Abstract
Previously, the immunophilin-like protein TWD1 from Arabidopsis has been demonstrated to interact with the ABC transporters AtPGP1 and its closest homologue, AtPGP19. Physiological and biochemical investigation of pgp1/pgp19 and of twd1 plants suggested a regulatory role of TWD1 on AtPGP1/AtPGP19 transport activities. To further understand the dramatic pleiotropic phenotype that is caused by loss-of-function mutation of the TWD1 gene, we were interested in other TWD1 interacting proteins. AtMRP1, a multidrug resistance-associated (MRP/ABCC)-like ABC transporter, has been isolated in a yeast two-hybrid screen. We demonstrate molecular interaction between TWD1 and ABC transporters AtMRP1 and its closest homologue, AtMRP2. Unlike AtPGP1, AtMRP1 binds to the C-terminal tetratricopeptide repeat domain of TWD1, which is well known to mediate protein-protein interactions. Domain mapping proved that TWD1 binds to a motif of AtMRP1 that resembles calmodulin-binding motifs; and calmodulin binding to the C-terminus of MRP1 was verified. By membrane fractionation and GFP-tagging, we localized AtMRP1 to the central vacuolar membrane and the TWD1-AtMRP1 complex was verified in vivo by coimmunoprecipitation. We were able to demonstrate that TWD1 binds to isolated vacuoles and has a significant impact on the uptake of metolachlor-GS and estradiol-β-glucuronide, well-known substrates of vacuolar transporters AtMRP1 and AtMRP2.
INTRODUCTION
FK506 binding proteins (FKBPs) form, together with the cyclosporin A (CsA) binding cyclophilins and parvulins, three structurally unrelated classes of proteins known to function as cis-trans-peptidylprolyl isomerases (PPIases; Schiene and Fischer, 2000). Small FKBPs, such as FKBP12, are thought to modulate signal transduction pathways (Luan, 1998). Cyclophilin-CsA and FKBP12-FK506 complexes have been shown to bind to calcineurin (PP2B), a Ca2+, calmodulin-regulated Ser/Thr-specific protein phosphatase, thereby blocking Ca2+-dependent signaling (Harrar et al., 2001) and leading to inhibition of T-cell activation. CsA and FK506 are therefore widely used to treat and prevent graft rejection in organ transplantation patients. Additionally, these products of soil-borne microorganisms have recently been shown to play a role in reversing multidrug resistance (MDR) in several types of cancer by inhibiting the efflux of anticancer drugs (Mealey et al., 1999). FKBP12 has been demonstrated to function as a physiological regulator of the cell cycle. Cells from FKBP-deficient (FKBP12-/-) mice are arrested in the G1 phase of the cell cycle (Agdhasi et al., 2001). Disruption of this gene also affects the proper function of calcium release channels in the heart muscle, leading to cardiovascular disorders in mutant mice (Shou et al., 1998).
High-molecular-weight FKBPs are composed of one or more FKBP12-like (also referred as PPIase) domains and differ from their small counterparts by containing a tetratricopeptide repeat (TPR) domain (Das et al., 1998; Pratt et al., 2001) and a C-terminus that in most cases contains a putative calmodulin-binding domain (Harrar et al., 2001). Mammalian FKBP52, the best investigated example, is associated with HSP90 via its TPR domain in the native steroid hormone receptor complex (Silverstein et al., 1999). Plant high-molecular-weight FKBPs seem to bind plant HSP90 by means of the same TPR interaction as the mammalian homologues (Pratt et al., 2001). FKBP73 and FKBP77 from wheat have been identified as part of a HSP90 hetero-complex in vitro (Reddy et al., 1998); and, very recently, TWD1 from Arabidopsis has been shown to bind both HSP90 and calmodulin (Kamphausen et al., 2002).
Although yeast seems to be viable without immunophilins, as demonstrated by a yeast mutant strain in which the entire set of immunophilin genes is disrupted (Dolinski et al., 1997), drastic phenotypes have been associated with mutations in individual plant immunophilins. Loss-of-function mutation in the cyclophilin40 homologue of Arabidopsis lead to reductions in the number and size of juvenile rosette leaves (Berardini et al., 2001). The chloroplast localized AtFKBP13 is responsible for import and accumulation of the Rieske protein subunit of the cytochrome bf complex in the thylakoid lumen. DsRNAi silenced plants show a substantial increase in the accumulation of Rieske protein in the chloroplast (Gupta et al., 2002). The Arabidopsis T-DNA mutant pasticcino1 (pas1), which lacks a 72-kDa FKBPs, is characterized by ectopic cell division, abnormally developed cotyledons and leaves, fusion of tissues, and impaired root development, as well as hypersensitivity toward cytokinin (Faure et al., 1998; Vittorioso et al., 1998). The Arabidopsis FKBP42 mutant twisted dwarf 1 (twd1) results in a drastic reduction of cell elongation combined with a disoriented growth behavior (Geisler et al., 2003). The twd1 and ultracurvata2 (ucu2) mutants are both defective in AtFKBP42 and phenotypically indistinguishable (Pérez-Pérez et al., 2004). TWD1 has been shown to be membrane-anchored and immunolocalized on both the central vacuole and the plasma membrane (Kamphausen et al., 2002; Geisler et al., 2003). TWD1 forms a protein-protein complex via the C-terminal domains of the ABC transporters AtPGP1 and AtPGP19 (Noh et al., 2001; Murphy et al., 2002; Geisler et al., 2003), which belong to the MDR subfamily of ABC transporters (recently renamed as ABCB in humans; Dean et al., 2001). The single gene mutation twd1 and double atpgp1/atpgp19 (atmdr1) mutants exhibit similar phenotypes including epinastic growth, reduced inflorescence size, and reduced polar auxin transport in hypocotyls (Noh et al., 2001; Geisler et al., 2003), suggesting a regulatory role of TWD1 on AtPGP1/AtPGP19 transport activities (Geisler et al., 2003).
FKBPs have been suggested to function as regulators of MDR-like ABC transporters (Hemenway and Heitman, 1996; Geisler et al., 2003). Moreover, MDR resistance can be partially overcome by immunosuppressant treatment (Cardenas et al., 1994; Mealey et al., 1999), but any attempts to link a direct association with a change in transport activity have failed so far. Here we show that TWD1 interacts with the C-termini of multidrug resistance-associated (MRP/ABCB)-like ABC transporters AtMRP1 and AtMRP2. Interacting domains in AtMRP1 and TWD1 were mapped. We demonstrate TWD1-AtMRP1 interaction on the membrane of central vacuoles and show that TWD1 can modulate ABC transporter uptake activities on isolated vacuoles.
MATERIALS AND METHODS
Yeast Two-hybrid Analysis
A stretch of TWD1 containing a TPR-like domain (E163-L314), which omits a calmodulin-binding domain (Q310-G328; Kamphausen et al., 2002) as well as the C-termini of Arabidopsis ABC transporters AtMRP1, AtMRP2, AtMRP4, AtMRP5, and AtMRP7 were amplified by PCR (for MIPS codes and primer sequences see Table 1) and cloned as GAL4-binding domain (BD) and activation domain (AD) fusions into the two-hybrid vectors pACT2 and pAS2 (Clontech, Palo Alto, CA), respectively.
Table 1.
Sequences of the oligonucleotide primers used for the PCR amplification reactions and origin of constructs described in this study
| Construct (MIPS code) | Upper primer/lower primer | RT primer sequence | GAL4 fusion | Template vector | Reference |
|---|---|---|---|---|---|
| pACT2-MRP1 | —a | — | AD | —a | This work |
| (At1g30400) | |||||
| pACT2-MRP1-CaMbd | 5′ cagggatccctgcctgttcttcatggagtttcg/ | — | AD | pRTΩ-MRP1 | This work |
| (At1g30400) | 5′ ctgctcgagcatctcgacattgtcccagtc | ||||
| pACT2-MRP2 | 5′ cagccatggaaggcaattatatagagattccg/ | — | AD | pGEM-MRP2 | Geisler and Klein, unpublished |
| (At2g34660) | 5′ gttgtgactctgcgcagtgttctcgagggg | ||||
| pACT2-MRP4 | 5′ cagggatcctcactgatattccctcagaatcc/ | — | AD | pNEV-MRP4 | Geisler and Klein, unpublished |
| (At2g47800) | 5′ ctgctcgagtattccggcagatcggagagc | ||||
| pACT2-MRP5 | 5′ cagggatccagtacagtcagattgtaggagag/ | — | AD | pNEV-MRP5 | Gaedeke et al. (2001) |
| (At1g04120) | 5′ ctgctcgagataattcagggattccagtag | ||||
| pACT2-MRP7 | —a | — | AD | —a | This work |
| (At3g13100) | |||||
| pACT2-MRP13 | 5′ cagggatcctggttctccagtacatggatgtg/ | 5′ taggcaagtccatgaagactc | AD | —b | This work |
| (At2g07680) | 5′ ctgctcgagctgagaagctctgacaaagc | ||||
| pAS2-BusB | — | — | BD | — | Geisler et al. (2003) |
| (At3g21640) | |||||
| pAS2-PPIase | — | — | BD | — | Geisler et al. (2003) |
| (At3g21640) | |||||
| pAS2-TPR | — | — | BD | — | Geisler et al. (2003) |
| (At3g21640) | |||||
| pAS2-TWD1-CaMbd | 5′ tggtcatatggccatggaggg/ | — | BD | pAS2-TWD1 | Geisler et al. (2003) |
| (At3g21640) | 5′ acgggatcccaaggctttctcttgctctgc | ||||
| pBDGal4-PAS1 | 5′ acggaattcaacttccagagcatcatggac/ | 5′ tctgagtctacagtgccgttg | BD | —b | This work |
| (At3g54010) | 5′ acggtcgacagcagcggtcgcatcagcttc | ||||
| pBDGal4-ROF1 | 5′ acggaattcgacatgaacactgaggagaag/ | 5′ cgttttcaagacctcaggtgc | BD | —b | This work |
| (At3g25230) | 5′ acggtcgactttctgctctagcttcacttc | ||||
| pBSC-MRP1c | 5′ ctccgtgagattcgaggattg/ | 5′ tgatcccagagtcatcaatgg | — | —b | This work |
| (At1g30400) | 5′ tgatcccagagtcatcaatgg | ||||
| 5′ tagcggccgcatggggtttgagccgttggattgg/ | |||||
| 5′ ggactctccgtacgtttaaataaactgtc |
Restriction sites used for subcloning are underlined.
Obtained by library screening (see MATERIALS AND METHODS).
Cloned by RT-PCR.
Two pairs of nested PCR primers were used in a nested RT-PCR (see MATERIALS AND METHODS).
The C-terminus of AtMRP13 as well as the TPR domains of PAS1 and ROF1 were cloned by two-step RT-PCR as described in Geisler et al. (2003) using a gene-specific primer located in the 3′ untranslated region of the corresponding genes (see Table 1 for primer sequences). The coding regions were amplified by PCR using Vent DNA Polymerase (New England Biolabs, Frankfurt, Germany) and inserted into the indicated sites of two-hybrid vectors pACT2 and pAS2 (see Table 1). All constructs were sequenced to verify the absence of PCR errors.
For interaction analysis, three to five independent transformants of two independent transformations for each construct were analyzed for activation of histidine growth reporter (HIS auxotrophy) and LacZ (β-galactosidase) reporter activity. Therefore, single colonies were resuspended in 1 ml of sterile water and 5 μl of each suspension were spotted on SD plates containing 25 mM 3-amino-1,2,4-triazole lacking leucine, tryptophan, and histidine (-HIS). Growth was judged after 3 days. β-galactosidase activity was quantified by liquid culture assay using standard protocols.
Recombinant Expression of the AtMRP1 C-terminus
The insert of clone pACT2-AtMRP1 (BE11) encoding the C-terminus of At-MRP1 was cut out from the two-hybrid vector using BglII sites flanking the insert and ligated in-frame into the BamHI site of pQE32 (Qiagen, Hilden, Germany). This way, the resulting peptide was expressed as an N-terminal 6× His-tagged fusion protein in Escherichia coli strain BL21D3 pLysC (Stratagene, La Jolla, CA). Due to an internal BglII site (base pairs 4172), the C-terminus was split into two halves, but only the N-terminal part of the interacting protein (E1217-S1392) was successfully expressed in E. coli.
In Vitro Binding Assays
A TWD1-affinity matrix was synthesized and incubated with cleared E. coli supernatants from cells overproducing the C-terminus of AtMRP1 or vector control lysates as described in Geisler et al. (2003). Some reactions were carried out in the presence of 500 μM spinach calmodulin (Sigma, Buchs, Switzerland) with (addition of 100 μM CaCl2) or without calcium (addition of 5 mM EGTA). Eluted proteins were detected by Western blot analysis using monoclonal anti-pentaHIS and anti-RGSHis (Qiagen).
Calmodulin Overlays
Total lysates of E. coli containing the expressed AtMRP1 C-terminus were transferred to filters and probed with 100 ng/ml biotinylated CaM (Calbiochem, Darmstadt, Germany) in TBS plus 2% BSA. Blots were washed with TNT (TBS plus 0.05% Tween 20) in the presence (500 μM CaCl2) or absence of calcium (5 mM EGTA). Bound CaM was detected with alkaline phosphatase coupled to streptavidine (Promega, Madison, WI).
Cloning of AtMRP1
The cDNA of AtMRP1 was isolated by screening a size selected Arabidopsis cDNA library made from plants, which were treated with the herbicide primisulfuron (Tommasini et al., 1997). A 1005-base pair AtMRP1-/AtMRP2-specific PCR fragment amplified from Arabidopsis genomic DNA (upper primer 5′ tgccaatcacttcataccatgtggtcgg, lower primer 5′ gagagagtgacgtaaa(c/t)gctcttgcagg) was used as radiolabeled probe. Positive lambda clones were purified and integrated plasmid vectors were excised via in vivo excision. Clone pBSC-24 contained the longest ORF of AtMRP1. Because pBSC-24 was lacking the first 387 base pairs of the predicted complete ORF, the 5′ end of AtMRP1 was cloned by one-step RT-PCR using the Titan RT-PCR system (Roche Diagnostics, Rotkreuz, Switzerland) and 1 μg of Arabidopsis RNA followed by a second PCR using a 10-fold dilution of the one-step RT-PCR reaction as template. Nested PCR primers contained NotI and SphI restriction sites (underlined in Table 1) that enabled completion of the ORF in pBSC-24 resulting in vector pBSC-MRP1.
Transgenic Plants
A NotI adapter was ligated into a XhoI site downstream the stop codon of vector pBsc-MRP1, and the AtMRP1 ORF was subcloned as NotI-XhoI fragment into vector pRTΩ (Überlacker and Werr, 1996), resulting in pRTΩ-MRP1. The entire gene of an enhanced GFP version (EGFP) was PCR amplified and inserted into the HindIII (base pairs 4526) and XbaI (base pairs 4729) sites of AtMRP1 (pRTΩ-MRP1), respectively (see Figure 5A). The cassette containing the CaMV 35S promoter, MRP1-EGFP and the polyadenylation signal was excised with AscI and inserted into vectors pGPTV-bar (Überlacker and Werr, 1996) and the resulting binary constructs pGPTV-MRP1-12 and pGPTV-MRP1-13 were used to transform Arabidopsis via vacuum infiltration using Agrobacterium strain GV3101. Resistant transformants were selected on soil by watering with a BASTA solution (1:20,000).
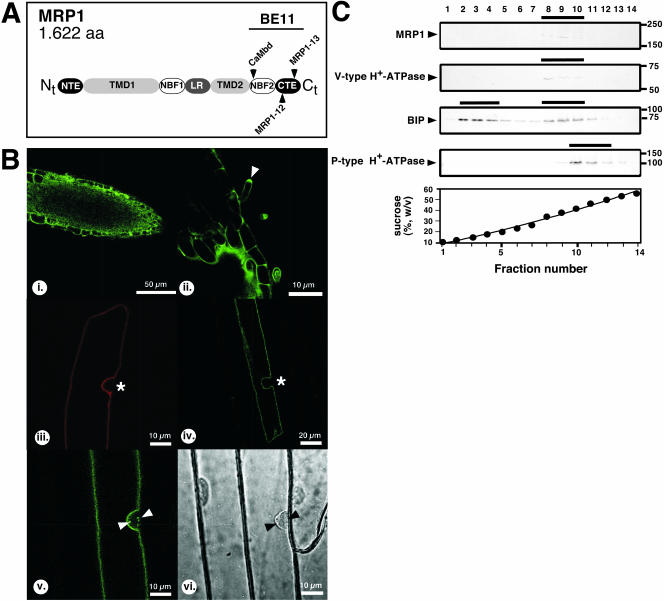
Figure 5.
AtMRP1 is localized on the central vacuolar membrane. (A) Structural model of AtMRP1. Functional domains of AtMRP1 are boxed. A putative calmodulin-binding domain (CaMbd) and extension of the isolated two-hybrid clone BE11 as well as the sites of insertion of enhanced GFPs (EGFP) into constructs pGPTV-MRP1-12 and pGPTV-MRP1-13 are indicated. Abbreviations are as follows: NTE, N-terminal extension; TMD, transmembrane domain; NBF, nucleotide binding fold; LR, linker region; CTE, C-terminal extension. (B) Laser scanning confocal analysis of plant material expressing a GFP-tagged version of AtMRP1. Images represent internal optical sections generated by CLSM from root material of heterozygous plants expressing ectopically (i and ii) and onion epidermis cells transiently expressing AtMRP1-EGFP (iv and v). The tonoplast surrounding the cytoplasma including the nucleus is indicated by asterisks; small GFP-labeled cytoplasmic structures obtained with construct pGPTV-MRP1-13 are marked by arrows. (i) Root apex. (ii) Close-up of the root hair zone. (iii) Onion epidermis cell transiently expressing the tonoplast marker KCO1-GFP (Schönknecht et al., 2002; red color). (iv) Onion epidermis cell expressing AtMRP1-EGFP (construct pGPTV-MRP1-12, green color). (v) Onion epidermis cell expressing AtMRP1-EGFP (construct pGPTVMRP1-13, green color). (vi) Same cells in a bright field. (C) Arabidopsis microsomal fractions from wild-type plants separated by linear sucrose gradient centrifugation were probed with anti-AtMRP1 antisera. Origin of immunopositive fractions was ascertained by Western blots using antisera against the marker proteins vacuolar V-type H+-ATPase, ER localized BIP, and the plasma membrane-bound P-type H+-ATPase (Geisler et al., 2000a).
Genetic nomenclature was used following the community standards for Arabidopsis genetics (Meinke and Koornneef, 1997).
Transfection of Onion Epidermis Cells and Confocal Laser Scanning Microscopy
Onion epidermis cell layers were transfected with both constructs pGPTVMRP1-12 and pGPTV-MRP1-13 after coating 0.6-μm gold microparticles (Bio-Rad, Hercules, CA) using a particle inflow gun as described in Ibrahim et al. (2000). After bombardment at low-pressure helium flow (26 psi), epidermal layers were incubated at RT for 16-24 h in the dark.
Transgenic Arabidopsis plants were grown for 7 days on MS plates including BASTA (1:20,000) under continuous light. Transfected onion epidermis cells and young Arabidopsis seedlings were analyzed by confocal laser scanning microscopy (CLSM). FITC fluorescence using the corresponding filter sets was recorded, and stored images were then colored as green or red colors using Adobe Photoshop 5.5 (Adobe Systems, San Jose, CA).
Membrane Fractionation
Arabidopsis microsomes were prepared and separated by continuous sucrose gradient centrifugation as described in Geisler et al. (2003). Nitrocellulose membranes were probed with anti-AtMRP1 and the following marker enzyme antibodies: anti-P-type H+-ATPase, anti-V-type H+-ATPase (2E7), anti-PPase and anti-BIP as described in Geisler et al. (2003). The anti-AtMRP1 antibodies were raised in rabbits by injecting 8 mg of synthetic peptide (C-NYIEIPSEAPLVIENNR), which was conjugated with keyhole limpet hemocyanin. The peptide sequence corresponds to the internal hydrophilic region of AtMRP1 (N1210-R1226).
For vacuolar pull down assays, Arabidopsis vacuolar membranes (50 μg protein) were incubated for 30 min with 20 μg TWD1-3 or 15 μg 14-3-3-GF14-ω protein in sorbitol buffer (400 mM sorbitol, 30 mM KCl, 20 mM HEPES, pH 7.2) at 4°C. Proteins were expressed from plasmids pTWD1-3 (Geisler et al., 2003) and pMP681 (Baunsgaard et al., 1998), purified by Ni-affinity chromatography, and dialyzed against sorbitol buffer. Vesicles were pelleted by ultracentrifugation and washed twice with sorbitol buffer. Equal volumes of pellets and supernatants (20 μl of 360 μl final volume) were separated by 15% PAGE, and TWD1-3 and 14-3-3-GF14-ω were detected using monoclonal anti-pentaHIS antibodies.
Coimmunoprecipitation
Immunoprecipitations using protein G-coupled anti-AtMRP1 were carried out as described in Geisler et al. (2003) with the following modifications: microsomal membranes prepared from Arabidopsis wild-type leaves were enriched for vacuolar membranes by pooling immunopositive TWD1 fractions obtained by sucrose gradient centrifugations (fractions 8-10; see Figure 5C). Immunoprecipitations using anti-HA affinity matrix were carried out according to the manufacturer (Roche Diagnostics, Rotkreuz, Switzerland) with microsomes derived from Arabidopsis plants expressing an N-terminal HA-tagged version of TWD1 (TWD1-HA; Geisler et al., 2003). After cross-linking, membrane proteins were solubilized using 0.1% (vol/vol) Brij 35 and 0.05% (wt/vol) CHAPS in 10 mM potassium phosphate, pH 7.8, 250 mM sucrose, and 20% (vol/vol) glycerol. Eluted proteins were separated by PAGE using 7.5%, 12.5% Laemmli gels, or 4-12% Criterion XT Bis-Tris gels (Bio-Rad Laboratories, Reinach, Switzerland) in the presence or absence of 1 mM DTT.
Vacuolar Uptake Experiments
Vacuoles were prepared from Arabidopsis cell suspension cultures (Axelos et al., 1992) and transport studies using the silicon oil centrifugation technique was carried out as described in Frangne et al. (2002). Some reactions were performed in the presence of 1 μM bovine brain calmodulin and 1 μM purified TWD1-3 protein (Geisler et al., 2003). Uptake was started by addition of 30 μl concentrated vacuole suspension and stopped after 10 min by centrifugation. 14C-metolachlor-GS and 3H-estradiol-β-glucuronide was determined by scintillation counting of aqueous phases and -ATP assays were considered as time 0. The vacuolar volume was calculated by the addition of 0.05 μCi 3H2O in separate assays. Conjugate-uptake experiments were performed with three independent vacuole preparations with five replicas each for each time point.
RESULTS
TWD1 Interacts Specifically with AtMRP1 and AtMRP2
Previously, we have isolated multidrug resistance (MDR)-like ABC transporter AtPGP1 as TWD1 interacting protein by screening an Arabidopsis cDNA library with the entire cytosolic domain of TWD1 as bait (Geisler et al., 2003). One of 48 sequenced prey clones encoded the C-terminal peptide (BE11) of multidrug resistance-associated (MRP)-like ABC transporter AtMRP1 (Lu et al., 1998). This interaction is specific because yeast strains expressing both proteins expressed high levels of β-galactosidase activity and were able to grow on medium lacking histidine, indicating a direct interaction between these two fusion proteins (Figure 1A). Negative controls of GAL4-binding domain (BD) or activation domain (AD) alone were not interacting with TWD1 or AtMRP1, respectively. Interestingly, as in the case of the AtPGP1 TWD1 interaction (Geisler et al., 2003), the AtMRP1 prey also coded for the C-terminus of AtMRP1. This domain comprises the C-terminal nucleotide binding fold covering the Walker A and B boxes and the intermediate ABC signature (Sanchez-Fernandez et al., 2001; Martinoia et al., 2002).
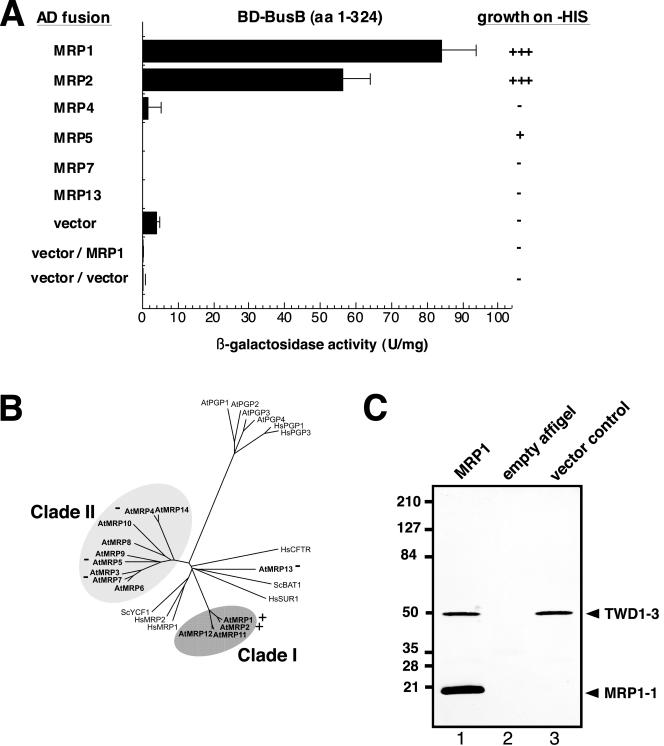
Figure 1.
TWD1 interacts specifically with the C-termini of AtMRP1 and AtMRP2. (A) Yeast two-hybrid analysis of TWD1 interacting clones. Two-hybrid screening of an Arabidopsis cDNA library using TWD1 (BD-BusB) resulted in the identification of AtMRP1 (clone BE11). Homologous stretches of selected AtMRPs were fused to a GAL4 activation domain and tested for interaction with the soluble part of TWD1 (BD-BusB). Negative controls are from top to bottom: BD-BusB/AD vector, BD vector/AD-MRP1, and AD vector/BD vector. Activation of histidine growth reporter (growth on -HIS) is indicated by + and -; LacZ reporter activities are displayed as units per mg; error bars represent standard deviations from three to five independent transformants. (B) Arabidopsis MRP-like ABC transporters cluster into two clades. The tree was modified from Kolukisaoglu et al. (2002) and identity and accession numbers can be deduced from there. Positive and negative two-hybrid interactions of AtMRPs tested against TWD1 in A are indicated with + and -, respectively. (C) In vitro interaction between TWD1 and AtMRP1. A TWD1 affinity matrix was incubated with cleared E. coli lysates containing the expressed C-termini of AtMRP1 (lane 1) or the vector control (lane 3). As negative control, empty Affigel beads were incubated with the C-terminus of AtMRP1-1 lysate (lane 2). Matrix-eluted proteins were separated by PAGE and immunoprobed against penta-His and anti-RGSHis recognizing the TWD1-3 and AtMRP1-1 peptides, respectively. Note that unproportional staining of TWD1-3 and AtMRP1-1 peptides is due to different affinities of the used antisera toward their antigens (see text).
We generated GAL4-BD fusions of homologous stretches of AtMRP2, the closest homologue of AtMRP1 sharing 85% sequence identity, and some selected members of the Arabidopsis MRP/ABCC gene family (Kolukisaoglu et al., 2002; Figure 1B). These peptides were tested for their ability to bind TWD1. AtMRP2—but not AtMRP4, AtMRP5, AtMRP7, and AtMRP13—interacted specifically with TWD1 in the yeast two-hybrid system as judged from β-galactosidase and His auxotrophy analysis. β-galactosidase activity (but not the growth assay on -HIS plates) for MRP2 was about 30% lower, possibly indicating a slightly lower magnitude of interaction.
To verify the two-hybrid data in vitro, a part of the two-hybrid peptide BE11 of AtMRP1 (E1217-S1392) was expressed in E. coli. This peptide was incubated as total lysate with purified TWD1-3 protein (M1-K337) immobilized on Affigel beads, constituting a highly specific TWD1 affinity matrix (Geisler et al., 2003). The TWD1 matrix was able to specifically sediment the AtMRP1 C-terminus of 20 kDa from soluble E. coli extracts (Figure 1C, lane 1). No proteins were detected in controls in which the empty Affigel resin or a vector control lysate (Figure 1C, lane 2 and lane 3, respectively) were used. Disproportional staining of TWD1-3 and AtMRP1-1 peptides is due to different affinities of the used anti-pentaHIS and anti-RGSHIS recognizing TWD1-3 and AtMRP1-1, respectively.
Interaction with AtMRP1 Is Mediated Mainly by the Putative TPR Domain of TWD1
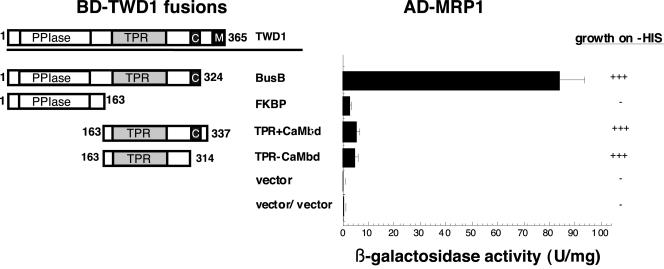
Homology modeling revealed that the putative TPR domain localized in the C-terminal part of TWD1 has the potential to form a scaffold (our unpublished results) known to act as protein-protein interaction surface (Owens-Grillo et al., 1996; Das et al., 1998; Pratt et al., 2001). To assess whether the TPR domain provided the interaction with AtMRP1, we tested GAL4-BD fusions covering the PPIase-like (M1-E163) and the TPR domains (E163-K337) of TWD1 in the yeast two-hybrid system. Indeed, AtMRP1 interacted with the C-terminal stretch comprising the TPR domain but not with the N-terminus containing the PPIase-like domain as monitored by β-galactosidase and His auxotrophy analysis (Figure 2). Because the TPR construct (TPR+CaMbd) used contained a motif with the potential to bind calmodulin downstream of the TPR repeats, we generated GAL4-BD fusions of the TPR motifs alone. This construct revealed similar two-hybrid profiles as the TPR+CaMbd construct proving that the TPR domain is mainly responsible for TWD1 complexation. Compared with the entire TWD1 bait, the TPR constructs displayed a reduced β-galactosidase activity, whereas the ability to grow on -HIS plates was in the same range. However, because the β-galactosidase activities (5.95 ± 0.53 and 5.41 ± 0.56 U/mg) were significantly higher than the vector control levels (0.98 ± 0.22 U/mg), the TPR domain alone seems to be sufficient for interaction with AtMRP1.
Figure 2.
The TPR domain of TWD1 domain is responsible for the interaction with AtMRP1. TWD1 fragments fused to an GAL4 binding domain tested for interaction with AD-MRP1 are represented by boxes as follows: PPIase, cis-trans-peptidyl prolyl isomerase domain; TPR, tetratrico peptide repeat; C, calmodulin-binding domain; M, membrane anchor. Amino acid positions are indicated. Negative controls are from top to bottom: BD-BusB/AD vector, BD vector/AD-MRP1, and AD vector/BD vector. Activation of histidine growth reporter (growth on -HIS) is indicated by + and - and LacZ reporter activities are displayed as mean units per mg; error bars represent standard deviations from three to five independent transformants.
The C-terminus of AtMRP1 Contains a Calmodulin-binding Domain
A search for candidate motifs that might be responsible for interaction to TWD1 revealed the existence of a putative calmodulin-binding domain (CaMbd) as well located in the C-terminus of AtMRP1 (W1231-Y1246; Figure 3A). Calmodulin-binding motifs are more conserved in terms of structural features than in terms of primary sequence homology (Geisler et al., 2000a, 2000b): many CaM targets contain hydrophobic residues at positions 5 and 8 that are flanked by aromatic residues at positions 1 and 15. Although this rule is not followed strictly in AtMRP1, in an α-helical wheel presentation the peptide shows a segregation of basic and polar residues to one side and hydrophobic residues to the other side (Geisler et al., 2000b), typical for CaMbd (our unpublished results). A putative CaMbd is also well conserved in AtMRP2 (Figure 3A).
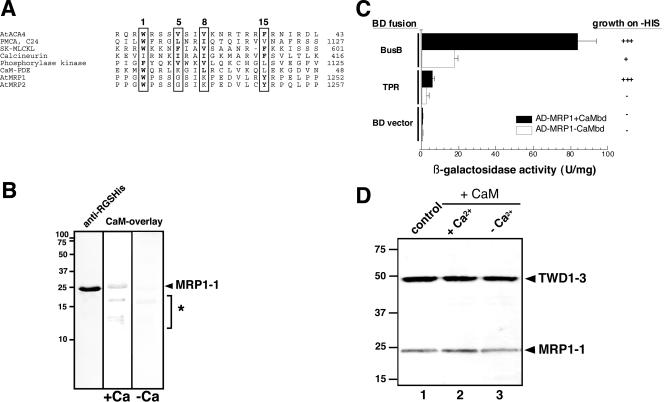
Figure 3.
A calmodulin-binding domain in AtMRP1 mediates interaction to TWD1. (A) Alignment of a putative calmodulin-binding domain (CaMbd) of AtMRP1 (MIPS code At1g30400) and AtMRP2 (At2g34660) with selected CaMbds. CaMbds of different origin were taken from Geisler et al. (2000b) and aligned using MegAlign (DNAStar, Madison, WI). Position of conserved residues essential for calmodulin binding are boxed, and conserved residues are printed in bold. (B) Calmodulin overlay the AtMRP1 C-terminus. Soluble E. coli extracts of cells expressing the MRP1 C-terminus (AtMRP1-1: residues 1217-1392) were transferred onto nitrocellulose and probed using anti-RGSHis antisera or biotinylated calmodulin (CaM-overlay) in the presence (+Ca) or absence of calcium (-Ca). The asterisk marks binding of E. coli proteins that were found also with vector control lysates. (C) The putative CaMbd domain of AtMRP1 is mainly responsible for the interaction with TWD1. AD-MRP1 fragment with (▪) and without putative CaMbd (□) are tested against indicated TWD1 fragments fused to a GAL4 binding domain. Activation of histidine growth reporter (growth on -HIS) is indicated by + and - and LacZ reporter activities are displayed as mean units per mg; error bars represent standard deviations from three to five independent transformants. (D) Calmodulin does not affect the interaction between TWD1 and AtMRP1 in vitro. A TWD1 affinity matrix was incubated with cleared E. coli lysates containing the expressed C-termini of AtMRP1 (control, lane 1). Some reactions were preincubated with spinach calmodulin in the presence (+Ca, lane 2) or absence of calcium (-Ca, lane 3). Matrix-eluted proteins were separated by PAGE and immunoprobed against anti-pentaHIS.
Indeed, by using calmodulin-overlays, we were able to show that the expressed C-terminus was able to bind calmodulin in a calcium-dependent manner. Calmodulin-positive bands matched exactly the size of the known molecular weight of AtMRP1 C-terminus as could be judged from Western analysis of samples run in parallel (Figure 3B). Smaller soluble peptides of the E. coli cell lysate also reacted with calmodulin (see asterisks in Figure 3B), but are probably unspecific because CaM binding took place in the absence of calcium and was also found with vector control lysates (our unpublished results).
The C-terminal Calmodulin-binding Domain of AtMRP1 Is Mainly Responsible for TWD1 Interaction
To elucidate whether the identified CaMbd motif in the AtMRPs corresponds to the acceptor for the TPR motif of TWD1, we deleted this peptide (E1217-P1251) from the GAL4-AD-MRP1 fusion construct. Two-hybrid analysis showed that with the entire soluble part of TWD1, β-galactosidase activity as well as growth on -HIS plates was heavily reduced (Figure 3C). Even more obvious was the effect with the C-terminus of TWD1, which contains mainly TPR stretches. In summary, this indicates that the putative CaMbd alone can function as acceptor for the TWD1 TPR domain.
On the basis of these findings, we asked whether calmodulin would affect the binding of the AtMRP1 C-terminus. Preincubations with a high concentration of spinach calmodulin (500 μM) before addition of the AtMRP1 target seemed to have no effect on AtMRP1 binding to the TWD1-matrix in pull-down assays (Figure 3D).
PAS1 Is Able To Interact with AtMRP1 But Uses a Different Interaction Domain
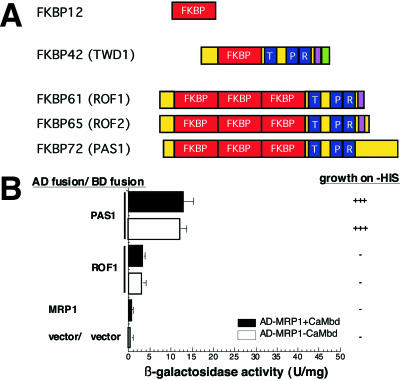
A FKBP gene family with 17 putative members (Harrar et al., 2001) has been identified in Arabidopsis. Members that contain three repetitions of TPR motifs are TWD1, both ROF isoforms, ROF1 and ROF2 (Vucich and Gasser, 1996; Harrar et al., 2001), and PASTICCINO1 (PAS1; Faure et al., 1998; Vittorioso et al., 1998; Figure 4A). To demonstrate specificity toward AtMRP1, we therefore tested TPR domains of ROF1 and PAS1 for AtMRP1 interaction in the yeast two-hybrid system. PAS1, but not ROF1 is able to interact with AtMRP1 (Figure 4B). Unlike TWD1, PAS1 seems not to interact with the CaMbd of AtMRP1 but with different regions of the C-terminus as two-hybrid analysis with AtMRP1 lacking the CaMbd showed very similar two-hybrid profiles (Figure 4B).
Figure 4.
The PAS1 but not ROF1 TPR domain of TWD1 interacts with AtMRP1 in yeast two-hybrid assays. (A) Domain structures of high-molecular-weight FKBPs from Arabidopsis. Functional domains of At-FKBP12, TWD1, ROF1, ROF2, and PAS1 are boxed and indicated as follows: cis-trans peptidy-prolyl isomerase domain (FKBP), red; tetratrico peptide repeats (TPR) blue; CaMbd, violet; membrane anchor, green. (B) Specificity of PAS1-AtMRP1 interaction in the yeast two-hybrid system. TPR fragments of PAS1 and ROF1 were fused to a GAL4 binding domain and tested for interaction with the C-terminus of AtMRP1 including the CaMbd (AD-MRP1+CaMbd) or lacking the CaMbd (AD-MRP1-CaMbd). Negative controls are from top to bottom: AD-MRP1/BD vector and AD vector/BD vector. Activation of histidine growth reporter (growth on -HIS) is indicated by + and - and LacZ reporter activities are displayed as mean units per mg; error bars represent standard deviations from three to five independent transformants.
AtMRP1 and TWD1 Form a Complex on the Central Vacuolar Membrane
The intracellular localization of AtMRP1 is unknown; however, the transport specificity of AtMRP1 approximates that of endogenous vacuolar GS-conjugate pumps (Lu et al., 1998, 2001; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002).
To determine precisely the intracellular localization of AtMRP1 we cloned AtMRP1 into a plant binary vector and constructed two independent C-terminal fusions with an enhanced version of the green fluorescent protein (EGFP). Both EGFPs were inserted into the C-terminal extension (CTE) after the second nucleotide binding fold of AtMRP1 (Figure 5A).
Confocal microscope analysis of heterozygous transgenic seedlings showed high levels of AtMRP1-EGFP fluorescence in the root apex and root epidermis (Figure 5Bi), whereas plants transformed with vector control revealed no GFP fluorescence (our unpublished results). Surprisingly, no fluorescence was found in the shoot. In the root hair zone, root hairs revealed fluorescent caps at their tips (arrows in Figure 5Bii). Fluorescence surrounded the cells, however, even from close-ups it was difficult to differentiate between tonoplast and plasma membrane, mainly because in fully turgid cells the cytoplasm is limited to just small stripes.
Therefore, we expressed AtMRP1-EGFP in onion epidermis cells that were transiently transformed by particle bombardment. The vacuolar GFP marker KC01, a component of the slow-vacuolar K+ channel (Schönknecht et al., 2002) labeled the tonoplast, that surrounds the central vacuole, sparing the cytoplasm, which is mainly restricted to a small spot (red color and asterisk in Figure 5Biii). Both EGFP constructs (green color in Figure 5B, iv and v) showed very similar expression patterns compared with the vacuolar control, suggesting that AtMRP1 resides on the tonoplast. In some onion cells small vesicles of unknown origin were labeled in addition to the tonoplast (arrows in Figure 5B, v and vi).
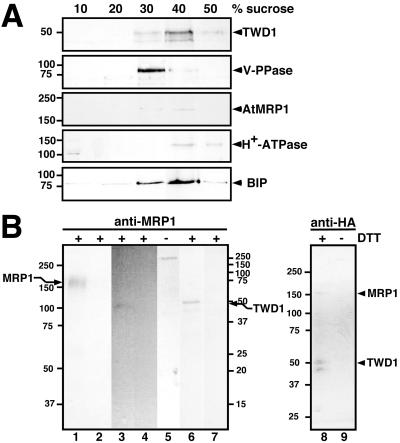
To immunologically verify these data, we probed Arabidopsis microsomes separated by linear sucrose gradient density centrifugation with a polyclonal antiserum that was raised against a 17-mer peptide of AtMRP1 (N1210-R1226). This antiserum detected a single band of the expected size (∼180 kDa) in fractions 8-10 (sucrose concentrations between 35 and 43%) of the sucrose gradient (Figure 5C). AtMRP1 colocalized with the vacuolar V-type H+-ATPase (same distribution of peak fraction), whereas markers for the plasma membrane (P-type H+-ATPase) or ER (BIP) cross-reacted with other fractions (Figure 5C).
A unique feature of TWD1 is the existence a C-terminal hydrophobic α-helical region, which anchors it apparently both in the plasma membrane and the central vacuolar membrane, the tonoplast. Using electron microscopy, a HA-tagged version of constitutively overexpressed TWD1 (TWD1-HA) has been immunolocalized in the plasma membrane and the vacuolar membrane (Kamphausen et al., 2002). In a parallel study, using membrane fractionation and cellular immunolocalization techniques, a plasma membrane location has been verified (Geisler et al., 2003). However, also in those continuous sucrose gradients TWD1-positive fractions show a clear overlap with vacuolar fractions (identified using the vacuolar V-type H+-ATPase marker) of lower sucrose percentage/higher membrane density (fractions 8-10; Geisler et al., 2003).
To verify the dual location of TWD1, we reanalyzed selected fractions derived from linear sucrose gradient separation of TWD1-HA microsomes. In contrast to our previous study, we analyzed equal protein amounts of each fraction and used as a vacuolar marker antisera directed against a mung bean vacuolar PPase. Western blotting clearly indicates that TWD1-HA-positive fractions overlap to similar extends with both plasma membrane (40 and 50% sucrose) and vacuolar fractions (30 and 40% sucrose; Figure 6A).
Figure 6.
In vivo interaction between TWD1 and AtMRP1. (A) TWD1-HA overlaps with vacuolar and plasma membrane fractions in a continuous sucrose gradient. Arabidopsis microsomal fractions from plants expressing TWD1-HA were separated by linear sucrose gradient centrifugation and equal amounts of protein of selected fractions were probed with anti-HA and anti-MRP1. Origin of immunopositive fractions was ascertained by Western blotting using antisera against the marker proteins vacuolar PPase, ER localized BIP and the plasma membrane-bound P-type H+-ATPase (Geisler et al., 2000a). (B) Immunoprecipitation of a TWD1-AtMRP1 complex. Microsomal membranes from Arabidopsis wild-type (lanes 1-5) or TWD-HA plants (lanes 6-9) were cross-linked with DTBP, solubilized using 0.1% Brij 35 and 0.05% CHAPS and immunoprecipitated using an anti-AtMRP1 or anti-HA affinity matrix, respectively. Immunoprecipitated proteins were separated by 7.5% (lanes 1, 2, and 5), 12.5% (lanes 3, 4, 6, and 7) or 4-12% PAGE (lanes 8 and 9) in the presence (+) or absence of DTT (-) and probed with anti-AtMRP1 (lanes 1-2, 8-9), anti-TWD1 (lanes 3-5), and anti-HA (lanes 6-9), respectively. As negative controls, unspecific binding of proteins to empty protein G was monitored (lanes 2 and 4). Note that the size difference of the coprecipitated wild-type TWD1 in lane 3 having a slightly smaller weight than the HA-epitope-tagged TWD1 (lane 6) is due to the lack of the HA-epitope. Molecular size markers on the left and right correspond to lanes 1-2, and 5 and lanes 3-4, 6-7, respectively; positions of TWD1 and AtMRP1 are indicated.
To demonstrate the TWD1-AtMRP1 complex in vivo, At-MRP1 and TWD1 were immunoprecipitated from solubilized wild-type and transgenic TWD1-HA (Geisler et al., 2003) microsomes, respectively, after cross-linking with thiol-cleavable DTBP (Figure 6B). Cross-linking was used to avoid disruption of protein-protein interactions during strong detergent treatments to solubilize both membrane proteins from the tonoplast.
Using immobilized anti-AtMRP1 antiserum for immunoprecipitation, wild-type TWD1 was detectable in a Western analysis (Figure 6B, lane 3), suggesting coprecipitation with AtMRP1 (Figure 6B, lane 1). The same was true for TWD1-HA being slightly bigger than the wild-type protein (Figure 6B, lane 6). This was the case under reducing conditions (+DTT), which cleave the DTBP cross-linker between AtMRP1 and TWD1. Under nonreducing conditions, a high-molecular-weight complex of more than 250 kDa was detected using anti-TWD1 (Figure 6B, lane 5), suggesting either multi-merization of more than one TWD1 with one AtMRP1 or the involvement of so far unknown components. No AtMRP1 or TWD1 could be detected in control experiments using empty protein G, respectively (lanes 2, 4, and 7).
Vice versa, using an anti-HA affinity matrix we were able to detect AtMRP1 beside TWD1-HA; however, the entire complex could not be analyzed by PAGE under nonreducing conditions (lane 9).
Purified TWD1 Protein Modulates Uptake of AtMRP-like ABC Transporter Model Substrates into Isolated Vacuoles
All cloned AtMRPs have been shown to transport GS conjugates, such as metolachlor-GS (MOC-GS), a glutathionated chloroacetanilide herbicide, to different degrees in vitro (Rea et al., 1998; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002). But only for AtMRP1 and AtMRP2 are quantitative data available (Liu et al., 2001). The overall transport capacity of AtMRP2, the sole AtMRP that has been roughly localized to vacuolar-enriched membranes (Liu et al., 2001), exceeds that of AtMRP1. The substrate specificity of AtMRP1 fits better that of an endogenous vacuolar GS-conjugate pumps than does AtMRP2 (Lu et al., 1998, 2001).
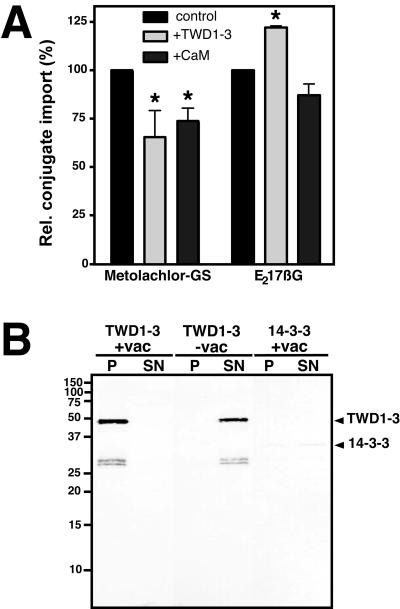
To investigate whether the interaction of TWD1 with vacuolar ABC transporters (AtMRP1 and AtMRP2) has a physiological impact on their transport activities, we measured the uptake of two ABC transporter model substrates into isolated vacuoles in the presence of purified TWD1-3 and calmodulin. Isolated Arabidopsis vacuoles offer an ideal test system, because the C-termini of transporters as putative interacting domains are thought to face the cytoplasm (Rea et al., 1998; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002) and are therefore accessible for interfering proteins in this assay. MOC-GS uptake was significantly reduced by both, TWD1-3 (34% inhibition) and calmodulin (26% inhibition) preincubation. On the other hand, TWD1-3 protein stimulated the uptake of 17β-estradiol 17-(β-d-glucuronide) (E217βG), a glucuronide test substrate, whereas calmodulin had no significant effect.
Vacuolar microsomes offered in excess were able to quantitatively sediment the TWD1-3 protein as demonstrated by Western analysis (Figure 7B, lane 1) when equal volumes of bound (P) and unbound fractions (SN) were used. No TWD1-3 protein was detected in bound fractions when microsomes were omitted from the assay (Figure 7B, lane 3), indicating the specificity of the interaction. Regarding the expected molar ratio of AtMRP1/TWD1, the high amount of sedimented TWD1 indicates that other transporters besides AtMRP1 (i.e., AtMRP2) might contribute to this effect. As a specific control, we tested the Arabidopsis 14-3-3 protein GF14-ω known to structurally resemble TPR domains (Das et al., 1998). This isoform of 14-3-3 (Baunsgaard et al., 1998) was also able to show some binding to vacuolar membranes but to a much lesser extend (<50%; Figure 7B, lanes 5 and 6).
Figure 7.
TWD1 binds to and modulates uptake of ABC transporter model substrates into vacuoles. (A) Uptake of 14C-metolachlor-GS and 3H-estradiol-β-glucuronide were carried out in the absence (control) or presence of each 1 μM purified TWD1-3 protein (+TWD1-3) and 1 μM calmodulin (+CaM), respectively. Conjugate uptake was measured with five replicas for each time point. Relative activities are displayed as means with standard deviations from three independent vacuole preparations. Import activities being statistically different (Mann-Whitney U test, p > 0.05) compared with control experiments are indicated by an asterisks. (B) Purified TWD1-3 protein binds specifically to vacuolar membranes. Arabidopsis vacuoles (+vac) were incubated with purified TWD1-3 or 14-3-3 protein (14-3-3-GF14-ω; Baunsgaard et al., 1998) and pelleted by ultracentrifugation. Equal volumes of pellets (P) and supernatants (SN) were separated by PAGE and TWD1-3, and 14-3-3-GF14-ω were detected using anti-pentaHIS antibodies.
DISCUSSION
Previously, TWD1 has been demonstrated to interact with Arabidopsis p-glycoprotein ABC-transporter AtPGP1 and its closest homologue, AtPGP19 (Geisler et al., 2003). Physiological and biochemical investigation of atpgp1 atpgp19 double mutants and twd1 plants suggest that a functional TWD1-AtPGP1/AtPGP19 complex is required for proper plant development. Therefore, a regulatory role of TWD1 on At-PGP1/AtPGP19 transport activities has been suggested (Geisler et al., 2003).
To further understand the dramatic pleiotropic phenotype that is caused by loss-of-function mutation of the TWD1 gene, we were interested in other TWD1-interacting proteins. AtMRP1, a full-size ABC transporter of the MRP/ABCC subclass (Kolukisaoglu et al., 2002; Martinoia et al., 2002), has been isolated in a yeast two-hybrid screen as TWD1-interacting protein. In this study, we demonstrate molecular interactions between TWD1 and Arabidopsis ABC transporters AtMRP1 and its closest homologue AtMRP2. Using GFP-tagging and membrane separation we localized AtMRP1, like TWD1, to the central vacuolar membrane and show that TWD1 has an impact on ABC transporter uptake activities into isolated vacuoles.
TWD1 Interacts Specifically with AtMRP1 and AtMRP2 via Its TPR Domain
Quantification of histidine reporter growth and the LacZ reporter of retransformed prey clones for isolated AtMRP1 with the TWD1 bait (BD-BusB) confirmed the interaction between the two proteins. In vitro interaction using a highly specific TWD1 affinity matrix (Geisler et al., 2003) verified the two-hybrid data (Figure 1). To further sustain specificity of interaction, we tested other C-termini of homologues of AtMRP1 in the two-hybrid system. Based on the AGI data (Arabidopsis Genome Initiative, 2000), 14 MRP-like transporter genes have been suggested for Arabidopsis (Kolukisaoglu et al., 2002; Martinoia et al., 2002) that can be assigned—with the exception of MRP13-to two clades (Figure 1B). None of the selected transporters, covering most subbranches of the phylogenetic tree of the AtMRP gene family, interacted with TWD1. The only other tested AtMRP to interact with TWD1 was AtMRP2, the closest homologue of AtMRP1. The fact that binding to TWD1 has been found only for AtMRP1 and AtMRP2 is an interesting finding and further proves specificity of this interaction.
A similar picture is true for AtPGPs interacting with TWD1 on the plasma membrane. Here, only AtPGP19, the closest homologue of AtPGP1 was shown to be a TWD1-interacting protein (Geisler et al., 2003). As with AtPGP1, the interacting AtMRP1 clone BE11 isolated from a two-hybrid screen covers nearly the entire C-terminus of AtMRP1. At-MRP1 and AtMRP2 bear—in contrast to all other AtMRPs and P-glycoproteins/MDRs/ABCBs (Liu et al., 2001)—a so-called C-terminal extension domain (CTE; see Figure 5A), which extends their C-terminus far longer (405 compared with 109 residues) than in AtPGP1. Based on in vitro interaction experiments, the TWD1-interacting region was further limited to 175-aa residues (E1217-S1392). However, identification of a putative calmodulin-binding domain just upstream of the Walker A motif of the C-terminal NBF allowed to map the TWD1 docking domain down to 34 aa (E1217-P1251) as deletion of this domain in the yeast two-hybrid construct abolishes interaction (Figure 3). This domain shows typical features of CaMbds (see above). Calmodulin binding to the expressed AtMRP1 C-terminus verifies the identity of this domain.
Vice versa, mapping of TWD1 motifs required for At-MRP1 binding demonstrated that AtMRP1 recognizes the C-terminal TPR domain, whereas AtPGP1 was shown to interact with the N-terminal PPIase-like domain (Geisler et al., 2003). This is not surprising because TPRs are often implicated in protein-protein interactions observed with diverse proteins and other FKBPs (Das et al., 1998; Harrar et al., 2001). Recently, a region of TWD1 containing the TPR domain has been shown to bind AtHsp90.1 and to prevent citrate synthase aggregation in analogy to the human PPIase TPR domains (Kamphausen et al., 2002).
However, besides TWD1, only the TPR domain of PAS1 but not of ROF1 was able to interact with AtMRP1, emphasizing the specificity of interaction. Interestingly, TWD1 and PAS1 seem to use different docking sites on the AtMRP1 molecule because deletion of the putative CaMbd did not reduce interaction with PAS1 (Figure 4B). Based on immunological data, PAS1 has a nuclear localization (Carol et al., 2001), making an interaction between PAS1 and vacuolar localized AtMRP1 (or AtMRP2) unlikely. This again highlights the role of TWD1 as the sole membrane-associated FKBP that interacts with integral membrane proteins such as the AtMRPs.
TWD1 Functionally Interacts with Vacuolar ABC Transporters
AtMRP1 has been identified by screening an ordered expression library with polyclonal antibodies raised against Arabidopsis plasmalemma-enriched membranes (Galaud et al., 1999). Using AtMRP1-specific antisera, we immunodetected AtMRP1 in microsomal fractions from vacuoles as shown by linear sucrose gradient centrifugation. Cross-reaction of the antibodies to the close homologue vacuolar-localized At-MRP2 (see below) cannot be ruled out because the 17-mer peptide used as antigen is also well conserved in the latter (88% sequence identity). However, because a band was exclusively observed in the tonoplast enriched fractions, our results indicate that even assuming a cross-reaction, both AtMRP1 and AtMRP2 reside on the vacuolar membranes.
GFP-tagging clearly showed that AtMRP1 resides on the tonoplast. In line with these data, transport activities assigned to AtMRP1 are found on vacuoles (Lu et al., 1997, 1998).
This is to our knowledge the first detailed localization of an MRP-like ABC transporter in plants. The closest homologue of AtMRP1, AtMRP2, has been detected in vacuolar-enriched fractions of Arabidopsis using polyclonal antisera directed against a short peptide of AtMRP2 (Liu et al., 2001; A1569-R1579). The AtMRP2 antibody has been suggested to be monospecific because it does not cross-react with heterologous produced AtMRP1. However, it seems very likely that both transporters reside on vacuolar membranes.
Considering the vacuolar location of AtMRP1 (and probably also of AtMRP2), we wanted to explore if the shown interaction of both proteins has an impact on vacuolar ABC transporter activities. Purified TWD1-3 protein inhibits metolachlor-GS conjugate uptake but stimulates estradiol-β-glucuronide import into vacuoles (Figure 7A). Both are well-described substrates for AtMRP1 and AtMRP2. Therefore these transporters are good candidates for catalyzing this uptake in vivo. Stronger effects of the TWD1 action might be masked by endogenous TWD1 still partially bound to the respective transporters in isolated vacuoles. In addition, other signaling components (like protein kinases) lacking in the in vitro assay might amplify the modulating effect of TWD1 in the cell. Finally, it could be, that activation of vacuolar AtMRP-mediated transport activities via TWD1 is increased under stress conditions. If this would be the case, modulation by TWD1 could already have taken place during vacuole isolation because treatment of plants with cellulases and pectinases is a typical stress inducing treatment.
Calmodulin, having the potential to bind to the same epitope as TWD1, could partially mimic TWD1 inhibition on metolachlor-GS uptake, but failed in stimulating estradiol-β-glucuronide transport. As the CaMbd, the most probable acceptor for the TPR domain of TWD1, is located in the direct proximity of the MRP1 C-terminal nucleotide-binding fold (NBF2; see Figure 5A), it is conceivable that interaction of this domain with TWD1 affects ATP binding and/or hydrolysis. Furthermore, up- and downregulation of transport activities via protein interaction is not unusual, especially because the two different substrates might use different substrate binding sites. Interestingly, TWD1 mimics an effect observed with glutathione conjugates: although GS-X transport is competitively inhibited by other GS-X conjugates, several GS-X are able to strongly enhance estradiol glucuronide transport in intact vacuoles (Klein et al., 1998) or by heterologously expressed AtMRP2 (Liu et al., 2001). On the other hand, the possibility that AtMRP1 and AtMRP2 are negatively regulated by TWD1 or CaM cannot be excluded.
In contrast to these results we found no interference of CaM on AtMRP1-TWD1 interaction with in vitro pull-down assays. This might indicate on the one hand, that calmodulin and TWD1 use different binding domains (thus CaM does not bind to the designated motif), which is unlikely because no other putative CaMbd could be identified in the AtMRP1 C-terminus. On the other hand, it might be possible that TWD1 and CaM do not interfere sterically while binding AtMRP1. This, however, is also not likely because both proteins are relatively space filling compared with the small stretch of maximal 34 residues acting as contact surface. Third, and most likely, it might be that the TPR of TWD1 owns a far higher affinity for the interaction domain of the AtMRPs compared with CaM.
Using a vacuolar pull-down assay, specific binding of TWD1-3 protein to vacuolar microsomes was demonstrated (Figure 7B). This is further support that the C-termini of interacting transporters as putative interacting domains are indeed facing the cytoplasm and are therefore accessible for TWD1 proteins in this assay. This fits well with current secondary structure models for AtMRPs (Rea et al., 1998; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002).
In contrast, the Arabidopsis 14-3-3 isoform GF14-ω (Baunsgaard et al., 1998), known to structurally resemble TPR domains although overall sequence identity is low (Das et al., 1998), binds to a lesser extent (<50%), emphasizing the specificity of TWD1-3 binding. Indeed, 14-3-3 proteins have been shown to downregulate currents of vacuolar SV channels by using whole-vacuole recordings (van den Wijngaard et al., 2001). Interestingly, docking of 14-3-3 proteins to the plasma membrane H+-ATPase is induced by phosphorylation (Baunsgaard et al., 1998), which is so far not known to be essential for binding of proteins to TPR domains.
Finally, our data indicate that TWD1 has a dual regulatory function on both the plasma membrane and vacuolar membrane. TWD1 forms complexes with pairs of ABC transporters of distinct subclasses: ABCB (AtPGP1/AtPGP19; Geisler et al., 2003) and ABCC (AtMRP1/AtMRP2). This unusual distribution of high-molecular-weight FKBPs might be a typical feature in higher plants. Recently, using quantitative immunogold electron microscopy wheat high-molecular-weight TaFKBP73 and TaFKBP77 have been shown to be present in the cytoplasm, but being transported into the nucleus upon heat-shock treatments (Dwivedi et al., 2003).
Specificity of interaction between TWD1 and ABC transporters is mediated by protein-protein interactions with distinct domains of the multidomain FKBP TWD1. The PPIase-like domain and the TPR domain seem to be responsible for specificity of interaction with different subclasses of ABC transporters.
In vivo association between TWD1 and AtMRP1 was established by coimmunoprecipitation assays (Figure 6B). Therefore, one might assume that absence of TWD1 (positively modulating a vacuolar transport complex consisting of AtMRP1 and AtMRP2) is responsible for certain aspects of the twd1 phenotype. This model is supported by several studies showing that immunophilins play a regulatory role in different multiprotein complexes (Cameron et al., 1995; Timerman et al., 1995; Hemenway and Heitman, 1996; Mealey et al., 1999). However, in contrast to atpgp1/atpgp19 plants resembling twd1 mutants, atmrp1 and atmrp2 single and atmrp1/atmrp2 double mutant plants, respectively, show no obvious phenotype (Ü. Kolukisaoglu, M. Klein, and E. Martinoia, unpublished results). Detailed morphological and biochemical analyses revealed that similar phenotypes of twd1 and atpgp1/atpgp19 plants are limited to early developmental stages. The twd1 mutant exhibits a far more drastic reduction of cell elongation that corresponds to further reduced height of the entire plant. Disorientation of growth behavior resulting in pronounced twisting of all organs can be observed only in twd1 plants. Compared with atpgp1/atpgp19 plants, polar auxin transport rate in hypocotyls is also further reduced in twd1 mutants (Geisler et al., 2003). Therefore, it cannot be ruled out that the combination of mutations of TWD1 interacting partners might reveal the entire picture of the phenotype that was observed in twd1 mutants. It is obvious that the differing aspects of the rather pleiotropic twd1 phenotype needs to be addressed separately to result in a genetic dissection of the “twd1 syndrome.” Generation of quadruple mutants that combine atmrp1, atmrp2, atpgp1, and atpgp19 mutations would certainly be a next step in recombining the genetic network of TWD1 function. However, the genetic establishment of these lines is no short-term task, as the genes for the ABC transporters AtMRP2 and AtPGP1 are in close genetic proximity on the long arm of chromosome 2.
Loss of a TWD1-AtMRP1/AtMRP2 complex could therefore contribute to the twd1 phenotype due to a lack of vacuolar import of yet unknown in vivo conjugate substrates. Regarding the role of AtPGP1 and AtPGP19 in in-dole-3-acetic acid (IAA) transport, it is tempting to speculate that AtMRP1 and AtMRP2 could act as transporters for IAA conjugated to amino acids. Removal of IAA-conjugates forming negatively charged organic substrates has been suggested to be catalyzed by ABC transporters such as AtMRP5 (Gaedeke et al., 2001; Luschnig, 2002) and for related HsMRP1 glutamate conjugate transport has been demonstrated (König et al., 1999).
Acknowledgments
We thank R. Flückiger for confocal microscope analysis of transgenic plants, M. Klein for help screening the Arabidopsis library, F. Keller and J. Blakeslee for critically reading the manuscript, and M.G. Palmgren and K. Palme for providing plasmids expressing 14-3-3-GF14-ω (pMP681) and KCO1-GFP. We are grateful to Z. Koncz-Kálmán and C. Koncz for supplying the yeast two-hybrid library and for their help during the screening and Felix Mauch for providing the particle inflow gun. The help of D. Bullis, P. Galli, and P. Hartmann during 14-3-3 purification is acknowledged. This work was supported by the Swiss National Foundation, Deutsche Forschungsgemeinschaft (Schu/821-2), the European Community (LATIN, BIOTEC 4) and the Ministerium für Schule, Wissenschaft, und Forschung des Landes NRW, Novartis and the Alexander von Humboldt-Foundation (Feodor-Lynen and Novartis fellowships to M.G.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0831. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0831.
Abbreviations used: FKBP(s), FK506 binding protein(s); PPIases, cis-trans-peptidylprolyl isomerases; MDR, multidrug resistance; TPR, tetratricopeptide repeat; twd1, twisted dwarf1; ABC, ATP-binding cassette; β-gal, β-galactosidase; PGP, P-glycoprotein; CaM, calmodulin; CaMbd, CaM-binding domain; EGFP, enhanced green fluorescent protein; aa, amino acid(s); PAGE, polyacrylamide gel electrophoresis.
References
- Aghdasi, B., Ye, K., Resnick, A., Huang, A., Ha, H.C., Guo, X., Dawson, T.M., Dawson, V.L., and Snyder, S.H. (2001). FKBP12, the 12-kDa FK506-binding protein, is a physiological regulator of the cell cycle. Proc. Natl. Acad. Sci. USA 98, 2425-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796-815. [DOI] [PubMed] [Google Scholar]
- Axelos, M., Curie, C., Mazzolini, L., Bardet, C., and Lescure, B. (1992). A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol. Biochem. 30, 123-128. [Google Scholar]
- Baunsgaard, L., Fuglsang, A.T., Jahn, T., Korthout, H.A., de Boer, A.H., and Palmgren, M.G. (1998). The 14-3-3 proteins associate with the plant plasma membrane H(+)-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J. 13, 661-671. [DOI] [PubMed] [Google Scholar]
- Berardini, T.Z., Bollmann, K., Sun, H., and Poethig, S. (2001). Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291, 2405-2407. [DOI] [PubMed] [Google Scholar]
- Cameron, A.M., Steiner, J.P., Roskams, A.J., Ali, S.M., Ronnett, G.V., and Snyder, S.H. (1995). Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell 83, 463-472. [DOI] [PubMed] [Google Scholar]
- Cardenas, M.E., Hemenway, C., Muir, R.S., Ye, R., Fiorentino, D., and Heitman, J. (1994). Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 13, 5944-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol, R.J., Breiman, A., Erel, N., Vittorioso, P., and Bellini, C. (2001). PASTICCINO1 (AtFKBP70) is a nuclear-localised immunophilin required during Arabidopsis thaliana embryogenesis. Plant Sci. 161, 527-535. [Google Scholar]
- Das, A.K., Cohen, P.T.W., and Barford, D. (1998). The structure of the tetratrico-peptide repeats of protein phosphatase 5, implications for the TPR-mediated protein-protein interactions. EMBO J. 17, 1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, M., Rzhetsky, A., and Allikmets, R. (2001). The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11, 1156-1166. [DOI] [PubMed] [Google Scholar]
- Dolinski, K., Muir, S., Cardenas, M., and Heitman, J. (1997). All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 13093-13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi, R.S., Breiman, A., and Herman, E.M. (2003). Differential distribution of the cognate and heat-stress-induced isoforms of high Mr cis-trans prolyl peptidyl isomerase (FKBP) in the cytoplasm and nucleoplasm. J. Exp. Bot. 54, 2679-2689. [DOI] [PubMed] [Google Scholar]
- Faure, J.D., Vittorioso, P., Santoni, V., Fraisier, V., Prinsen, E., Barlier, I., Van Onckelen, H., Caboche, M., and Bellini, C. (1998). The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125, 909-918. [DOI] [PubMed] [Google Scholar]
- Frangne, N., Eggmann, T., Koblischke, C., Weissenbock, G., Martinoia, E., and Klein, M. (2002). Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H(+)-antiport and ATP-binding cassette-type mechanisms. Plant Physiol. 128, 726-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedeke, N. et al. (2001). The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 20, 1875-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaud, J.P., Carriere, M., Pauly, N., Canut, H., Chalon, P., Caput, D., Pont-Lezica, R.F. (1999). Construction of two ordered cDNA libraries enriched in genes encoding plasmalemma and tonoplast proteins from a high-efficiency expression library. Plant J. 17, 111-118. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Frangne, N., Gomès, E., Martinoia, E., and Palmgren, M.G. (2000a). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Phys. 124, 1814-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, M. Axelsen, B.K., Harper, J.F., and Palmgren, G.M. (2000b). Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim. Biophys. Acta 1465, 52-78. [DOI] [PubMed] [Google Scholar]
- Geisler, M. et al. (2003). TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell 14, 4238-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R., Mould, R.M., He, Z., and Luan, S. (2002). A chloroplast FKBP interacts with and affects the accumulation of Rieske subunit of cytochrome bf complex. Proc. Natl. Acad. Sci. USA 99, 15806-15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrar, Y., Bellini, C., and Faure, J.D. (2001). FKBPs: at the crossroads of folding and transduction. Trends Plant Sci. 6, 426-431. [DOI] [PubMed] [Google Scholar]
- Hemenway, C.S., and Heitman, J. (1996). Immunosuppressant target protein FKBP12 is required for P-glycoprotein function in yeast. J. Biol. Chem. 271, 18527-18534. [DOI] [PubMed] [Google Scholar]
- Ibrahim, M., Si-Ammour, A., Celio, M.R., Mauch, F., and Menoud, P. (2000). Construction and application of a microprojectile system for the transfection of organotypic brain slices. J. Neurosci. Methods 101, 171-179. [DOI] [PubMed] [Google Scholar]
- Kamphausen, T., Fanghänel, J., Neumann, D., Schulz, B., and Rahfeld, J.-U. (2002). Characterisation of Arabidopsis thaliana AtFKBP42 that is membrane bound and interacts with Hsp90. Plant J. 32, 263-276. [DOI] [PubMed] [Google Scholar]
- Klein, M., Martinoia, E., and Weissenbock, G. (1998). Directly energized uptake of beta-estradiol 17-(beta-d-glucuronide) in plant vacuoles is strongly stimulated by glutathione conjugates. J. Biol. Chem. 273, 262-270. [DOI] [PubMed] [Google Scholar]
- König, J., Nies, A.T., Cui, Y., Leier, I., and Keppler, D. (1999). Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity and MRP2-mediated drug resistance. Biochim. Biophys. Acta 1461, 377-394. [DOI] [PubMed] [Google Scholar]
- Kolukisaoglu, H. Ü., Bovet, L., Klein, M., Eggmann, T., Geisler, M., Wanke, D., Martinoia, E., and Schulz, B. (2002). Family business: the multidrug-resistance related protein (MRP) ABC transporter genes in Arabidopsis thaliana. Planta 216, 107-119. [DOI] [PubMed] [Google Scholar]
- Liu, G., Sanchez-Fernandez, R., Li, Z.S., and Rea, P.A. (2001). Enhanced multispecificity of Arabidopsis vacuolar multidrug resistance-associated protein-type ATP-binding cassette transporter, AtMRP2. J. Biol. Chem. 276, 8648-8656. [DOI] [PubMed] [Google Scholar]
- Lu, Y.P., Li, Z.S., and Rea, P.A. (1997). AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc. Natl. Acad. Sci. USA 94, 8243-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.P., Li, Z.S., Drozdowicz, Y.M., Hortensteiner, S., Martinoia, E., and Rea, P.A. (1998). AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with Atmrp1. Plant Cell 10, 267-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, S. (1998). Immunophilins in animals and higher plants. Bot. Bull. Acad. Sin. 39, 217-223. [Google Scholar]
- Luschnig, C. (2002). Auxin transporters: ABC transporters join the club. Trend Plant Sci. 7, 329-333. [DOI] [PubMed] [Google Scholar]
- Martinoia, E., Klein, M., Geisler, M., Bovet, L., Forestier, C., Kolukisaoglu, Ü., Müller-Röber, B., and Schulz, B. (2002). Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214, 345-355. [DOI] [PubMed] [Google Scholar]
- Mealey, K.L., Barhoumi, R., Burghardt, R.C., McIntyre, B.S., Sylvester, P.W., Hosick, H.L., and Kochevar, D.T. (1999). Immunosuppressant inhibition of P-glycoprotein function is independent of drug-induced suppression of peptide-prolyl isomerase and calcineurin activity. Cancer Chemother. Pharmacol. 44, 152-158. [DOI] [PubMed] [Google Scholar]
- Meinke, D., and Koornneef, M. (1997). Community standards for Arabidopsis genetics. Plant J. 12, 247-253. [Google Scholar]
- Murphy, A.S., Hoogner, K.R., Peer, W.A., and Taiz, L. (2002). Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol. 128, 935-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh, B., Murphy, A.S., and Spalding, E.P. (2001). Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 13, 2441-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens-Grillo, J.K., Stancato, L.F., Hoffmann, K., Pratt, W.B., and Krishna, P. (1996). Binding of immunophilins to the 90 kDa heat shock protein (hsp90) via a tetratrico-peptide repeat domain is a conserved protein interaction on plants. Biochemistry 35, 15.249-15.255. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez, J.M., Ponce, M.R., and Micol, J.L. (2004). ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol. 134, 101-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt, W.B., Krishna, P., and Olsen, L.J. (2001). Hsp90-binding immunophilins in plants: the protein movers. Trends Plant Sci. 6, 54-58. [DOI] [PubMed] [Google Scholar]
- Rea, A.P., Ze-Sheng, L., Yu-Ping, L., Drozdowicz, Y.M., and Martinoia, E. (1998). From vacuolar GS-X pumps to multispecific ABC transporters. Annu. Rev. Plant Physiol. 49, 727-760. [DOI] [PubMed] [Google Scholar]
- Reddy, R.K., Kurek, I., Silverstein, A.M., Chinkers, M., Breiman, A., and Krishna, P. (1998). High-molecular-weight FK506-binding proteins are components of heat-shock protein 90 heterocomplexes in wheat germ lysate. Plant Physiol. 118, 1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fernandez, R., Davies, T.G.E., Coleman, J.O.D., and Rea, P.A. (2001). The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 276, 30231-30244. [DOI] [PubMed] [Google Scholar]
- Schiene, C., and Fischer, G. (2000). Enzymes that catalyse the restructuring of proteins. Curr. Opin. Struct. Biol. 10, 40-45. [DOI] [PubMed] [Google Scholar]
- Schönknecht, G. et al. (2002). KCO1 is a component of the slow-vacuolar (SV) ion channel. FEBS Lett. 511, 28-32. [DOI] [PubMed] [Google Scholar]
- Shou, W., Aghdasi, B., Armstrong, D.L., Guo, Q., Bao, S., Charng, M.J., Mathews, L.M., Schneider, M.D., Hamilton, S.L., and Matzuk, M.M. (1998). Nature 391, 489-492. [DOI] [PubMed] [Google Scholar]
- Silverstein, A.M., Galigniana, M.D., Kanelakis, K.C., Radanyi, C., Renoir, J.M., and Pratt, W.B. (1999). Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J. Biol. Chem. 274, 36.980-36.986. [DOI] [PubMed] [Google Scholar]
- Timerman, A.P., Wiederrecht, G., Marcy, A., and Fleischer, S. (1995). Characterization of an exchange reaction between soluble FKBP-12 and the FKBP ryanodine receptor complex. Modulation by FKBP mutants deficient in peptidyl-prolyl isomerase activity. J. Biol. Chem. 270, 2451-2459. [DOI] [PubMed] [Google Scholar]
- Tommasini, R., Vogt, E., Schmid, J., Fromentau, M., Amrhein, N., and Martinoia, E. (1997). Differential expression of genes coding for ABC transporters after treatment of Arabidopsis thaliana with xenobiotics. FEBS Lett. 411, 206-210. [DOI] [PubMed] [Google Scholar]
- Überlacker, B., and Werr, W. (1996). Vectors with rare-cutter restriction enzyme sites for expression of open reading frames in transgenic plants. Mol. Breeding 2, 293-295. [Google Scholar]
- van den Wijngaard, P.W., Bunney, T.D., Roobeek, I., Schonknecht, G., and de Boer, A.H. (2001). Slow vacuolar channels from barley mesophyll cells are regulated by 14-3-3 proteins. FEBS Lett. 488, 100-104. [DOI] [PubMed] [Google Scholar]
- Vittorioso, P., Cowling, R., Faure, J.D., Caboche, M., and Bellini, C. (1998). Mutation in the Arabidopsis PASTICCINO1 gene, which encodes a new FK506-binding protein-like protein, has a dramatic effect on plant development. Mol. Cell. Biol. 18, 3034-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucich, V.A., and Gasser, C.S. (1996). Novel structure of a high molecular weight FK506 binding protein from Arabidopsis thaliana. Mol. Gen. Genet. 252, 510-517. [DOI] [PubMed] [Google Scholar]