Abstract
Objective: To study the effects of RhoA siRNA on the malignant phenotypes of human colorectal cancer cell line LoVo. Methods: The siRNA expression vector pGPU6/GFP/Neo-shRNA-RhoA targeting the mRNA of RhoA and vector pGPU6/GFP/Neo-NC (as a control) were constructed, and then transfected into LoVo cells. The expression of Survivin was detected by real-time fluorescent quantitative polymerase chain reaction and Western blot. The malignant phenotypes of transfected LoVo cells, including invasive activities and adhesive capabilities, were analyzed. Results: RhoA mRNA and protein level were decreased after the pshRNA-RhoA transfection. The cell adhesion rates significantly decreased in the cells transfected with pshRNA-RhoA. The migrating number of LoVo cells (26.5 ± 0.9) transfected with pshRNA-RhoA was also significantly decreased as compared with the control group (53.7 ± 1.4). Conclusions: The sequence specific shRNA against RhoA constructed in the study can block the expression of RhoA in LoVo cell effectively and specifically; Blocking the expression of RhoA in LoVo cells transfected with pshRNA-RhoA can reduce their invasive and adhesive capabilities.
Keywords: Colonic cancer, LoVo cells, RNA interference, RhoA
Introduction
As a member of the Rho subfamily in the ras related small molecule GTP enzyme superfamily, the biological effect basis of RhoA is to regulate various biological behaviors including cellular morphology, polarity, proliferation, adhesion, apoptosis and so on [1,2], through changing the structure of cytoskeleton actin. Based on researches in recent years, there is the signal of RhoA playing a vital role in the occurrence and metastasis of tumors [3,4]. Currently, studies related to the connection between RhoA and the invasion as well as metastasis ability of malignant tumors are still rather rare [5]. In this paper, a small interruption RNA (siRNA) segment has been designed against the RhoA coded area; an shRNA eukaryotic expression vector of the target RhoA has also been constructed in order to observe its impacts on the malignant phenotype of colon cancer cells; in addition, the relativity between the gene expression of RhoA and the occurrence as well as metastasis of colon cancer has been discussed so that new methods of therapy can be provided for this type of tumor.
Materials and methods
Cell line
The colon cancer LoVo cell line is from the Xiangya Central Laboratory of Central South University.
Regeants
RPMI-1640 nutrient fluid (Gbico), calf serum (Hangzhou Sijiqing), Lipofeetamine 2000 (Invitrogen), Opti-MEN I (Gbico), RT-PCR kit (QIAGEN), TRIzol (Invitrogen), dimethyl sulfoxide DMSO (Sigma). Mouse anti-human GAPDH monoclonal antibody, mouse anti-human RhoA monoclonal antibody (Abnova, China Taipei), SYBR Premix Ex TaqTM (TaKaRa).
Design of RhoA siRNA and the construction of Its recombined plasmid
The interference sequences of RhoA siRNA small hairpin structure containing the double enzyme digestion of BamH I and Pst I (354-384 bp of NM-001664CDS) have been designed as follow; 5-CACCGAAGGATCTTCGGAATGATTCAAGAGATCATTCC GAAGATCCTCTTTTTTG-3 is the positive-sense strand, where as the antisense strand is 5-GATCCAAAAAAGAAGGATCTTCGGAATGATGATCTCTTGAATCATCATTCCGAAGATCCTTC-3. T4 ligase has been in the oriented connection between double stranded oligonucleotides and the pGPU6/GFP/Neo linear vector before transforming the Escherichia coli DH5α; Kanamycin resistant colonies have been selected for enlargement culturing then a small amount of plasmid has been quickly prepared to be sent to Shanghai Genepharma Ltd. for the DNA sequencing identification. In addition, the RhoA interruption recombined plasmid has been named PshRNA-RhoA whereas the negative control one has been called pshRNA-NC.
Cells culturing
Place the RPMI-1640 culture medium containing 10% calf serum into the 5% CO2 cell culturing box at 37°C in order to culture human colon cancer LoVo cells; then digest the cells with 0.25% tryptase as soon as they have grown till 90% fusing; afterwards, blow the cells of with the 10% calf serum RPMI-1640 culture medium so that their numbers can be adjusted to a certain level for the following experiments.
Filtering and identifying the recombinant transfected LoVo cells as well as the positive clone
Inoculate 3×105 LoVo cells onto a six-well plate then use PshRNA-RhoA and pshRNA-NC to transfect them as soon as the degree of fusion reaches 90%. After 48 hours, dilute the passage with a ratio of 1:6 while changing the culture medium to the selective one (constant potency of 400 μg/ml) in order to filter 14 d; subsequently, clone the generated cells onto the culturing plate for enlargement culturing, breeding and constructing lines, they are named PshRNARhoA and pshRNA-NC cells.
The impact of RhoA siRNA on the RhoA expression of LoVo cells
The experiment has been divided into three groups, including LoVo, PshRNA-RhoA cell group and pshRNA-NC cells with three complex holes in each group.
Real-time quantitative PCR
Collect 1×106 of cells from each group from which the total RNA can be obtained and reverse transcribed into cDNA in order to carry out real-time quantitative PCR reaction. The RhoA upstream primer is 5’-agttcccagaggtgtatgtg-3’; whereas the downstream primer is 5’-ggacagaaatgcttgacttc-3’, with 238 bp as the amplification product. In addition, the upstream primer for beta-actin, the internal control, is 5’-AGCGAGCATCCCCCAAAGTT-3’; while 5’-GGGCACGAAGGCTCATCATT-3’ for downstream, along with the 285 bp amplification products. The reaction happens under the condition of 10 seconds at 95°C, 45 seconds at 61°C, then another 10 seconds at 61°C, such cycle goes on for 40 times. Set LoVo cells as the internal control and calculate the expression amounts of pshRNA-NC and RhoAmRNA towards LoVo cells according to the 2-ΔΔCT formula.
Western blots
Split the cells with decomposition solution which is then boiled for 5 minutes prior to the Western blots; Mouse anti-human GAPDH monoclonal antibody (1:1000 diluted, Abnova, China Taipei) and mouse anti-human RhoA monoclonal antibody (1:1000 diluted, Abnova, China Taipei) have been used as the primary antibodies; whereas goat anti mouse IgG has been appointed the secondary antibody, with DAB and NBT/BCIP for coloration.
Cell adhesion
Every hole of a 96-well plate is covered by 100 μl fibronectin (10 μg/ml), while 100 μg/ml polylysine and the 1% bovine serum albumin peridium hole have been set as the maximum and minimum adhesion control. Prepare LoVo/silence (+) and LoVo/silence (-) into 5× 105/ml cell suspension, fill each hole with 100 μl, then hatch at 37°C and saturated humidity with 5% CO2 for 3 hours. Subsequently, rinse away the non-adhesive cells with phosphate buffer before the fixation with paraformaldehyde, then color them with 1% methylene blue solution for 20 minutes; afterwards, add 100 μl 1 mol/L HCl into each well and keep it at 37°C for 40 minutes; lastly, use the enzyme-labeled meter to measure the absorbance value (value A) at 600 nm, hence the cell adhesion rate = (experimental value A-bovine serum albumin hole value A)/(polylysine hole value A-bovine serum albumin hole value A) ×100%.
Matrigel invasion
Place 100 μl Matrigel in the upper chamber of Transwell which is then hatched at 37°C for 2 hours, then add in 1×105 cells from each of the non-serum solution diluted LoVo interruption and LoVp negative control groups; while 1 ml culturing medium with FBS is poured into the lower chamber for incubation in the culturing box for 24 hours. Take out the chambers, wipe away the non-invaded cells and Matrigel on the upper chamber with cotton buds, then fix with 95% alcohol for 15 minutes and color the nucleus with haematoxylin for 10 minutes; after that, remove the filter membrane and place the cells under the 200 times optical microscope in order to calculate them in 5 random visual fields so that the average value can be obtained.
Statistical method
Use the SPSS13.0 statistic software for the paired tests between the two experimental groups at the same time.
Results
Identifying the enzyme digestion of recombined plasmids
Both RhoAshRNA-1 and RhoAshRNA-2 have been testing with BamH I as well as Pst I for the identification of enzyme digestion. The obtained positive-recombinant vectors of plasmids ought to have the possibility of being cut open by BamH I but not Pst I. After the double enzyme digestion, a small 5 200 DNA band has emerged in the 25 g/L agarose gel electrophoretic analysis which fulfills the characteristics of the pGPU6/GFP/Neo plasmid (Figure 1).
Figure 1.
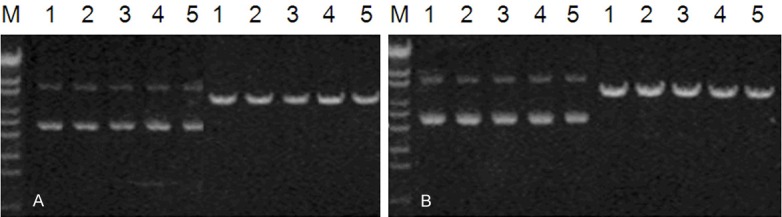
Enzyme digestion identification of the recombined vector. A: The enzyme digestion result of BamH I and Pst I for RhoAshRNA-1; B: the enzyme digestion result of BamH I and Pst I for RhoAshRNA-2. M: DNA markers 1 to 5 all stand for 5 individual positive clones.
Changes in the expression of RhoA mRNA
After the PshRNARhoA being transfected by LoVo cells, in comparison with the negative control group (5. 87% ± 0.57%), the expression amount of RhoAmRNA has significantly reduced, with a restraining rate of up to 74.87% ± 3.24%.
Testing the protein expression of RhoA with Western blots
Tested the protein expression of RhoA for the cells in each group with Western marks (Figure 2), the expression amount of RhoA protein in pshRNA-RhoA cells has reduced comparing with non-transfected LoVo cells; according to the grey value analysis, the relative expression amount of its RhoA equals to 36.93% of the non-transfected LoVo cells.
Figure 2.
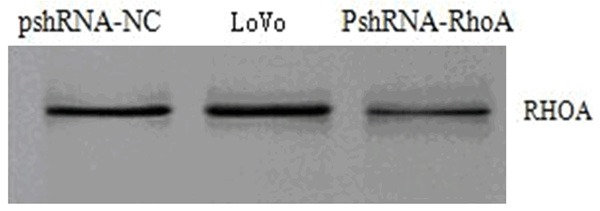
The protein expression of RhoA for the cells in each group (Western blot).
Cell adhesion experiment
The transfected PshRNA-RhoA group has an adhesion rate of 9.24% ± 2.54% whereas 42.83% ± 1.37% for the negative control group, the difference is of statistical significance (P<0.05).
Cell invasion experiment
After transfecting the recombined plasmid of RhoA shRNA, the number of LoVo cells passed through the membrane is 26.5 ± 0.9which is obviously lower than those 53.7 ± 1.4 of negative control plasmid transfected cells, such difference is of statistical significance (P<0.05), as shown in (Figure 3).
Figure 3.
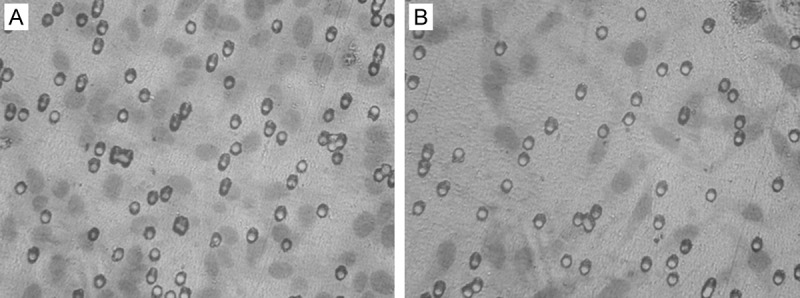
Cell invasion experiment (HE, ×200). A: The negative control group; B: The recombined plasmid with RhoA shRNA transfection group.
Discussions
Metastasis is the most basic biological characteristics of malignant tumors; it is a result of the interactions between various internally related approaches, including the invasion, migration and growth of cancer cells, to name but a few; every step consists of multiple factors making it complicated. Due to the fact that it is so far the major cause of death in cancer patients, studies on the metastasis of tumors has become one of the most concentrated leading edge of oncology researches. The Rho subfamily is a group of guanine nucleotide binding protein which contains 20-30 kD molecules and GTP enzyme activity; its RhoA, RhoB and RhoC are highly homologous with approximately 85% of their amino acid sequences identical [6,7]. Based on researches, the expression amount of the RhoA in colon cancer tissues is higher than its corresponding control group; also, the incidence rate of lymphatic metastasis is rather high in the cases of positive expressed RhoA [8-10] that has been confirmed in the early studies of this paper, it implies that RhoA genes play a crucial role in the process of colon cancer metastasis. Hence in this research, the shRNA expression vector of target RhoA has been successfully designed while the colon cancer LoVo cells have been transfected of which its impacts on the adhesion and invasion as well as other biological behavior of cells have been further investigated [11].
As a heredity phenomenon in natural organisms, the RNA interruption (RNAi) is able to induce target gene silencing, as well as efficiently and specifically restrain target genes. RNAi possesses a lot of advantages that many traditional methods could not rival, including specification, efficiency and transmissibility; it has been widely used in the studies of genomic functions, treating cancers and viral diseases, etc. ever since its discovery [12-14]. Furthermore, LoVo cells are the highly expressed RhoA gene of human colon cancer cells [15]. Through the construction of the eukaryotic expression vector for the target PshRNA-RhoA and the stable transfection of human colorectal cancer LoVo cells, the real-time quantitative RT-PCR and Western blot tests have detected that both the RhoA mRNA expression amount and protein content of cells from the interruption group have become lower than normal LoVo cells; its RhoA restrain rate at the mRNA level reaches 74.87% whereas the siRNA of the negative control group does not have any significant effect (5.87%) on the expression of RhoA; hence, it has been conveyed that the RhoA genes in LoVo cells have already been successfully reduced, in another words, the siRNA constructed by us has not only great power but also specification. Based on this research background, the eternal adhesion experiment shows that after transfecting the expression vector of PshRNA-RhoA, the adhesion ability of cells have decreased, this proves that RhoA is related to the adhesion ability of colorectal cancer cells. In addition, results of the invasion experiment plot that after the RhoA expression being restrained; the number of cells passing through the artificial basement membrane is significantly less than the negative control group which means indicates the close relationship between the RhoA protein expression amount and the invasion as well as metastasis of colorectal cancer cells.
All the above evidences show that RhoA is possibly related to the invasion and metastasis of tumors, hence RhoA has a high potential of being regarded as the new target for researches on cancer gene therapy. Nevertheless, the internal influence of shRNA on the genomic expression of RhoA along with the invasion and metastasis of colorectal cancer cells is yet to be studied.
Disclosure of conflict of interest
None.
References
- 1.Chang YW, Bean RR, Jakobi R. Targeting RhoA/Rho kinase and p21-activated kinase signaling to prevent cancer development and progression. Recent Pat Anticancer Drug Discov. 2009;4:110–24. doi: 10.2174/157489209788452830. [DOI] [PubMed] [Google Scholar]
- 2.Hiyoshi H, Okada R, Matsuda S, Gotoh K, Akeda Y, Iida T, Kodama T. Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway. PLoS Pathog. 2015;11:e1004694. doi: 10.1371/journal.ppat.1004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouzahzah B, Albanese C, Ahmed F, Pixley F, Lisanti MP, Segall JD, Condeelis J, Joyce D, Minden A, Der CJ, Chan A, Symons M, Pestell RG. Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol Med. 2001;7:816–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Li XR, Ji F, Ouyang J, Wu W, Qian LY, Yang KY. Overexpression of RhoA is associated with poor prognosis in hepatocellular carcinoma. EJSO. 2006;32:1130–4. doi: 10.1016/j.ejso.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Takemoto K, Ishihara S, Mizutani T, Kawabata K, Haga H. Compressive Stress Induces Dephosphorylation of the Myosin Regulatory Light Chain via RhoA Phosphorylation by the Adenylyl Cyclase/Protein Kinase A Signaling Pathway. PLoS One. 2015;10:e0117937. doi: 10.1371/journal.pone.0117937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. Accumulation of GTP-bound RhoA during cytokinesis and acritical role of ECT2 in this accumulation. JBiol Chem. 2000;275:17233–6. doi: 10.1074/jbc.C000212200. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 8.Takami Y, Higashi M, Kumagai S, Kuo PC, Kawana H, Koda K, Miyazaki M, Harigaya K. The activity of RhoA is correlated with lymph node metastasis in human colorectal cancer. Dig Dis Sci. 2008;53:467–73. doi: 10.1007/s10620-007-9887-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Chen Y, Cao D, Zhang Y, Meng R, Lu J. The expression of RhoA gene in colorectal cancer. Zhonghua Yi Xue Za Zhi. 2002;82:348–51. [PubMed] [Google Scholar]
- 10.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–7. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999;59:2004–10. [PubMed] [Google Scholar]
- 12.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 13.Artamonov MV, Jin L, Franke AS, Momotani K, Ho R, Dong XR, Majesky MW, Somlyo AV. Signaling pathways that control RhoA activity maintain the embryonic epicardial progenitor state. J Biol Chem. 2015;290:10353–67. doi: 10.1074/jbc.M114.613190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briz V, Zhu G, Wang Y, Liu Y, Avetisyan M, Bi X, Baudry M. Activity-Dependent Rapid Local RhoA Synthesis Is Required for Hippocampal Synaptic Plasticity. J Neurosci. 2015;35:2269–82. doi: 10.1523/JNEUROSCI.2302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao K, Tang W, Li Y, Zhang P, Wang D, Yu L, Wang C, Wu D. Front-signal-dependent accumulation of the RHOA inhibitor FAM65B at leading edges polarizes neutrophils. J Cell Sci. 2015;128:992–1000. doi: 10.1242/jcs.161497. [DOI] [PMC free article] [PubMed] [Google Scholar]


