Abstract
Background and objective: Emerging evidences indicate that miR-155-5p is associated with some cancer tumorigenesis, but their specific effects on proliferation, invasion and metastasis of colorectal carcinoma (CRC) are still poorly understood. The aim of the study is to investigate miR-155-5p effect on proliferation and invasion metastasis of CRC. Methods: Retrospectively analyzed clinicopathological parameters and fresh tissue samples of 372 colon cancer patients receiving radical surgery. HT-29 cells were transfected with mimics and inhibitors of miR-155-5p, respectively. Real-time reverse transcription-PCR was performed to measure miR-155-5p relative levels of tissues and cells. Results: miR-155-5p expression in cancer group was higher than that in normal group, with statistical differences (P<0.05). miR-155-5p expression was associated with tumor location, tumor grade, TNM staging and distant metastasis (P<0.05 for all parameters). Cell number of mimics group was higher than control group (P<0.01), and that of inhibitor group was lower than control group (P<0.05). Invasion and metastasis effect of mimics group were the highest and those of inhibitor group were the lowest. Conclusions: miR-155-5p expression is up-regulated in most CRC and promotes proliferation, invasion and metastasis of CRC cells. It may play an essential role in tumorigenesis and tumor progression of CRC.
Keywords: miR-155-5p, colorectal carcinoma, HT-29 cell, tumorigenesis, proliferation, invasion
Introduction
Colorectal cancer (CRC) ranks the third most common cancer worldwide and it is regarded as one of the most frequent cancers, greatly influence human health [1,2]. Although some progression has been achieved in treating CRC in the past decades, the overall survival rate of patients with CRC has not expectantly changed. CRC development involves a multi-step process including both genetic and epigenetic changes, which leads to activation of oncogenes and inactivation of tumor suppressor genes in cancer cells [3].
MicroRNAs (miRNAs) are non-coding RNA molecules. They exert their functions by binding to the 39-untranslated regions of their corresponding mRNA targets [4]. Approximate one-third of the total human genes are considered to be regulated by miRNAs, suggesting that miRNAs have critical roles in physiological and pathological processes [5,6]. Plenty of studies indicate that miRNAs are implicated in human malignance [7,8]. The abnormal miRNAs expression can lead to corresponding aberrant protein expression which may contribute to acquiring malignance hallmarks. Consequently, the function of miRNAs is supposed to be tumor suppressors or oncogenes.
Recently, convincing evidences showed that a series of miR-155 play crucial roles in CRC tumorigenesis and tumor progression. Svrcek et al [9] reported that detection and monitoring of miR-155 field defect might have implications for the prevention and treatment of inflammatory bowel disease related CRCs with microsatellite instability. Valeri et al [10] pointed out there was an inverse correlation between the expression of miR-155 and the expression of MLH1 or MSH2 proteins in human colorectal cancer. Hiroyuki et al [11] confirmed that miR-155 overexpression could down-regulate expression of MLH1, MSH2, and MSH6, resulting in tumorigenesis. However, miR-155 effect on CRC proliferation and invasion metastasis has been far from being fully understood.
In the present study, we firstly investigated miR-155-5p expression and found it was up-regulated in 81.45% CRC patients. CRC cells were transfected with mimics and inhibitors of miR-155-5p, respectively. RT-PCR results showed that miR-155-5p could promote CRC cells proliferation. Transwell test indicated it could enhance invasion metastasis effect of CRC cells. These results suggested that miR-155-5p play a significant role in CRC tumorigenesis and tumor progression.
Materials and methods
Patients and clinical samples
Clinicopathological parameters and fresh tissue samples of 372 colon cancer patients (205 males, 167 females) who received radical surgery in the Tumor Affiliated Hospital of Xinjiang Medical University in China between January 1st 2011 and May 1st 2014 were collected. The mean patient age was 60.07±13.89 years old. All patients had their colorectal cancer diagnosis histopathologically confirmed. The adjacent normal tissue samples were obtained from the normal colorectal tissue located 5 cm away from the tumor. The clinicopathological data of all the patients were listed in Table 1. Tumor-Node-Metastasis (TNM) stage was determined according to the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM staging system of colorectal cancer (2010, Seventh Edition). No patients received preoperative chemotherapy or immunotherapy. The presence of complex metastases (e.g. uncertain lumps, micrometastases [particularly in the liver], and abdominal/pelvic lymph node metastases) were diagnosed using enhanced Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography-Computed Tomography (PETCT) and puncture biopsies.
Table 1.
Relationship of miR-155-5p and clinicopathologic features
| miR-155-5p | n | χ2 | P | ||
|---|---|---|---|---|---|
|
| |||||
| lower | upper | ||||
| Tumor location | 14.456 | 0.001 | |||
| right hemicolon | 27 | 56 | 83 | ||
| left hemicolon | 14 | 100 | 114 | ||
| rectal | 28 | 147 | 175 | ||
| Tumor size (cm) | 3.438 | 0.179 | |||
| <4 | 23 | 111 | 134 | ||
| 4-6 | 21 | 115 | 136 | ||
| ≥6 | 25 | 77 | 102 | ||
| Tumor grade | 4.120 | 0.042 | |||
| low | 46 | 237 | 283 | ||
| high | 23 | 66 | 89 | ||
| TNM staging | 13.092 | 0.004 | |||
| I | 8 | 33 | 41 | ||
| III | 34 | 86 | 120 | ||
| III | 23 | 140 | 163 | ||
| IV | 4 | 44 | 48 | ||
| Distant metastasis | 5.295 | 0.021 | |||
| M0 | 65 | 249 | 314 | ||
| M1 | 4 | 54 | 58 | ||
| Lymphatic invasion | 0.148 | 0.700 | |||
| Yes | 9 | 45 | 54 | ||
| No | 60 | 258 | 318 | ||
| Peripheral nerve infiltration | 0.052 | 0.819 | |||
| Yes | 3 | 18 | 21 | ||
| No | 66 | 285 | 351 | ||
The study design and procedures described below were approved by our institutional review board, and written informed consent was obtained from each patient. Patient samples were obtained following informed consent according to an established protocol approved by the Institute Research Ethics Committee of Affiliated Tumor Hospital of Xinjiang Medical University (No. W-201321), which acts to meet the demands of the Declaration of Helsinki (2000) of the World Medical Association.
Cell culture and transfection
HT-29 cell lines were obtained from Cancer Research Institute, Central South University. The HCT116 cells were cultured in RPMI 1640 (Gibco Co., Ltd., USA). The media were supplemented with 10% FBS (fetal bovine serum) (Gibco Co., Ltd., USA). The miR-155-5p mimics (No. miR10000646-1-5) and inhibitors (No. miR 20000646-1-5) were synthesized and purified by TaKaRa Biotechnology Co., Ltd., Japan. Both the single mimics and inhibitors were transfected using LipofectamineTM 2000 reagent (Invitrogen Co., Ltd., California, USA), and collected for RT-PCR and Transwell test.
Proliferation assay
Cells were grown in RPMI-1640 medium containing 10% fetal serum. 1×103 cells were seeded in flat-bottom 96 well plates and incubated at 37°C in 5% CO2. Cell number was directly counted by Coulter Counter (Inno-Alliance Biotech, Co., Ltd., USA) on 2nd day.
Real-time reverse transcription-PCR (RT-PCR)
The expression of miR-155-5p in the fresh tissue samples or transfected cell lines was quantified by SYBR Green assays with primers and SYBR Green from TaKaRa Biotechnology. The mean cycle threshold was determined by triplicate PCR runs, and gene expression was calculated relative to U6 snRNA. The primer for miR-155-5p was 5’-TTAATGCTAATCGTGATAGGGGT-3’ and the primer for U6 was 5’-ATTGGAACGATACAGAGAAGATT-3’.
Cell migration/invasion assays
Cell motility and invasiveness were determined by a 24 well transwell plate (8 µM pore size; Costar) [12]. Briefly, for transwell migration assays, 1×104 cells were placed on the top chamber lined with the noncoated membrane. For invasion assays, 3×104 cells were placed on the upper chamber of each insert coated with 200 mg/ml of Matrigel (BD Biosciences, CA, USA).
Statistical analysis
Statistics were analyzed with the SPSS statistical software package, version 13.0 ((SPSS Inc., Chicago, IL, USA)). The Students’ t test were performed for RT-PCR. The correlation between miR-155-5p and clinicopathological factors was analyzed by Chi-square test. A value of P<0.05 indicated a significant difference.
Results
miR-155-5p relative expression in colorectal cancer tissue
Malignant tumor tissues and matched adjacent normal tissues of 372 colorectal cancer patients were measured by RT-PCR. The results showed that in cancer group miR-155-5p expressions were up-regulated in 303 cases (81.45%) and down-regulated in 69 cases (18.55%). Their miR-155-5p relative mean expression level of cancer tissues were higher than those of matched adjacent normal tissues in 303 up-regulated miR-155-5p cases, with obvious statistical significance (P<0.01); Meanwhile, their miR-155-5p relative mean expression level of cancer tissues were a little lower than those of matched adjacent normal tissues in 69 down-regulated miR-155-5p cases, with no statistical significance (P>0.05) (Figure 1).
Figure 1.

miR-155-5p relative expression in colorectal cancer tissue and matched adjacent normal tissues. miR-155-5p relative expression in 372 patients with colorectal cancer was measured by RT-PCR. Statistic analysis showed miR-155-5p expression in cancer group was higher than in normal group, with statistical differences (P<0.05).
Correlation between miR-155-5p and clinicopathologic parameters
Statistical analyses were performed to assess any relationships between miR-155-5p and clinicopathological factors (Table 1). miR-155-5p levels were associated with tumor location, tumor grade, TNM staging and distant metastasis (P<0.05 for all parameters). Meanwhile, miR-155-5p was not related to the patient’s tumor size, lymphatic invasion and peripheral nerve infiltration (P>0.05 for all parameters).
Proliferation effect of miR-155-5p in vitro
HT-29 cell lines were treated with different methods, Group A, as a control group, treated with the same dose of D-Hanks liquid; Group B, as a overexpression group, transfected with miR-155-5p mimics; Group C, as a silence group, transfected with miR-155-5p inhibitor. The results indicated that cell number of Group B was higher than group A (P<0.01), and that of group C was lower than group A (P<0.05) (Figure 2). It suggested that miR-155-5p could promote colorectal cancer cell proliferation in vitro.
Figure 2.
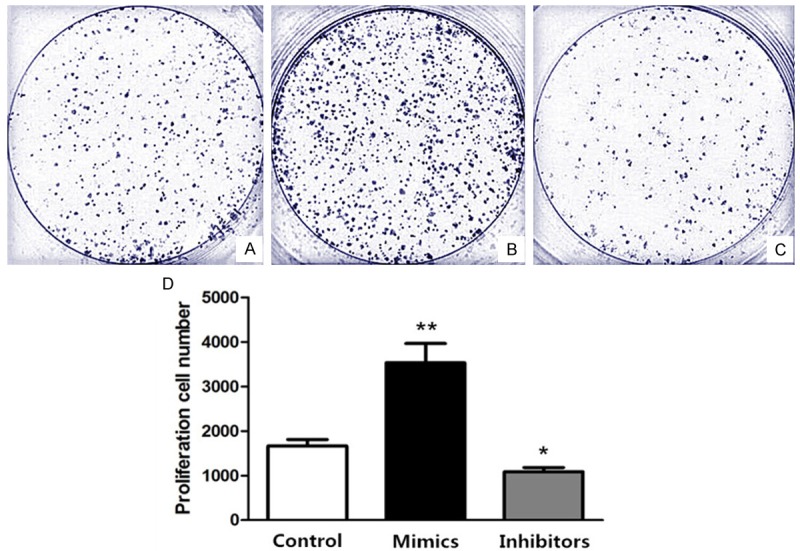
Proliferation effect comparison among differently treated HT-29 cells. Group A. Treated with the same dose of D-Hanks liquid; Group B. Transfected with miR-155-5p mimics; Group C. Transfected with miR-155-5p inhibitor. D. Cell number was counted by Coulter Counter on 2nd day and statistically analyzed, compared with control group, *P<0.05, **P<0.01.
Invasion metastasis effect of miR-155-5p in vitro
HT-29 cell lines were treated with three different methods, the same as the above. The results indicated that invasion metastasis effect of Group B was the highest and that of group C was the lowest among the three groups (Figure 3). It suggested that miR-155-5p could promote colorectal cancer cell invasion metastasis in vitro.
Figure 3.
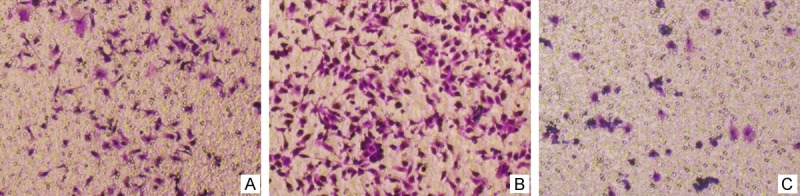
Invasion metastasis effect comparison among differently treated HT-29 cells by Transwell test. Group A. Treated with the same dose of D’ Hanks liquid; Group B. Transfected with miR-155-5p mimics; Group C. Transfected with miR-155-5p inhibitor. Invasion metastasis effect of HT-29 cells with transfection of miR-155-5p mimics and inhibitor were the best and the worst, respectively.
Discussion
Current research suggests that differential expression level of miRNAs in CRC can be involved in the development and progression of CRC and may serve as biomarkers for CRC diagnosis and prognosis [13-15]. In CRC, several miRNAs are known to be differentially expressed and implicated in colorectal carcinogenesis. Studies presented more miRNAs, when dysregulated in CRC, have been demonstrated to be associated with cell proliferation [16], apoptosis [17,18], invasiveness and metastasis [19]. For example, aberrant miR-106a expression could act as a marker of disease-free survival and overall survival independent of tumor stage.
However, in our study, we aimed to explore new miRNAs which can be potentially developed into novel biomarkers for CRC diagnosis and prognosis. We discovered that miR-155-5p expression in colorectal cancer tissues was higher than those in matched adjacent normal tissues. Li [20] reported that up-regulation of miR-155 is related to colitis-associated carcinogenesis. Svrcek [9] pointed out that miR-155 up-regulation was closely related with inflammatory bowel diseases, which increased risk of developing CRC. Pan [21] indicated that miR-155 overexpression in pancreatic cancer. Jurkovicova [22] said that altered expression of miR-155 has been described in multiple cancerous and other diseases, reflecting staging, progress and treatment outcomes. miR-155 became a potential biomarker and candidate for clinical utilization as predictor of the presence of cancer, its staging and prognosis.
For miR-155 effect, there are several different arguments previously reported [23-26]. Valeri et al [10] reported that mismatch repair and genomic stability was modulated by miR-155. Farooqi et al [27] pointed out that miR-155 could modulate cell apoptosis. Huang et al [28] said that regulation of miR-155 affected pancreatic cancer cell invasiveness and migration. Palma et al [29] indicated microRNA-155 could induce apoptosis and cell differentiation in acute myeloid leukemia. Yuhas et al [30] demonstrated that upregulation of miR-155 by nitric oxide provides a new link between nitric oxide, inflammation, and cancer in human hepatic epithelial cells. In our study, the results demonstrated overexpression miR-155-5p could promote CRC cells proliferation and enhanced Invasion metastasis effect of CRC cells by measuring different transfected HT-29 cell lines. It was consistent with some other research conclusion, that expression of miR-155 is upregulated in cells with high proliferative activity and decreased apoptotic capability. Zhang et al [31] proved that miR-155 promoted proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. Yu et al [32] also said that miR-155 could promote tumor metastasis of bone marrow. However, Gasparini et al [33] reported miR-155 in breast cancer, as a protective role, targeted impairs homologous recombination through RAD51 after irradiation. Li et al [34] reported that mir-155-5p was down-regulated in gastric cancer. The reason of the above may be that heterogeneity lies in biological function. In detail, miR-155 may play an opposite effect in different cancers and it might also play a different role in different location or stage of a certain type of cancer.
In summary, overall results herein presented indicate that miR-155-5p expressions are up-regulated in malignancy tissue of most patients with CRC. miR-155-5p expressions are closely associated with some malignant phenotype of CRC, such as tumor location, tumor grade, TNM staging and distant metastasis. Proliferation, invasion and metastasis of CRC cells can be modulated by miR-155-5p expression. Up-regulated miR-155-5p can promote proliferation, invasion and metastasis of CRC. Therefore, miR-155-5p in CRC might play a crucial role in regulating tumorigenesis, and tumor progression. It is prospective to be a diagnostic factor or a drug target after researching deeper mechanism of biological functions.
Acknowledgements
This study was supported by Wu Jieping Medical Fund Project of China (No. 320.6750.13330) and Science & Technology Innovation Fund of Xinjiang Medical University (No. XJC201267). The authors thank Drs Lanying Ma and Chunlei Xu for their support in collecting the surgical specimens needed to perform this study.
Disclosure of conflict of interest
None.
References
- 1.Fan NJ, Kang R, Ge XY, Li M, Liu Y, Chen HM, Gao CF. Identification alpha-2-HS-glycoprotein precursor and tubulin beta chain as serology diagnosis biomarker of colorectal cancer. Diagn Pathol. 2014;9:53. doi: 10.1186/1746-1596-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Wang L, Liao Q, Huang Y, Ye H, Chen F, Xu L, Ye M, Duan S. Hypermethylation of EDNRB promoter contributes to the risk of colorectal cancer. Diagn Pathol. 2013;8:199. doi: 10.1186/1746-1596-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F, Xiong Y, Zhao Y, Tao L, Zhang Z, Zhang H, Liu Y, Feng G, Li B, He L, Ma J, Qin S, Yang Y. Identification of aberrant microRNA expression pattern in pediatric gliomas by microarray. Diagn Pathol. 2013;8:158. doi: 10.1186/1746-1596-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai EC. Micro RNAs are complementary to 39UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 5.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 39UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezzanzanica D, Bagnoli M, De Cecco L, Valeri B, Canevari S. Role of microRNAs in ovarian cancer pathogenesis and potential clinical implications. Int J Biochem Cell Biol. 2010;42:1262–1272. doi: 10.1016/j.biocel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Gong J, Zeng H, Chen N, Huang R, Huang Y, Nie L, Xu M, Xia J, Zhao F, Meng W, Zhou Q. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res. 2010;70:2728–2738. doi: 10.1158/0008-5472.CAN-09-3718. [DOI] [PubMed] [Google Scholar]
- 9.Svrcek M, El-Murr N, Wanherdrick K, Dumont S, Beaugerie L, Cosnes J, Colombel JF, Tiret E, Fléjou JF, Lesuffleur T, Duval A. Overexpression of microRNAs-155 and 21 targeting mismatch repair proteins in inflammatory bowel diseases. Carcinogenesis. 2013;34:828–834. doi: 10.1093/carcin/bgs408. [DOI] [PubMed] [Google Scholar]
- 10.Valeri N, Gasparini P, Fabbri M, Braconi C, Veronese A, Lovat F, Adair B, Vannini I, Fanini F, Bottoni A, Costinean S, Sandhu SK, Nuovo GJ, Alder H, Gafa R, Calore F, Ferracin M, Lanza G, Volinia S, Negrini M, McIlhatton MA, Amadori D, Fishel R, Croce CM. Modulation of mismatch repair and genomic stability by miR-155. PNAS. 2010;107:6982–6987. doi: 10.1073/pnas.1002472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Adachi Y, Taniguchi H, Kunimoto H, Nosho K, Suzuki H, Shinomura Y. Interrelationship between microsatellite instability and microRNA in gastrointestinal cancer. World J Gastroenterol. 2012;18:2745–2755. doi: 10.3748/wjg.v18.i22.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Zhang Z, Sun L, Chai N, Tang S, Jin J, Hu H, Nie Y, Wang X, Wu K, Jin H, Fan D. MicroRNA-499-5p promotes cellular invasion and tumor metastasis in colorectal cancer by targeting FOXO4 and PDCD4. Carcinogenesis. 2011;32:1798–1805. doi: 10.1093/carcin/bgr213. [DOI] [PubMed] [Google Scholar]
- 13.Liu WJ, Zhao YP, Zhang TP, Zhou L, Cui QC, Zhou WX, You L, Chen G, Shu H. MLH1 as a direct target of MiR-155 and a potential predictor of favorable prognosis in pancreaticcancer. J Gastrointest Surg. 2013;17:1399–1405. doi: 10.1007/s11605-013-2230-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li J, Tong L, Zhang J, Zhai A, Xu K, Wei L, Chu M. The prognostic value of miR-21 and miR-155 in non-small-cell lung cancer: a meta-analysis. Jpn J Clin Oncol. 2013;43:813–820. doi: 10.1093/jjco/hyt084. [DOI] [PubMed] [Google Scholar]
- 15.Xu TP, Zhu CH, Zhang J, Xia R, Wu FL, Han L, Shen H, Liu LX, Shu YQ. MicroRNA-155 expression has prognostic value in patients with non-small cell lung cancer and digestive system carcinomas. Asian Pac J Cancer Prev. 2013;14:7085–7090. doi: 10.7314/apjcp.2013.14.12.7085. [DOI] [PubMed] [Google Scholar]
- 16.Tsang WP, Kwok TT. The miR-18a microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 2009;30:953–959. doi: 10.1093/carcin/bgp094. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 18.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M, Xia M, Chen X, Lin Z, Xu Y, Ma Y, Su L. MicroRNA-141 regulates Smad-interacting protein 1 (SIP1) and inhibits migration and invasion of colorectal cancer cells. Dig Dis Sci. 2010;55:2365–2372. doi: 10.1007/s10620-009-1008-9. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Han W, Zhao X, Wang H. Changes of expression of miR-155 in colitis-associated colonic carcinogenesis. Zhonghua Zhong Liu Za Zhi. 2014;36:257–262. [PubMed] [Google Scholar]
- 21.Pan W, Tang W, Yuan W, Yu Q, Zuo W, Xu C, Ma J. Expression and clinical significance of plasma small RNA in patients with pancreatic cancer. Zhonghua Zhong Liu Za Zhi. 2014;36:351–354. [PubMed] [Google Scholar]
- 22.Jurkovicova D, Magyerkova M, Kulcsar L, Krivjanska M, Krivjansky V, Gibadulinova A, Oveckova I, Chovanec M. miR-155 as a diagnostic and prognostic marker in hematological and solid malignancies. Neoplasma. 2014;61:241–251. doi: 10.4149/neo_2014_032. [DOI] [PubMed] [Google Scholar]
- 23.Sochor M, Basova P, Pesta M, Dusilkova N, Bartos J, Burda P, Pospisil V, Stopka T. Oncogenic microRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer. 2014;14:448. doi: 10.1186/1471-2407-14-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui B, Chen L, Zhang S, Mraz M, Fecteau JF, Yu J, Ghia EM, Zhang L, Bao L, Rassenti LZ, Messer K, Calin GA, Croce CM, Kipps TJ. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124:546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hongliang C, Shaojun H, Aihua L, Hua J. Correlation between expression of miR-155 in colon cancer and serum carcinoembryonic antigen level and its contribution to recurrence and metastasis forecast. Saudi Med J. 2014;35:547–553. [PubMed] [Google Scholar]
- 26.Dinami R, Ercolani C, Petti E, Piazza S, Ciani Y, Sestito R, Sacconi A, Biagioni F, le Sage C, Agami R, Benetti R, Mottolese M, Schneider C, Blandino G, Schoeftner S. miR-155 Drives Telomere Fragility in Human Breast Cancer by Targeting TRF1. Cancer Res. 2014;74:4145–4156. doi: 10.1158/0008-5472.CAN-13-2038. [DOI] [PubMed] [Google Scholar]
- 27.Farooqi AA, Qureshi MZ, Coskunpinar E, Naqvi SK, Yaylim I, Ismail M. MiR-421, miR-155 and miR-650: emerging trends of regulation of cancer and apoptosis. Asian Pac J Cancer Prev. 2014;15:1909–1912. doi: 10.7314/apjcp.2014.15.5.1909. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Li H, Wu W, Jiang T, Qiu Z. Regulation of miR-155 affects pancreatic cancer cell invasiveness and migration by modulating the STAT3 signaling pathway through SOCS1. Oncol Rep. 2013;30:1223–1230. doi: 10.3892/or.2013.2576. [DOI] [PubMed] [Google Scholar]
- 29.Palma CA, Al Sheikha D, Lim TK, Bryant A, Vu TT, Jayaswal V, Ma DD. MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukaemia. Mol Cancer. 2014;13:79. doi: 10.1186/1476-4598-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuhas Y, Berent E, Ashkenazi S. Effect of nitric oxide on microRNA-155 expression in human hepatic epithelial cells. Inflamm Res. 2014;63:591–596. doi: 10.1007/s00011-014-0730-8. [DOI] [PubMed] [Google Scholar]
- 31.Zhang CM, Zhao J, Deng HY. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci. 2013;20:79. doi: 10.1186/1423-0127-20-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu F, Jia X, Du F, Wang J, Wang Y, Ai W, Fan D. miR-155-deficient bone marrow promotes tumor metastasis. Mol Cancer Res. 2013;11:923–936. doi: 10.1158/1541-7786.MCR-12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparini P, Lovat F, Fassan M, Casadei L, Cascione L, Jacob NK, Carasi S, Palmieri D, Costinean S, Shapiro CL, Huebner K, Croce CM. Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc Natl Acad Sci U S A. 2014;111:4536–4541. doi: 10.1073/pnas.1402604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Xie S, Liu M, Chen Z, Liu X, Wang L, Li D, Zhou Y. The clinical significance of downregulation of mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric cancer tumorigenesis. Int J Oncol. 2014;45:197–208. doi: 10.3892/ijo.2014.2415. [DOI] [PubMed] [Google Scholar]


