Abstract
Obliterative bronchiolitis (OB) is characterized by sub-epithelial inflammatory and fibrotic narrowing of the bronchioles, and it is the predominant factor limiting long-term survival after lung transplantation. To explore molecular mechanism of OB, we investigated the interaction of transcription factor (TF), microRNA, long noncoding RNA (lncRNA), and gene expression in the mice model of OB by integrated analysis of TF array, miRNA microarray, and lncRNA and mRNA microarray. After 28 days of orthotopic tracheal transplantation in mice, 42 TFs were significantly up-regulated in allogeneic graft compared to syngeneic graft; 62 miRNAs including miR-376-5p were up-regulated and 17 miRNAs including miR-338-3p were down-regulated over 2-fold; 137 mRNAs were down-regulated and 129 mRNAs were up-regulated over 2-fold; 234 lncRNAs were up-regulated and 212 lncRNAs were down-regulated over 2-fold in the allogeneic model compared to that in the syngeneic control group. We further analyzed potential interaction between TFs, miRNAs, lncRNAs and target genes by different algorithms. Four differentially expressed TFs (Myc/Max, FOXO1, FOXM1, and SMAD) were predicted to regulate 3 different miRNAs, 17 mRNAs, and 16 lncRNAs. These findings suggest that modulation of altered transcription factors such as Myc/Max and FOXO1, and miRNAs such as miR-376-5p and miR-338-3p may become a preventive or therapeutic targets in the chronic lung allograft dysfunction.
Keywords: Obliterative bronchiolitis, orthotopic tracheal transplantation, molecular mechanism
Introduction
Lung transplantation is the definitive therapy for many end-stage pulmonary disorders such as interstitial fibrosis and COPD [1,2]. Compared with other solid organ transplantations, the operation and preservation of donor techniques have been developed and the result is satisfactory in short-term [2,3]. However, long-term results are still not compelling and the major death reason is largely due to obliterative bronchiolitis (OB), which is considered as chronic rejection presenting as a progressive decline in FEV1 [4] and the loss of allograft function. Chronic rejection, now known as chronic lung allograft dysfunction (CLAD) can be represented by different histological patterns with OB being the most common. Pathological feature of OB is characterized with luminal obliteration, bronchiolar epithelial cell injury and cicatrix formation, which lead to airway fibrosis and disappearance of small airways. While a peri-bronchiolar or peri-vascular infiltration of leukocytes and lymphocytes is considered as initial pathogenesis of OB, little is known about its cellular and molecular pathogenesis.
An increased expression of many proteins is believed to be involved in the complex immune response and inflammatory response in OB [1]. Expression of these proteins such as cytokines, inflammatory enzymes, receptors and adhesion molecules, is mainly regulated by gene transcription through an activation or inactivation of certain transcription factors (TFs), the DNA-binding proteins. The regulatory mechanism of gene expression and protein synthesis is coordinated and interactive regulation, including transcription factors, short noncoding RNAs (miRNAs) and long noncoding RNAs (lncRNAs) [5]. Better understanding interactive regulation of these proteins and RNAs is crucial to explore the pathogenesis of many immune disorders including obliterative bronchiolitis. Thus, the current study is designed to explore pathogenesis of obliterative bronchiolitis through analyze interaction network between TF, miRNA, lncRNA and their target gens. To accomplish this, allogeneic orthotopic tracheal transplantation in mice, a known animal model of obliterative bronchiolitis, was performed and used to accomplish the aims of the current study [6,7]. Specifically, expression of transcription factor, miRNA, lncRNA, and mRNA was profiled in the lung tissues of the animal models 28 days after orthotopic tracheal transplantation. Interactive regulation of TFs, miRNAs, and lncRNAs on gene expression was then analyzed using freely available online tools.
Materials and methods
Animals
Specific pathogen-free, female C57BL/6 (H-2b) mice and Balb/C (H-2d) mice (Tianjin Medical University Laboratory), weighing 20-24 g, were used. All animals were housed in a specific pathogen-free facility (Tianjin Medical University, Tianjin, China) and had access to water and food. All animal studies were performed in compliance with the Principles of Laboratory animal care (NIH publication Vol 25, No. 28 revised 1996) and the Tianjin Medical University Animal Care and Use Committee Guidelines. Tracheas from C57BL/6 mice were implanted into Balb/c mice (allogeneic) or C57BL/6 mice (syngeneic). Each donor tracheal graft was orthotopically implanted. Total 8 pairs of animals in each group were transplanted. Grafts were harvested on day 28 after transplantation and used for histological analysis, and expression analysis of transcription factors, miRNAs and LncRNAs.
Histology
Recipient mice were euthanized by CO2 and the grafts were harvested on day 28. Tracheal grafts were cut into longitudinal sections for hematoxylin and eosin (H&E) staining. Each graft segment for H&E staining was fixed in 4% formalin at room temperature for 24 h followed by embedding in paraffin, cutting into 4-μm sections and then staining with H&E.
Preparation of RNA extraction from the tissues
After 28 days of allogeneic or syngeneic transplantation, trachea grafts were harvested from CO2 euthanized mice. The tissues were immediately washed using 0.9% NaCl (RNase-free), quickly dipped into RNase Inhibitor (Epicentre, USA) following the manufacturer’s instructions, and were stored at -80°C for further RNA extraction.
Transcription factor array analysis
Nuclear proteins were extracted using a commercial Kit (Panomics Nuclear Extraction Kit, Cat#: K266. Redwood City, CA) following the manufacturer’s instruction. Protein concentration of nuclear extract was determined using bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Transcription factor array analysis was performed and activity of 345 transcription factors was analyzed using a transcription factor pathfinder array (Panomics TranSignal kit, Redwood City, CA). To highlight TFs that characterize each group, a per-gene on median normalization was performed, which normalized the expression of every TF on its median among samples using saved data in an Excel spreadsheet and calculating the ratio of the data collected from the images. Any spots with a two-fold increase or decrease are considered as significant.
MicroRNA isolation and microarray analysis
Total RNA was extracted using TRIzol (Life Technology, Grand Island, NY) and purified using miRNeasy mini kit (QIAGEN China, Shanghai, China) following manufacturer’s instructions. After RNA quantification with a NanoDrop 1000, the samples were labeled using Hy3/Hy5 Power labeling kit and hybridized on the miRCURY™ LNA Array (v.18.0) (Exiqon, Denmark) following the manufacture’s instruction, which contains 3100 capture probes, covering all human, mouse, and rat miRNAs annotated in miRBase 18.0. After washing, the hybridized slides were scanned using Axon GenePix 4000B microarray scanner (Molecular Device, Sunnyvale, CA).
Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Replicated miRNAs were averaged and miRNAs with intensity ≥30 were chosen for calculating normalization factor. Expressed data were normalized using the Median normalization. After normalization, differentially expressed miRNAs were identified through Fold Change filtering. Finally, hierarchical clustering was performed to identify distinguishable miRNA expression.
LncRNA microarray analysis
Total RNA from each sample was extracted using TRIzol (Life Technology, Grand Island, NY) and quantified using the NanoDrop ND-1000. RNA integrity was then assessed using standard denaturing agarose gel electrophoresis. For microarray analysis, Agilent Array platform (Agilent Technologies, Santa Clara, CA) was employed following the manufacturer’s standard protocols with minor modifications. Briefly, mRNA was purified from total RNA by removal of rRNA (mRNA-ONLY™ Eukaryotic mRNA Isolation Kit, Epicentre). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 3’ bias utilizing a random priming method. The labeled cRNAs were hybridized onto the Mouse LncRNA Array v2.0 (8×60K, Arraystar, Rockville, MD), which can detect 31,423 LncRNAs and 25,376 coding transcripts. After washing, the arrays were scanned by the Agilent Scanner G2505C.
Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v12.0 software package (Agilent Technologies, Santa Clara, CA). After quantile normalization of the raw data, LncRNAs and mRNA that at least 1 out of 2 samples have flags in Present or Marginal (“All Targets Value”) were chosen for further data analysis. Differentially expressed LncRNAs and mRNAs between two samples were identified through Fold Change filtering. The threshold is Fold Change ≥2.0.
Bioinformatics analysis
Association of miRNA and gene expression
Target gene prediction was identified through the following three software from indicated websites: PicTar 2005: http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi; miRanda v5: http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/; TargetScan 6.2: http://www.targetscan.org/.
The result was considered reliable if a predicted target was identified by at least two of the aforementioned software. The identified targets were then intersected with mRNAs that significantly altered in expression.
Association of TF, miRNA and lncRNA expression
Databases of starBase (v2.0), TransmiR, TRED, ITFP (Integrated Transcription Factor Platform) and TFe (Transcription Factor encylopedia) were used to analyze potential association between TF level and expression of miRNAs or lncRNAs. Cytoscape software (http://www.cytoscape.org) was then used to analyze and obtain the ultimate network graph of gene regulation by miRNAs or lncRNAs.
Results
Histological studies
On day 28 of post-transplantation, a mid-portion of the tracheal graft was harvested to determine histological features. In the syngeneic graft group, none of syngeneic allograft animals had obliterative bronchilolitis (OB)-like airway disease, that is, normal luminal ciliated mucosa and without evidence of cellular infiltrate or edema. In the allogeneic allograft group, in contrast, 8 of 8 (100%) allogeneic orthotopic trachea transplanted animals suffered from OB-like airway disease characterized by edema and lymphocyte infiltration into the airway wall, and >30% luminal obliteration, although severity of the OB-like features was variable in each animal. Lymphocytic inflammation of trachea and epithelial ulcerations were noted in the allografts after the allogeneic transplant. In addition, mononuclear cell infiltration was also seen within the host tracheal segments adjacent to the graft. During the 28 days of observation, aforementioned pathological alterations of the allografts progressively developed into obliterative airway disease characterized by formation of granulation tissue within the airway lumen with spindle-shape cells, matrix deposition, and inflammatory cells (Figure 1).
Figure 1.
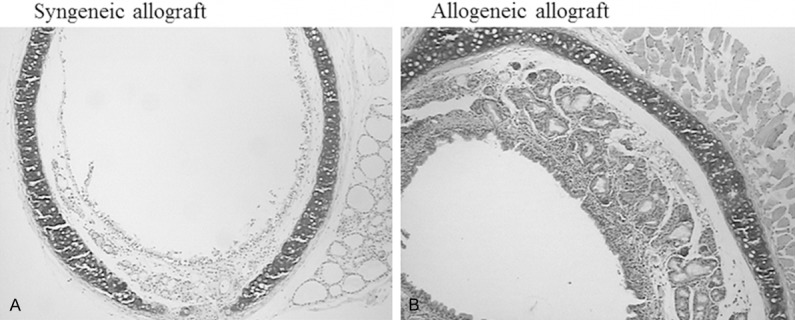
Obliterative bronchiolitis-like features following allogeneic orthotopic tracheal transplantation. After 28 days of syngeneic (A) or allogeneic (B) orthotopic tracheal transplantation, the allograft tissues were harvested and processed for H&E staining as described in the method. Data presented was one representative of 8 pairs syngeneic and 8 pairs of allogeneic transplantation.
Findings of protein/DNA array
Status of TF activation in bronchus of allogeneic grafts was compared to that of syngeneic control mice. After 28 days of transplantation, of the 345 tested TFs, 42 TFs were significantly up-regulated in the allogeneic group compared to the syngeneic group (Table 1). Of the significantly up-regulated 42 TFs, 7 TFs were associated with immune function, 20 TFs were associated with cell proliferation, apoptosis, differentiation or organ formation, and the rest 15 transcription factors or cis-elements were unclear function.
Table 1.
Altered expression of transcription factors in obliterative bronchiolitis (OB)
| Transcription factor | Description | Fold change |
|---|---|---|
| Immune function | ||
| CD28RC, NF-IL2B | T-cell accessory molecule CD28 | 92.5 |
| AAF | An IFN-gamma-regulated DNA-binding factor | 46.1 |
| XBP1, X2BP | X-box-binding protein 1 | 46.0 |
| LR1 | LR1 is a regulator of immunoglobulin class switch recombination in B lymphocytes | 32.4 |
| IRF-1, IRF-2 | Interferon regulatory factor 1/2 binding element | 27.7 |
| Ikaros | Ikaros protein (zinc-finger protein) | 15.2 |
| RORE | RAR-related orphan receptor | 7.3 |
| Cell proliferation and differentiation | ||
| Smad SBE | MADH: MAD, mothers against decapentaplegic homolog | 1234.3 |
| Myc/Max | Myc-associated factor X | 866.4 |
| FKHR | Forkhead box O1A (rhabdomyosarcoma) human | 460.0 |
| CBFB | CCAAT-binding factor | 44.1 |
| HOXA4 | Chox-1.4; Chox1.4; Hox-1.4 (mouse) | 39.8 |
| NRF-1 | Nuclear respiratory factor 1 | 23.8 |
| myc-PRF | A transcriptional repressor of c-myc | 20.4 |
| LF-B2 | Liver-specific factors | 19.8 |
| CdxA/NKX2 | Caudal-type homeodomain protein/ cardiac-specific homeo box | 19.1 |
| C/EBPa/g | CCAAT/enhancer binding protein alpha, gamma | 16.9 |
| Pax2 | Paired box gene 2 (gene5/gene8) | 14.1 |
| HLF | Hepatic leukemia factor | 13.9 |
| LF-A1 (2) | Liver-specific transcription factor | 13.5 |
| GATA1 (2) | 11.9 | |
| CBF | Mouse CCAAT-binding factor, CP1 (human, rat); NF-Y | 10.0 |
| AFP1 | Alpha-fetoprotein | 9.8 |
| TGT3 | TTF-1 (Thyroid Transcription Factor 1) binding element | 7.0 |
| WAP BP | Whey acidic protein | 6.5 |
| AP3 | Activator protein 3 | 6.4 |
| IL-6 RE-BP | Interleukin-6 response element binding protein | 12.4 |
| Unknown function | ||
| DE I | Rat DE1 element from albumin gene | 38.6 |
| ZNF174 | Zinc-finger protein 174 | 20.9 |
| Elf-1 | E74-like factor 1, a novel Ets family member | 20.0 |
| SIF3 | SI promoter 3 | 18.1 |
| Freac-2 (1) | Forkhead box F2 (mouse) | 13.1 |
| Freac-7 | Forkhead box L1 | 12.8 |
| PO-B | Stimulatory factor, binds at -15 in proopiomelanocortin (POMC) gene | 11.9 |
| HNF-3 (a, b, g) | Hepatocyte nuclear factor 3 (a, b, g) | 11.0 |
| Myc-CF1 | Common factor 1; CF1 | 10.6 |
| SIF1 | Sucrase-isomaltase (SI) is an enterocyte-specific gene | 9.0 |
| Pur-1 | MYC-associated zinc finger protein (purine-binding transcription factor) | 8.6 |
| GKLF | Gut-enriched krueppel-like factor; EZF; Epithelial zinc-finger | 8.3 |
| PUR | Pur factor | 6.2 |
| SPERM1 | A pou domain gene transiently expressed prior to meiosis I in the male germ cell | 6.0 |
| GBF1/2/3/HY5 | G-box binding factor 1 | 5.0 |
Findings of miRNA microarray analysis
All murine miRNAs annotated in miRBase 18.0 as well as all viral miRNAs related to mouse were analyzed in both allogeneic and syngeneic graft groups. It was found that 62 miRNAs were up-regulated and 17 miRNAs were down-regulated in the tissues of OB compared to that of syngeneic control group (Table S1). Interestingly, of those significantly up- or down-regulated miRNAs, miR-302C-3p, miR-338-3p, miR-205-5p, miR-302a-3p, miR-124-3p, and miR-690 are associated with innate or adaptive immune function, and miR-376c-5p is known to be involved in idiopathic pulmonary fibrosis through regulating epithelial-mesenchymal transition.
Findings of lncRNA microarray analysis
As shown in Table S2, 234 lncRNAs were up-regulated, while 212 lncRNAs were down-regulated in the animals with OB-like pathological features compared to the animals without OB-like change. In addition, 137 mRNAs were down-regulated, while 129 mRNAs up-regulated in the animals with OB-like alteration compared to the animals received syngeneic graft (Table S3).
Potential biological effect of altered miRNA on gene expression
As described above in 3.3, total 79 miRNAs were up-regulated (62 miRNAs) or down-regulated (16 miRNAs). In order to further study potential biological effect of these miRNAs, predicted target genes for these miRANs was analyzed using three different online programs (PicTar 2005; miRandav5; and TargetScan 5.1). As shown in Table S4, of the 79 miRNAs, 9 miRNAs (miR-883b-5p, miR-709, miR-675-5p, miR-446f-3p, miR-446e-5p, miR-446a-3p, miR-151-5p, miR-690, and miR-338-3p) were found specifically targeting multiple genes, which was confirmed by both miRanda and Targetscan programs. Of them, miR-151-5p, miR-690, and miR-338-3p have been reported to be involved in regulating innate or adaptive immune function.
Potential interaction network of TFs, miRNAs, lncRNAs and mRNAs
In order to further study whether altered miRNAs and lncRNAs were regulated by the transcription factors identified in the current study, online available programs including starBase (v2.0), TransmiR, TRED, ITFP (Integrated Transcription Factor Platform) and TFe (Transcription Factor encylopedia) were used. It was found that total of 3 miRNAs, 16 lncRNAs, and 17 mRNAs might be modulated by the following 4 transcription factors: Myc/Max, FOXO1, FOXM1, and SMAD (Figure 2).
Figure 2.
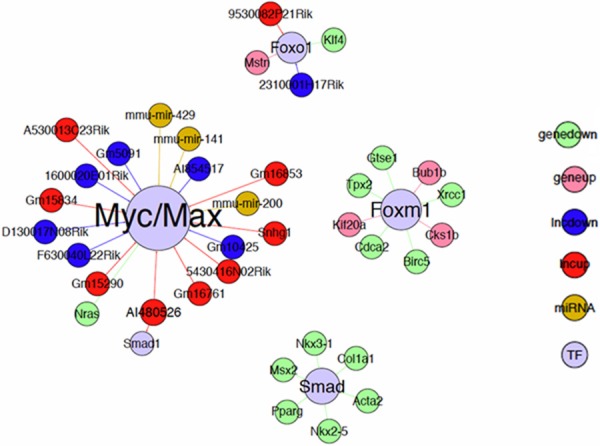
Predicted interaction network of transcription factors, miRNAs, lncRNAs and mRNAs in the development of obliterative bronchiolitis after allogeneic lung transplantation.
Discussion
In the present study, using integrated analysis of protein/DNA array, miRNA microarray and lncRNA microarray, we have investigated alteration of transcription factors, miRNAs, lncRNAs and their interaction in regulating gene expression in the airway tissues of allogeneic and syngeneic orthotopic tracheal transplantation, a well-known animal model of obliterative bronchiolitis. Compared to the syngeneic graft orthotopic trachea transplant, allogeneic graft transplant animals developed obliterative bronchiolitis in all 8 pairs of animals as evidenced by histological features of edema and lymphocyte infiltration into the airway wall, and >30% luminal obliteration. Compared to non-OB-like animal tissues, 42 TFs were significantly up-regulated and 79 miRNAs, 276 mRNA, and 446 lncRNAs were up- or down-regulated in the mice developed with OB after allogeneic tracheal transplantation. Furthermore, 9 of 79 significantly altered miRNAs were identified to target multiple genes as predicted by two different computer programs. Integrated analysis of interaction network of those altered TFs, miRNAs, lncRNAs and mRNAs indicated that 4 TFs (Myc/Mx, FOXO1, FOXM1, and SMAD) likely modulate 3 miRNAs, 16 lncRNAs and 17 mRNAs.
Despite significant advances in lung transplantation, long-term survival rate of lung transplantation recipients has not been dramatically improved [8]. Chronic lung allograft dysfunction, in particular obliterative bronchiolitis, is the primary limiting factor [8]. While innate and adaptive immune response to the transplanted graft plays an important role in the development of obliterative bronchiolitis [1], pathogenesis of OB is not fully defined. Thus, in the current study, an animal model of OB was established by allogeneic and orthotopic tracheal transplantation, which was characterized by edema, lymphocyte infiltration into airway walls and over 30% luminal obliteration. A comprehensive analysis of transcription factor pathways associated with miRNA and lncRNA in OB after syngeneic or allogeneic and orthotopic tracheal transplantation was then further conducted in the airway tissues of the animal models.
Transcription factors have been implicated in human disease such as cancer and inflammation, and it represent one of the most powerful drug development targets [9,10]. Thus, main focus of this study was not only to compare expression of TFs between OB-like animal and control group, but also to screen potential transcription factors that regulate expression of miRNA or lncRNA, and to identify their potential target genes. By protein/DNA binding array analysis, of the 345 tested transcription factors, 42 TFs were significantly up-regulated in the animals with OB-like alteration. Furthermore, of the 42 significantly altered TFs, 7 TFs were associated with immune function and 20 TFs were associated with cell proliferation, apoptosis, differentiation or organ formation, suggesting aberrant immune response and cellular dysfunction in proliferation and differentiation may contribute to the development of OB. In addition, the following 4 TFs, Myc/Max, FOXO1, FOXM1, and SMAD, seemed to modulate miRNAs, lncRNAs or mRNA expression.
Myc, which heterodimerizes with Max (Myc/Max) regulates a large number of genes including genes involved in ribosome biogenesis, and control cancer progression through the cell cycle and cell growth in somatic cells [11]. The Myc/Max heterodimer binds to the DNA sequence CACGTG (E box element) and activates transcription through transactivation domain of Myc, and by which mechanism, Myc/Max regulates growth arrest, differentiation, and cell survival [12]. The current study demonstrated that Myc/Max was up-regulated in OB animals and it seemed to regulate multiple miRNAs and lncRNAs, suggesting Myc/Max may play an important role in the development of OB in the animal models following allogeneic and orthotopic tracheal transplantation.
FKHR (FOXO1) is a member of forkhead transcription factor family, which is characterized by the presence of highly conserved, monomeric DNA-binding domain. FOXO members may play a decisive role in the life and death of immune cells. In response to stimuli such as stress, Forkhead transcription factors translocate into the nucleus and up-regulate a series of target genes, thereby promoting cell cycle arrest, stress resistance, or apoptosis [10]. In allogeneic allograft-induced OB, FOXO proteins may translate environmental stimuli into changes in gene expression, and by which mechanism, it may lead to the progression of OB.
Forkhead Box M1 (FOXM1) is transcription factor, which regulates gene expression throughout embryonic and fetal development as well as during adult tissue homeostasis and repair [6,13,14]. Mouse genetic deletion studies have revealed that FOXM1 plays a critical role in the formation of respiratory epithelium during initial stages of lung development, whereby deletion of foxm1 gene impairs lung maturation and results in respiratory failure after birth [15]. Conversely, expression of a constitutively active form of FOXM1 in the lung epithelium causes epithelial hyperplasia, and inhibits both lung sacculation and expression of the type II epithelial cell marker [15]. In addition, FOXM1 is a key regulator of EMT during adult lung tissue repair [13,14,16], which is engaged in wound healing, organ fibrosis and metastasis of cancer progression. In the current study, we have demonstrated that FOXM1 was significantly up-regulated and it seemed to modulate cell cycle-associated proteins. Therefore, abnormal expression of FOXM1 may be caused by stimuli of allogeneic transplantation, and subsequently lead to OB through interfering the reparation and homeostasis of lung.
SMAD is an important signal transduction protein in the classic signaling of TGF-β families. TGF-β is a transforming growth factor secreted by numerous cell types to regulate fibroblast proliferation, differentiation, migration and synthesis of extra-cellular matrix. Activation of TGF-ß/SMAD signal pathway in airway cells, in particular fibroblasts, leads to fibrotic tissue formation [17,18]. Consistently, TGF-ß1 up-regulation and Smad3 activation have been noticed in obliterative bronchiolitis [17,19,20]. Thus, up-regulation and activation of SMAD protein in allogeneic lung transplantation may contribute to the fibrotic tissue formation after transplantation. Interestingly, SMAD is one of the 4 TFs that significantly up-regulated in the animals with OB-like alteration in the current study.
In addition to transcription factor array, profiling of miRNAs and lncRNAs were also performed in that these non-coding RNAs play an important role in controlling post-transcriptional protein synthesis. MiRNAs are 21-23 nucleotide RNA molecules that regulate the stability or translational efficiency of target messenger RNAs. The regulation of miRNA is important in development and in many normal biological processes such as cellular differentiation, proliferation and apoptosis. MiRNA has also been implicated in cancer, cardiovascular diseases, and immune function [21-23]. In this study, 62 miRNAs were significantly up-regulated while 17 miRNAs were dramatically down-regulated in the animals with OB compared to control animals, suggesting miRNAs may contribute to the pathogenesis of chronic lung allograft dysfunction. Interestingly, limited number of publications has been reported on the role of miRNA in the pathogenesis of OB. Here, we report that miRNAs that associated with innate or adaptive immune regulation, including miR-302C-3p, miR-338-3p, miR-205-5p, miR-302a-3p, miR-124-3p, and miR-690, are significantly altered in OB animals, and that miR-376c-5p, a miRNA known to be involved in idiopathic pulmonary fibrosis through regulating epithelial-mesenchymal transition [24], are significantly up-regulated in OB animals. These findings suggest dynamic alteration of miRNAs such as miR-376c-5p may play an important role in the development of chronic lung allograft dysfunction.
Long non-coding RNAs (lncRNA) are transcribed RNA molecules greater than 200 nucleotides in length and do not appear to have any protein-coding potential [25]. LncRNAs are emerging as important regulatory factors in mammalian genomics. LncRNAs are pervasively transcribed and believed to be critical regulators of the epigenome [13]. New evidence is indicating that lncRNAs are associated with enhancer regions and that such non-coding transcription correlate with the increased activity of the neighboring genes. In the current study, therefore, expression pattern of lncRNAs in animals with or without OB were also assessed. Intriguingly, 234 lncRNAs were significantly up-regulated while 212 lncRNAs were down-regulated in the animals with OB compared to the control animals. While 9 of the 234 up-regulated lncRNAs and 7 of 212 down-regulated lncRNAs may be controlled by Myc/Max or FOXOX1 (Figure 2), potential biological activities of these lncRNAs remains to be further determined.
Taken together, using allogeneic orthotopic tracheal transplantation-induced animal models of OB, expression profiling of transcription factors, miRNAs, lncRNAs and mRNAs in obliterative bronchiolitis was analyzed in the current study. It was found that 42 TFs were significantly up-regluated in the animals with OB, and that transcription factors such as Myc/Max, FOXO1, FOXM1, and SMAD may play important roles in the pathogenesis of OB through regulating downstream miRNAs, lncRNAs or mRNAs. In addition, 62 miRNAs including miR-376c-5p were significantly up-regulated, while 17 miRNA including miR-338-3p were remarkably down-regulated in the OB animals compared to control animals. These findings suggest that modulation of altered transcription factors such as Myc/Max and FOXO1, and miRNAs such as miR- miR-376c-5p and miR-338-3 may become a preventive or therapeutic goal in the chronic lung allograft dysfunction.
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program: 2012AA021003), Science and technology foundation of Tianjin Health Bureau (2014KZ127), National Natural Science Foundation of China (21177091) and Tianjin Science and Technology Support Program (12ZCZDSY03400).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–8. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–95. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Hachem RR, Trulock EP. Bronchiolitis obliterans syndrome: pathogenesis and management. Semin Thorac Cardiovasc Surg. 2004;16:350–5. doi: 10.1053/j.semtcvs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Groves S, Galazka M, Johnson B, Corcoran T, Verceles A, Britt E, Todd N, Griffith B, Smaldone GC, Iacono A. Inhaled cyclosporine and pulmonary function in lung transplant recipients. J Aerosol Med Pulm Drug Deliv. 2010;23:31–9. doi: 10.1089/jamp.2009.0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Nayak D, Yang W, Baskaran G, Ramachandran S, Sarma N, Aloush A, Trulock E, Hachem R, Patterson GA, Mohanakumar T. Dysregulated MicroRNA Expression and Ch- ronic Lung Allograft Rejection in Recipients With Antibodies to Donor HLA. Am J Transplant. 2015;15:1933–47. doi: 10.1111/ajt.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan K, Qiao XW, Nie J, Yuan L, Guo HZ, Zheng ZK, Li JS, Wang JJ, Jiang K. Orthotopic and heterotopic tracheal transplantation model in studying obliterative bronchiolitis. Transpl Immunol. 2013;28:170–5. doi: 10.1016/j.trim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL, Shilling R, Wu Q, Weber DJ, Wagner SR, Lasaro M, Devore D, Wang Y, Sandusky GE, Lipking K, Pandya P, Reynolds J, Love R, Wozniak T, Gu H, Brown KM, Wilkes DS. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191:4431–9. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verleden SE, Ruttens D, Vandermeulen E, Bellon H, Van Raemdonck DE, Dupont LJ, Vanaudenaerde BM, Verleden G, Vos R. Restrictive chronic lung allograft dysfunction: Where are we now? J Heart Lung Transplant. 2015;34:625–30. doi: 10.1016/j.healun.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Latchman DS. Transcription factors as potential targets for therapeutic drugs. Curr Pharm Biotechnol. 2000;1:57–61. doi: 10.2174/1389201003379022. [DOI] [PubMed] [Google Scholar]
- 10.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 11.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–9. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Stover JS, Whitby LR, Vogt PK, Boger DL. Small molecule inhibitors of Myc/Max dimerization and Myc-induced cell transformation. Bioorg Med Chem Lett. 2009;19:6038–41. doi: 10.1016/j.bmcl.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin Cancer Res. 2014;20:1477–88. doi: 10.1158/1078-0432.CCR-13-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, Li Y, Azmi AS, Miele L, Sarkar FH. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, Park HJ, Whitsett JA, Kalinichenko VV. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol. 2010;347:301–14. doi: 10.1016/j.ydbio.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu N, Jia D, Chen W, Wang H, Liu F, Ge H, Zhu X, Song Y, Zhang X, Zhang D, Ge D, Bai C. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PLoS One. 2013;8:e59412. doi: 10.1371/journal.pone.0059412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6:2080–8. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez AM, Nunley DR, Rojas M, Roman J. Activation of Tissue Remodeling Precedes Obliterative Bronchiolitis in Lung Transplant Recipients. Biomark Insights. 2008;3:351–9. doi: 10.4137/bmi.s686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vittal R, Fan L, Greenspan DS, Mickler EA, Gopalakrishnan B, Gu H, Benson HL, Zhang C, Burlingham W, Cummings OW, Wilkes DS. IL-17 induces type V collagen overexpression and EMT via TGF-beta-dependent pathways in obliterative bronchiolitis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L401–14. doi: 10.1152/ajplung.00080.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan DL, Merrick BA, Gerrish KE, Stockton PS, Wang Y, Foley JF, Gwinn WM, Kelly FL, Palmer SM, Ton TV, Flake GP. Gene expression in obliterative bronchiolitis-like lesions in 2,3-pentanedione-exposed rats. PLoS One. 2015;10:e0118459. doi: 10.1371/journal.pone.0118459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong W, Zhao JJ, He L, Cheng JQ. Strategies for profiling microRNA expression. J Cell Physiol. 2009;218:22–5. doi: 10.1002/jcp.21577. [DOI] [PubMed] [Google Scholar]
- 23.Sonkoly E, Pivarcsi A. microRNAs in inflammation. Int Rev Immunol. 2009;28:535–61. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- 24.Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D, Martinez FJ, Hogaboam CM. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One. 2011;6:e21253. doi: 10.1371/journal.pone.0021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


