase activity was blocked using 3% H2O2 for 10 min. Slides were washed with PBS for 5 min. Slides were then blocked with 10% normal goat serum in PBS for 30 min and incubated overnight in the presence of anti-Tra2β antibody (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA, 1:500). The slides were washed 3 times using PBS, incubated with goat anti-rabbit antibody (Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA, 1:100) for 30 min. All slides were digitalized using Aperio System (Vista, CA, USA). Automated image quantification generates a final score ranging from 0 (negative) to 1 (weak positive), 2 (moderate positive), or 3 (strong positive). The 0 and 1 were classified as low expression and 2 and 3 as high expression for further statistical analysis.
Statistical analysis
Statistical analysis was performed using SPSS version 17.0. Data were analyzed using Student’s t-test and expressed as mean ± SD. The correlation between Tra2β expression and the clinicopathological parameters was assessed by Chi-square test. Kaplan-Meier and log-rank tests were used when assessing overall survival and biochemical recurrence free survival rate, while COX regression analysis was used for the univariate and multivariate analysis. Multivariate survival analysis was performed on all parameters that were found to be significant on univariate analysis. Differences were considered statistically significant when P<0.05.
Results
Tra2β protein expression is up-regulated in PCa tissues
The function of Tra2β in PCa has not been well defined. Therefore, western blot was performed on paired samples of 30 fresh PCa tissue and noncancerous tissue adjacent to the cancer lesion isolated from the same patient for the expression levels of Tra2β in samples from PCa and adjacent noncancerous tissues. Our data reveal that the expression of Tra2β at the protein level was significantly increased in the PCa tissues, compared with the paired adjacent non-carcinoma tissues (P<0.001, Figure 1A). As indicated in Figure 1B, the semi-quantitative analysis of band intensity showed the consistent trends.
Figure 1.
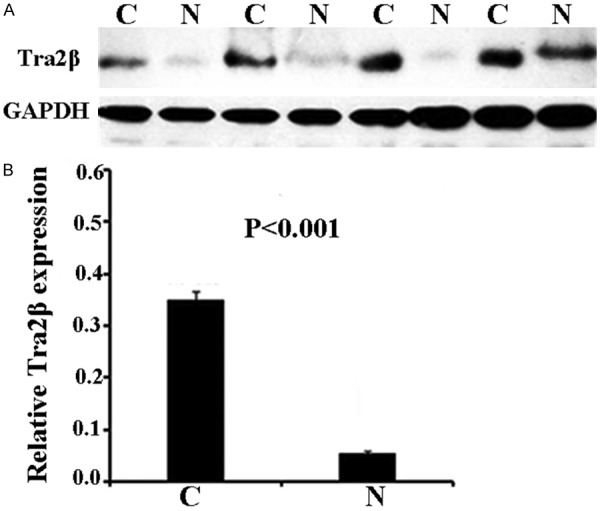
Increased Tra2β protein expression in prostate cancer. A. Representative Western blots of Tra2β protein in 4 patients and corresponding normal tissues. B. Relative protein expression levels of Tra2β/GAPDH in all 30 fresh samples. N, Non-tumor tissue; C, Cancer tissues).
Relationship between Tra2β expression and clinicopathologic variables in PCa patients
Furthermore, we investigated the Tra2β protein expression levels in 160 PCa patient samples and paired adjacent non-carcinoma tissues from the same patient by IHC staining. The 160 PCa samples were subdivided into two groups with respectively low or high amounts of Tra2β. As shown in Figure 2, IHC showed that 95 out of 160 PCa tissues (59.38%) were classified as Tra2β high. In contrast, 10 out of 160 (6.25%) control tissues were classified as Tra2β high. Tra2β protein expression is up-regulated in PCa tissues by IHC (P<0.001).
Figure 2.
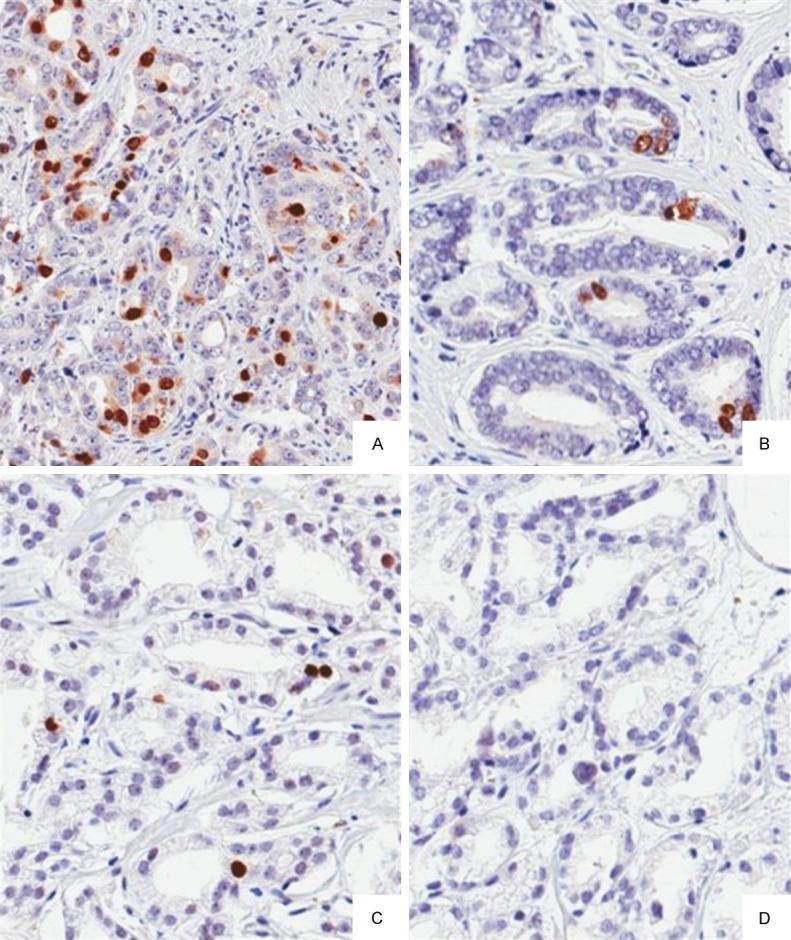
Representative immunohistochemical staining results for Tra2β in prostate cancer observed at 200 magnification showing 3+ staining (A) 2+ staining (B) 1+ staining (C) and negative staining (D).
In PCa, the relationship between the expression of Tra2β and patient clinicopathologic characteristics is shown in Table 1. High expression of Tra2β was found to significantly correlate with lymph node metastasis (P=0.002), clinical stage (P=0.015), preoperative prostate-specific antigen (P=0.003), Gleason score (P=0.001), and biochemical recurrence (P=0.021). No significant difference in Tra2β expression was observed with age, surgical margin status and seminal vesicle invasion (P>0.05).
Table 1.
Clinicopathologic characteristics of 160 prostate cancer patients
| Variable | Tra2β expression | P value | |||
|---|---|---|---|---|---|
|
| |||||
| Group | High | Low | Total | ||
| Age | 0.098 | ||||
| <65 | 55 (65.5%) | 29 (34.5%) | 84 | ||
| ≥65 | 40 (52.6%) | 36 (47.4%) | 76 | ||
| Lymph node metastasis | 0.002 | ||||
| Absence | 75 (54.7%) | 62 (45.3%) | 137 | ||
| Presence | 20 (87%) | 3 (13%) | 23 | ||
| Surgical margin status | 0.426 | ||||
| Absence | 71 (49.4%) | 35 (50.6%) | 106 | ||
| Presence | 24 (57.1%) | 30 (42.9%) | 54 | ||
| Seminal vesicle invasion | 0.63 | ||||
| Absence | 62 (62.6%) | 37 (37.4%) | 99 | ||
| Presence | 33 (54.1%) | 28 (45.9%) | 61 | ||
| Clinical stage | 0.015 | ||||
| T1 | 45 (55.6%) | 36 (44.4%) | 81 | ||
| T2/T3 | 50 (63.3%) | 29 (36.7%) | 79 | ||
| Preoperative PSA | 0.0013 | ||||
| <4 | 2 (28.6%) | 5 (71.4%) | 7 | ||
| 4-10 | 20 (40%) | 30 (60%) | 50 | ||
| >10 | 71 (68.9%) | 32 (31.1%) | 103 | ||
| Gleason score | |||||
| <7 | 24 (32.9%) | 49 (67.1%) | 73 | <0.001 | |
| 7 | 29 (74.4%) | 10 (25.6%) | 39 | ||
| >7 | 42 (87.5%) | 6 (12.5%) | 48 | ||
| Biochemical recurrence | 0.021 | ||||
| Absence | 56 (54.4%) | 47 (45.6%) | 103 | ||
| Presence | 39 (68.4%) | 18 (31.6%) | 57 | ||
Relationship between Tra2β expression and biochemical recurrence free survival
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. Differences in survival times were assessed using the log rank test. First, to confirm the representativeness of the PCa in present study, we analyzed established prognostic predictors of PCa patient survival. Kaplan-Meier analysis demonstrated a significant impact of well-known clinicopathological prognostic parameters, such as seminal vesicle invasion, and Gleason score (P<0.05, Table 2). Assessment in total PCa revealed that the high expression level of Tra2β was correlated with adverse biochemical recurrence free and overall survival of PCa patients (Figure 3). Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of Tra2β and those clinicalopathological parameters that were significant in univariate analysis were further examined in multivariate analysis. The results showed that the high expression of Tra2β was an independent prognostic factor for biochemical recurrence-free survival (relative risk: 1.642, 95% CI: 1.154-2.337, P=0.006, Table 2). With regard to other parameters, Gleason score or seminal vesicle invasion status was shown to be an independent prognostic factor for biochemical recurrence-free survival.
Table 2.
Prognostic value of Tra2β expression for the biochemical recurrence free survival in univariate and multivariate analyses by Cox regression
| Covariant | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Exp (B) | 95% CI | P value | Exp (B) | 95% CI | P value | |
| Tra2β expression | 1.716 | 1.207-2.439 | 0.003 | 1.642 | 1.154-2.337 | 0.008 |
| Gleason score | 1.703 | 1.280-2.265 | <0.001 | 1.674 | 1.259-2.225 | 0.001 |
| Seminal vesicle invasion | 1.505 | 1.132-2.003 | 0.005 | 1.443 | 1.084-1.920 | 0.012 |
| Preoperative PSA | 1.241 | 0.705-2.188 | 0.454 | |||
| Surgical margin status | 1.017 | 0.709-1.459 | 0.925 | |||
| PCa Stage | 1.090 | 0.921-1.291 | 0.316 | |||
| Lymph node metastasis | 1.140 | 0.850-1.528 | 0.381 | |||
| Age | 1.068 | 0.804-1.419 | 0.650 | |||
Figure 3.
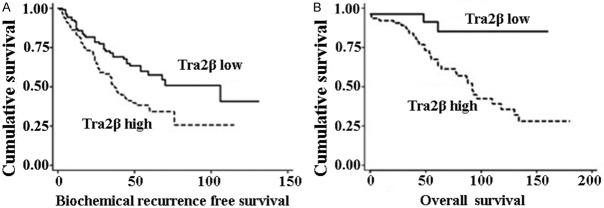
Kaplan-Meier plots of biochemical recurrence free survival and overall survival. The P-values for two-sided log-rank statistics are given, comparing patients with Tra2β low (IHC score=0 and 1) or high (IHC score=2+ or 3+) immunoreactivity.
Relationship between Tra2β expression and overall survival
In terms of overall survival, patients with high Tra2β expression had a poorer overall survival than patients with low Tra2β expression. PCa patients with high Tra2β expression had shorter overall survival. Multivariate analysis indicated that high amount of Tra2β was independently associated with poorer overall survival (Table 3). The detailed data between Tra2β expression and overall survival are shown in Table 3.
Table 3.
Prognostic value of Tra2β expression for the overall survival in univariate and multivariate analyses by Cox regression
| Covariant | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Exp (B) | 95% CI | P value | Exp (B) | 95% CI | P value | |
| Tra2βexpression | 1.629 | 1.038-2.555 | 0.034 | 1.751 | 1.098-2.792 | 0.019 |
| Gleason score | 2.526 | 1.788-3.568 | <0.001 | 1.953 | 1.870-2.784 | 0.003 |
| Preoperative PSA | 2.034 | 1.338-23.092 | 0.001 | 2.025 | 2.313-4.123 | 0.001 |
| PCa Stage | 4.131 | 2.888-5.911 | 0.006 | 4.094 | 1.213-3.043 | 0.015 |
| Age | 1.282 | 0.917-1.792 | 0.146 | |||
| Surgical margin status | 1.101 | 0.703-1.724 | 0.674 | |||
| Lymph node metastasis | 1.044 | 0.746-1.462 | 0.800 | |||
| Seminal vesicle invasion | 1.358 | 0.956-1.928 | 0.087 | |||
Discussion
In this study, we analyzed Tra2β expression by western blot in 30 fresh PCa samples and immunohistochemistry in PCa using 160 surgical specimens. We analyzed the association between Tra2β expression and traditional clinicopathogical characteristics in PCa. The present data showed that Tra2β overexpression was associated with poor survival by analyzing the overall survival and biochemical recurrence free survival. High Tra2β expression was significantly correlated with higher PCa stage, seminal vesicle invasion, lymph node metastasis, higher preoperative PSA, and higher Gleason score. Moreover, our data also demonstrated that the patients with high Tra2β expression had significantly poor overall survival and biochemical recurrence free survival. Multivariate analysis demonstrated that Tra2β expression was an independent prognostic factor for both overall survival and biochemical recurrence free survival in patients with PCa. To the best of our knowledge, this is the first study to demonstrate in detail an association of clinicopathologic parameters and prognostic significance of Tra2β overexpression in PCa. These results suggest that high Tra2β expression plays a critical role in the progression of PCa and is significantly associated with a poor prognosis independently of other factors. This raises the possibility that Tra2β may be a prognostic parameter for PCa that is as or more reliable than the clinicopathologic factors currently in use and suggests the possibility to use Tra2β in individualization of both patient prognosis and therapy.
Previous studies have shown that Tra2β participated in various cellular processes such as cell proliferation, diversification and apoptosis [9,11]. However, the effect of Tra2β expression on Tra2β is still unknown. Alternative splicing is considered as an important mechanism in regulating gene expression and associated with tumorigenesis and metastasis of a wide variety of human cancers including PCa [12]. Tra2β belongs to SR-like protein family; it binds to the highly degenerated purine-rich sequence motif (GAARGARR) and influences various alternatively spliced exons [13]. Tra2β is related to multiple biological processes and various diseases by alternative splicing of target mRNA [10]. Tra2β mRNA level was found being induced in advanced International Federation of Gynecology and Obstetrics (FIGO) stages [14]. Tra2β protein nuclear levels were elevated in poorly differentiated and lymph node metastases in endometrial cancer (EC) and the antagonistic functional effects of Tra2β on alternative splicing correlate directly to their opposite clinical effects on EC patient outcome [14]. The involvement of Tra2β in exon recognition and alternative splicing may be important for gene regulation of alternatively spliced genes like CD44 with potential functional consequences in epithelial ovarian cancer leading to progression and metastasis [15]. Known Tra2 β splicing targets have important roles in cancer cells, where they affect metastasis, proliferation, and cell survival. Tra2 β protein is also known to interact directly with the RBMY protein which is implicated in liver cancer [10]. Our results suggested that high expression of Tra2β might play an important role in the development and progression of PCa. However, further studies are necessary to elucidate the molecular mechanisms of Tra2β in PCa pathogenesis.
In summary, our studies showed that Tra2β was up-regulated in PCa and associated with poor prognosis. Therefore, Tra2β might serve as a novel molecular target for the diagnosis and treatment of PCa.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Carroll PR. Early stage prostate cancer-do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173:1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 3.Andrén O, Fall K, Franzén L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year follow up of a population based cohort in Sweden. J Urol. 2006;175:1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Pina F, Mota-Pinto A, Lopes C, Medeiros R. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31:32. doi: 10.1186/1756-9966-31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. J Cancer. 2011;2:1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford ED, Bennett CL, Andriole GL, Garnick MB, Petrylak DP. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int. 2013;112:548–560. doi: 10.1111/bju.12061. [DOI] [PubMed] [Google Scholar]
- 7.Grellscheid SN, Dalgliesh C, Rozanska A, Grellscheid D, Bourgeois CF, Stévenin J, Elliott DJ. Molecular design of a splicing switch responsive to the RNA binding protein Tra2β. Nucleic Acids Res. 2011;39:8092–8104. doi: 10.1093/nar/gkr495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayler O, Cap C, Stamm S. Human transformer-2-beta gene (SFRS10): complete nucleotide sequence, chromosomal localization, and generation of a tissue-specific isoform. Genomics. 1998;53:191–202. doi: 10.1006/geno.1998.5471. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Ennajdaoui H, Edmondson C, Wirth B, Sanford JR, Chen B. Splicing factor TRA2B is required for neural progenitor survival. J Comp Neurol. 2014;522:372–392. doi: 10.1002/cne.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Best A, Dagliesh C, Ehrmann I, Kheirollahi-Kouhestani M, Tyson-Capper A, Elliott DJ. Expression of Tra2β in Cancer Cells as a Potential Contributory Factor to Neoplasia and Metastasis. Int J Cell Biol. 2013;2013:843781. doi: 10.1155/2013/843781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajita K, Kuwano Y, Kitamura N, Satake Y, Nishida K, Kurokawa K, Akaike Y, Honda M, Masuda K, Rokutan K. Ets1 and heat shock factor 1 regulate transcription of the Transformer 2β gene in human colon cancer cells. J Gastroenterol. 2013;48:1222–1233. doi: 10.1007/s00535-012-0745-2. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang DY, Xu LH, He XH, Zhang YT, Zeng LH, Cai JY, Ren S. Autophagy is differentially induced in prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing profiles of ATG5. Autophagy. 2013;9:20–32. doi: 10.4161/auto.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mende Y, Jakubik M, Riessland M, Schoenen F, Rossbach K, Kleinridders A, Köhler C, Buch T, Wirth B. Deficiency of the splicing factor Sfrs10 results in early embryonic lethality in mice and has no impact on full-length SMN/Smn splicing. Hum Mol Genet. 2010;19:2154–2167. doi: 10.1093/hmg/ddq094. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang YQ, zur Hausen A, Orlowska-Volk M, Jäger M, Bettendorf H, Hirschfeld M, Tong XW, Stickeler E. Expression levels of hnRNP G and hTra2-beta1 correlate with opposite outcomes in endometrial cancer biology. Int J Cancer. 2011;128:2010–2019. doi: 10.1002/ijc.25544. [DOI] [PubMed] [Google Scholar]
- 15.Iborra S, Hirschfeld M, Jaeger M, Zur Hausen A, Braicu I, Sehouli J, Gitsch G, Stickeler E. Alterations in expression pattern of splicing factors in epithelial ovarian cancer and its clinical impact. Int J Gynecol Cancer. 2013;23:990–996. doi: 10.1097/IGC.0b013e31829783e3. [DOI] [PubMed] [Google Scholar]


