Abstract
The present study aims to test whether Lycium barbarum L. has anti-hypertensive effect through regulating expression of lncRNA sONE in a rat model of salt-sensitive hypertension. Nine weeks old borderline hypertensive rats (BHRs) were divided into 4 groups receiving high (8% NaCl), medium (0.25% NaCl, as control group), and low salt diet (0.015% NaCl) for 16 weeks, respectively, while the fourth group (high salt + L. barbarum group) fed with high salt diet for 12 weeks, then followed by 8% NaCl and L. barbarum treatment for 4 weeks. Body weight and blood pressure were recorded biweekly. Salt-sensitive hypertension was successfully induced by 12-week high salt diet in BHR model. Blood pressure was significantly increased in the model (P < 0.05), and L. barbarum treatment reversed the elevated blood pressure to normal level. Expression of lncRNA sONE was significantly reduced and eNOS expression level was dramatically improved in the hypertension model rats with the L. barbarum compared with that receiving high salt diet. Our results indicated that L. barbarum L. had anti-hypertensive effect and might lower blood pressure by suppressing the expression of lncRNA sONE in BHR model.
Keywords: Lycium barbarum L., salt-sensitive hypertension, endothelial nitric oxide synthase, lncRNA sONE
Introduction
Hypertension is a major public health challenge due to its high prevalence, major risk factor for cardiovascular disease and all-cause mortality [1]. As a complex trait, hypertension is influenced by multiple environmental and genetic determinants, as well as their interactions [2]. Dietary sodium intake is the most common and important etiological factor for hypertension [3]. The previous study has suggested that blood pressure (BP) varies among individuals in response to dietary sodium intake, described as salt sensitivity of BP [4].
The release of nitric oxide (NO) from the endothelium can increase vasodilation and regional blood flow as well as reduce BP [5,6]. Endothelial nitric oxide synthase (eNOS) catalyzes the generation of NO in endothelial cells [7]. It has been reported that the T-786C polymorphism of eNOS gene is related to sodium intake, leading to the increasing risk of hypertension [8,9]. Additionally, Robb et al. [10] find the existence of an antisense mRNA (sONE), which is long noncoding RNA (lncRNA) derived from a transcription unit (NOS3AS) on the opposite DNA strand of human eNOS. RNA interference-mediated expression inhibition of sONE in vascular smooth muscle cells increases eNOS expression, while overexpression of sONE in endothelial cells reduces the expression of eNOS. Therefore, the antisense lncRNA sONE may be involved in salt-sensitive hypertension.
Lycium barbarum L. (L. barbarum), also called Goji berry or wolfberry, is a traditional Chinese medicine used for preventing and treating various diseases, such as diabetes, hyperlipidemia, thrombosis, immunodeficiency and cancer [11]. The polysaccharides extracted from L. barbarum (LBP) has been reported to show a remarkable antioxidant [12], immunomodulation and antitumor activity [13], meanwhile it also has protective effects on retinal ganglion cells in both chronic ocular hypertension model rat [14] and acute ocular hypertension model mouse [15], suggesting that LBP may be the primary active chemical constituent of L. barbarum. In this study, we tried to investigate whether L. barbarum had anti-hypertensive effect on salt-sensitive hypertension in borderline hypertensive rat (BHR) which was considered as a model for environmentally-induced hypertension [16]. In addition, we also monitored the expression levels of lncRNA sONE and eNOS in renal vascular endothelia cells of BHR (in vivo) fed with different concentrations of salt diets and L. Barbarum and the cultured human umbilical vein endothelial cells (HUVEC, in vitro) treated with NaCl only or plus LBP (a substitute of L. Barbarum), exploring the underlying molecular mechanisms of blood pressure regulation by L. Barbarum.
Materials and methods
Animals
BHR, which is a genetic model of environmentally induced hypertension, become permanently hypertensive when subjected to a time limited period of exposure to environmental stress or to increased dietary sodium intake [17]. BHRs used in the study were born in our animal facility as F1 offspring of male normotensive Wistar and female spontaneous hypertensive rats purchased from Vital River Laboratories (Beijing, China). At 5 weeks old, BHRs (16 female and 16 male) were weaned and housed in a controlled environment (21-24°C, 45-55% relative humidity, 12:12 h light-dark cycle) and maintained on a pellet diet (New Brunswick, New jersey, USA) and tap water. All procedures were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Fuwai hospital Animal Care and Use Committee. All human studies have been approved by China Ethics Committee and performed in accordance with the ethical standards.
Salt-sensitive hypertension model and experimental design
These BHRs were fed with normal salt diet (0.25% NaCl) for 4 weeks. Then, animals were randomly divided into 4 groups with 4 male and 4 female BHRs in each group. Medium salt (control) group, high salt group and low salt group received 0.25%, 8% and 0.015% NaCl diet, respectively for 16 weeks. The fourth group (high salt + L. Barbarum group) received high salt diet (8% NaCl) for 12 weeks (optimal BP changes could be observed at this time-point), and then fed 8% NaCl and L. barbarum (Ningxia wolfberry conglomerate company, Ningxia, China; 10 g/day for female and 20 g/day for male) for 4 weeks.
Blood pressure and body weight measurement
The systolic blood pressure (SBP) and body weight (BW) of rats in each group were respectively measured by tail-cuff method (SoftronBP-98A, Beijing, China) and weight scale every 2 weeks.
Separation of rat renal vascular endothelial cells
All rats were anesthetized by intraperitoneal injecting of ketamine-xylazine mixture (100 mg/ml; 0.1 ml/100 g body weight) and the kidneys were rapidly removed. Renal cortex was isolated and placed into Rat Endothelial Cell Growth Medium (Cell Applications, San Diego, California, USA), then cut into pieces (< 1 mm3). Minced tissues were collected into a 50 mL centrifuge tube with the medium containing collagenase type II and dispase (Invitrogen, Carlsbad, California, USA), then suspended and put into water bath at 37°C for 30 min with stirring. DNaseI (Sigma, St. Louis, Missouri, USA, 75 μL/10 mL suspension) was added into the tube. After another 30 min at 37°C with stirring, the tube was placed on ice, and added pre-cool medium (5 mL for 10 mL suspension). The suspension was mixed completely and filtrated by 75 μm cell strainer (BD, San Diego, California, USA). Cell suspension was centrifuged at 400 g for 5 min at room temperature and cells were collected and resuspended in Ficoll isolation medium (rat endothelial cell growth medium + sodium citrate (1% v/v, 2 M)). After that, cell suspension was gently transferred to another 50 mL centrifuge tube containing 7.5 mL Ficoll-Paque and kept on the top. After centrifugation at 400 g for 30 min at 18°C, the white flocculent cells at interlayer were transferred into a new 50 mL centrifuge tube, and resuspended with 2-fold volume medium, then centrifuged at 400 g for 5 min. Subsequently, cells were counted and resuspended in the medium (≤ 107/mL), and incubated with mouse anti-rat CD31 Ab (R & D Systems, Minneapolis, Minnesota, USA, 500 mg/mL, 1 μg/106 cells) at 4°C for 45 min with slow stirring. After washing, goat-anti-mouse Dynabeads (110-33, Invitrogen) were added (25 μL/107 cells), and the tube was incubated again at 4°C for 45 min with slow stirring. The beads were washed four times, and cryopreserved with 1 mL Trizol at -20°C for further use.
Cell culture and treatment
HUVEC cells were cultured using VascuLife VEGF Medium Complete Kit (Lifeline cell technologies, Frederick, Maryland, USA). When 80~90% confluence was reached, 150 mM NaCl only or plus 500 μg/mL LBP (a gift from professor Kwok-Fai So of Hong Kong University) was added for 1 h, 3 h and 6 h, respectively. A NaCl and LBP dose dependent curve had been generated previously (data not shown), and optimal changes could be observed at this concentration.
Gene expression analysis
Total RNA was extracted from cells (rat renal vascular endothelial cells and HUVEC cells) using Trizol (Invitrogen) reagent following the manufacturer’s instruction and reverse transcribed using PrimeScript® RT Master Mix (TAKARA, Dalian, China). The sequences of primers (Shenggong Bioengineering Co., Shanghai, China) used for quantification measurement of lncRNA sONE and eNOS mRNA were shown in Table 1, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as reference gene. Non-template controls were run to test for DNA-contaminated primers. Real-time reactions were run and analyzed by using a Real-Time PCR system (BIO-RAD, Hercules, California, USA). The melting curves for PCR samples were made to guarantee amplification of the correct genes. The amplification was used to calculate the CT value (ΔCT) of the target genes and the difference between the ΔCT of those genes and GAPDH gene. In addition, the equation 2-ΔΔCT was used to determine the relative amount of mRNA in specific target genes.
Table 1.
The sequences of the primers used for qRT-PCR
| Primer | Sequences (5’-3’) | |
|---|---|---|
| lncRNA sONE | Forward | GCAAGGAGAGGAGATGCTGATGGC |
| Reverse | GCAGTTCTCAGGCTCGTGATCG | |
| eNOS | Forward | TGAGACCTTCTGTGTGGGAGAG |
| Reverse | CGGATTGTAGCCTGGAACAT | |
| GAPDH | Forward | GTG GAT ATT GTT GCC ATC AAT GAC C |
| Reverse | GCC CCA GCC TTC TTC ATG GTG GT | |
| Rat-lncRNA sONE | Forward | GCG GGG ACT CAC CAA GAG GTT GGC C |
| Reverse | TGG CAC ACC CAC AAT GGC AGC CAC C | |
| Rat-eNOS | Forward | GCA CAG GAA ATG TTC ACC TAC A |
| Reverse | GGT CCA GCC ATG TTG AAT ACA G | |
| Rat-GAPDH | Forward | ACA GCA ACA GGG TGG TGG AC |
| Reverse | TTT GAG GGT GCA GCG AAC TT |
Statistical analysis
Data were reported as mean ± SD of at least three independent experiments. Comparisons among multiple groups were made by analysis of variance (ANOVA) using SPSS 16.0. P < 0.05 and P < 0.01 were considered statistically significant.
Results
Effects of different salt concentration and L. barbarum diets on SBP and BW of BHRs
SBP of rats receiving high salt diet was significantly higher than that of rats in control group and low salt group from 19 to 25 weeks (P < 0.05). There was no significant difference of SBP between low salt and control group (P > 0.05) (Figure 1A). With L. barbarum diet treatment for 4 weeks, the SBP of rats in high salt + L. barbarum group exerted significant decline in comparison with that in high salt group (P < 0.05) and recovered to the normal level at 25 weeks old (Figure 1A). The BW of rats fed low salt diet showed slight decrease compared with other groups at 19 weeks of age (P < 0.05), while there was no statistical difference among 4 groups at other period (P > 0.05) (Figure 1B).
Figure 1.
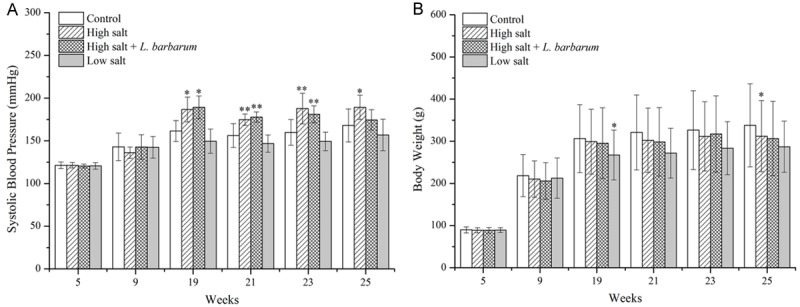
Effects of high salt (8% NaCl), low salt (0.015% NaCl), medium salt (0.25% NaCl) and high salt + L. barbarum diets on (A) systolic blood pressure and (B) body weight of borderline hypertensive rats from 5 to 25 weeks old. Data are mean ± SD of 4 female and 4 male rats in each group. *P < 0.05 and **P < 0.01 indicate significant differences.
Alternations of mRNA levels of lncRNA sONE and eNOS in rat renal vascular endothelial cells
The relative mRNA level of lncRNA sONE in renal vascular endothelial cells of high salt group was significantly higher than that of other groups (P < 0.05). The declined expression of lncRNA sONE was observed in BHRs receiving L. barbarum diet compared with high salt (Figure 2A). The expression of eNOS increased apparently after L. barbarum treatment, and its expression level in salt + L. Barbarum group was higher (P < 0.01) than that in other groups (Figure 2B).
Figure 2.
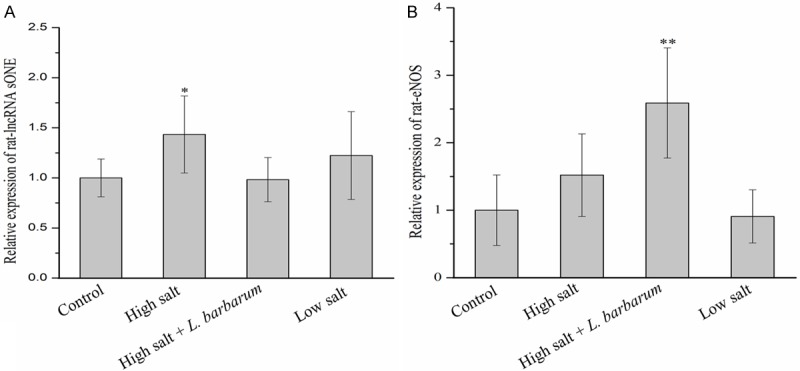
Effects of high salt (8% NaCl), low salt (0.015% NaCl), medium salt (0.25% NaCl) and high salt + L. barbarum diets on the relative expression levels of (A) lncRNA sONE and (B) eNOS mRNA in renal vascular endothelia cells of BHR model rats at 25 weeks of age. Data are mean ± SD (n = 6). *P < 0.05 and **P < 0.01 indicate significant differences.
Effects of NaCl and LBP on the expression levels of lncRNA sONE and eNOS in HUVEC
LncRNA sONE expression in HUVEC was significantly up-regulated at 1 h after NaCl treatment (P < 0.05), while the elevated expression dramatically declined and returned to normal level with addition of LBP (Figure 3A). The markedly increased expression of eNOS was observed in HUVEC at 1 h treated by high salt (P < 0.05) and it was reverted by LBP treatment (Figure 3B), while there was no significant difference at other time points between both treatments.
Figure 3.
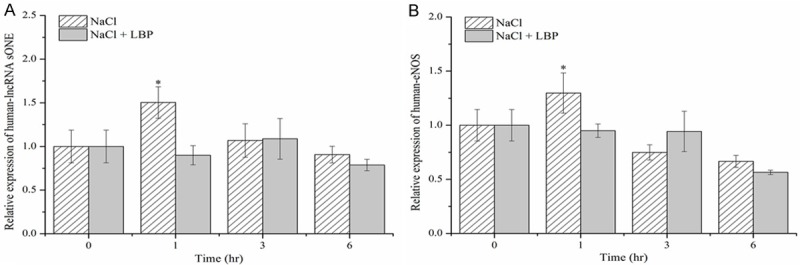
Effects of 150 mM NaCl and 500 μg/mL L. barbarum polysaccharides (LBP) on the relative expression levels of (A) lncRNA sONE and (B) eNOS mRNA in Human umbilical vein endothelial cells (HUVEC). Data are mean ± SD (n = 6). *P < 0.05, NaCl vs. NaCl + LBP.
Discussion
Salt-sensitive hypertension is highly sensitive to sodium chloride intake [18]. In this study, salt-sensitive hypertension was successfully induced by 12-week high salt diet in BHR model. L. Barbarum was found to have anti-hypertensive effect, as it reduced the increased SBP induced by high salt diet to normal level. The expression level of lncRNA sONE was upregulated in renal vascular endothelial cells of rat model with salt-sensitive hypertension as well as high salt-treated HUVEC, indicating lncRNA sONE might be involved in the pathogenesis of salt-sensitive hypertension. However, L. barbarum could suppress the elevated lncRNA sONE expression in vivo and in vitro. It was speculated that the anti-hypertensive effect of L. barbarum might be via down-regulating expression of lncRNA sONE.
L. barbarum has been widely used as health food or one component of traditional medicine for thousands of years in China and other oriental countries. In several earlier studies, it has been reported to have various functions, including anticancer [19], immunomodulatory [20], antioxidant [21], and neuroprotective [22]. In addition, orally administrated LBP could efficiently protect the pressure-induced loss of retinal ganglion cells (RGCs) [14], while LBP also exerts protection of RGCs in a mouse model of acute ocular hypertension [15]. In the present study, the dietary 8% NaCl intake was able to induce an increase in systolic pressure as those previously observed in BHR model or hypertensive parent [17]. However, daily feeding L. barbarum could revert the elevated blood pressure without effect on body weight of hypertension rat. It was indicated that L. barbarum also has potential to ameliorate blood pressure in the salt-sensitive hypertension BHR model.
NO synthesis by the vascular endothelium is extremely important to control blood pressure in humans [23]. In addition, disruption of the eNOS gene can lead to hypertension in mouse [24]. These previous results strongly implicate that the eNOS gene is essential for hypertension. However, the majority of hypertensives are salt sensitive and the molecular basis of salt-sensitive hypertension remains unclear [25]. Blood pressure of salt sensitivity mice deficient in eNOS exhibits a 2.5-fold increase compared with wild-type controls in response to high salt intake, suggesting that eNOS is also an important regulator of salt sensitivity hypertension [26]. In the present study, high salt diet did not induce significant change in expression level of eNOS in BHR renal vascular endothelial cells in comparison with low or salt normal diets, while eNOS expression was dramatically up-regulated in salt-sensitive hypertension rat fed L. barbarum. In previous study, relaxin can also ameliorate salt-sensitive hypertension by increasing eNOS expression [18]. Moreover, nicorandil treatment significantly lower blood pressure, which is accompanied by an increase in eNOS expression in the kidneys [27]. Therefore, it was speculated that L. barbarum lowered blood pressure and ameliorated salt-sensitive hypertension by up regulating the expression level of eNOS in vascular endothelial cells. However, the elevated eNOS expression was not observed in HUVEC after LBP. Other bioactive components from L. barbarum, tissue specific expression or additional reasons may be involved, probably deserving further investigation.
NOS mRNA in endothelial cells is highly stable, as a protein complex containing translation elongation factor 1-alpha 1 (eEF1A1) binding to its 3’ untranslated region (UTR) to maintain the stability [28]. LncRNA NOS3AS (sONE deriving from NOS3AS) may competitively inhibit the protein complex binding to 3’ UTR of eNOS mRNA, or its binding with eNOS mRNA impedes the formation of the protein complex to negatively regulate eNOS expression [29]. Our results showed that the expression levels of lncRNA sONE in were higher in renal vascular endothelial cells of salt-sensitive hypertensive rats and HUVEC with high salt treatment than that in the other groups, suggesting that lncRNA sONE might also be an important correlation factor for salt-sensitive hypertension. However, the increased lncRNA sONE expression could be downregulated by L. Barbarum treatment in vivo and in vitro. Therefore, it was audaciously inferred that L. Barbarum improved the expression of eNOS via suppressing lncRNA sONE expression, exerting anti-hypertensive effect on salt-sensitive hypertension. Whereas, changes of eNOS protein level and local NO level have not been detected yet, which deserve further study.
In summary, these experimental findings reveal that L. barbarum L. can lower blood pressure by downregulating the expression of lncRNA sONE in the salt-sensitive hypertension BHR model.
Acknowledgements
This work was supported by the Key Project in the National Science & Technology Pillar Program during the 12th 5-Year Plan Period (2011BAI11B04 to Rutai Hui), the 973 program (2011CB503901 to Rutai Hui, 2014CB541601 to Jingzhou Chen) and the National Natural Science Foundation of China (81270356 to Jingzhou Chen). Funding from Logistic University of the Chinese People’s Armed Police Force (WHB201409) and Tianjin Key Laboratory of Cardiovascular Remodeling and Target Organ Injury (TJC1405).
Disclosure of conflict of interest
None.
References
- 1.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–646. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 5.Oberleithner H, Kusche-Vihrog K, Schillers H. Endothelial cells as vascular salt sensors. Kidney Int. 2010;77:490–494. doi: 10.1038/ki.2009.490. [DOI] [PubMed] [Google Scholar]
- 6.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415–424. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 7.Li XA, Everson W, Smart EJ. Nitric oxide, caveolae, and vascular pathology. Cardiovascular toxicology. 2006;6:1–13. doi: 10.1385/ct:6:1:1. [DOI] [PubMed] [Google Scholar]
- 8.Dengel DR, Brown MD, Ferrell RE, Reynolds TH, Supiano MA. A preliminary study on T-786C endothelial nitric oxide synthase gene and renal hemodynamic and blood pressure responses to dietary sodium. Physiol Res. 2007;56:393–401. doi: 10.33549/physiolres.931002. [DOI] [PubMed] [Google Scholar]
- 9.Miyaki K, Tohyama S, Murata M, Kikuchi H, Takei I, Watanabe K, Omae K. Salt intake affects the relation between hypertension and the T-786C polymorphism in the endothelial nitric oxide synthase gene. Am J Hypertens. 2005;18:1556–1562. doi: 10.1016/j.amjhyper.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Robb GB, Carson AR, Tai SC, Fish JE, Singh S, Yamada T, Scherer SW, Nakabayashi K, Marsden PA. Post-transcriptional regulation of endothelial nitric-oxide synthase by an overlapping antisense mRNA transcript. J Biol Chem. 2004;279:37982–37996. doi: 10.1074/jbc.M400271200. [DOI] [PubMed] [Google Scholar]
- 11.Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Li X, Zhou A. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. European Polymer Journal. 2007;43:488–497. [Google Scholar]
- 13.Gan L, Hua Zhang S, Liang Yang X, Bi Xu H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum . Int Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Mi XS, Feng Q, Lo AC, Chang RC, Lin B, Chung SK, So KF. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One. 2012;7:e45469. doi: 10.1371/journal.pone.0045469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawler JE, Sanders BJ, Chen YF, Nagahama S, Oparil S. Hypertension produced by a high sodium diet in the borderline hypertensive rat (BHR) Clin Exp Hypertens A. 1987;9:1713–1731. doi: 10.3109/10641968709158968. [DOI] [PubMed] [Google Scholar]
- 17.DiBona GF, Jones SY. Renal manifestations of NaCl sensitivity in borderline hypertensive rats. Hypertension. 1991;17:44–53. doi: 10.1161/01.hyp.17.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Kumagai H, Suzuki A, Kobayashi N, Ohkawa S, Odamaki M, Kohsaka T, Yamamoto T, Ikegaya N. Relaxin ameliorates salt-sensitive hypertension and renal fibrosis. Nephrol Dial Transplant. 2011;27:2190–2197. doi: 10.1093/ndt/gfr618. [DOI] [PubMed] [Google Scholar]
- 19.Tang WM, Chan E, Kwok CY, Lee YK, Wu JH, Wan CW, Chan RY, Yu PH, Chan SW. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology. 2012;20:307–314. doi: 10.1007/s10787-011-0107-3. [DOI] [PubMed] [Google Scholar]
- 20.Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009;12:1159–1165. doi: 10.1089/jmf.2008.0300. [DOI] [PubMed] [Google Scholar]
- 21.Yin YP, Wei WH, Wang HC, Zhu BY, Yu YH, Chen XS, Peeling RW, Cohen MS. Performance of serological tests for syphilis in sexually transmitted diseases clinics in Guangxi Autonomous Region, China: implications for syphilis surveillance and control. Sex Health. 2009;6:5–9. doi: 10.1071/sh08027. [DOI] [PubMed] [Google Scholar]
- 22.Rui C, Yuxiang L, Yinju H, Qingluan Z, Yang W, Qipeng Z, Hao W, Lin M, Juan L, Chengjun Z, Yuanxu J, Yanrong W, Xiuying D, Wannian Z, Tao S, Jianqiang Y. Protective effects of Lycium barbarum polysaccharide on neonatal rat primary cultured hippocampal neurons injured by oxygen-glucose deprivation and reperfusion. J Mol Histol. 2012;43:535–542. doi: 10.1007/s10735-012-9420-4. [DOI] [PubMed] [Google Scholar]
- 23.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;334:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 24.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 25.Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J Cardiovasc Pharmacol. 2008;51:239–245. doi: 10.1097/FJC.0b013e318162011f. [DOI] [PubMed] [Google Scholar]
- 26.Li J, White J, Guo L, Zhao X, Wang J, Smart EJ, Li XA. Salt Inactivates Endothelial Nitric Oxide Synthase in Endothelial Cells. J Nutr. 2009;139:447–451. doi: 10.3945/jn.108.097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashiro Y, Yogo K, Serizawa K, Endo K. Nicorandil suppresses urinary protein excretion and activates eNOS in Dahl salt-sensitive hypertensive rats. Clin Exp Nephrol. 2015;19:343–9. doi: 10.1007/s10157-014-0998-6. [DOI] [PubMed] [Google Scholar]
- 28.Yan G, You B, Chen SP, Liao JK, Sun J. Tumor necrosis factor-alpha downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-alpha 1. Circ Res. 2008;103:591–597. doi: 10.1161/CIRCRESAHA.108.173963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fish JE, Matouk CC, Yeboah E, Bevan SC, Khan M, Patil K, Ohh M, Marsden PA. Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J Biol Chem. 2007;282:15652–15666. doi: 10.1074/jbc.M608318200. [DOI] [PubMed] [Google Scholar]


