Abstract
Special AT-rich sequence binding protein-2 (SATB2) is selectively expressed in the lower gastrointestinal tract mucosa and has been identified as a sensitive marker for colorectal adenocarcinomas. The goal of this study was to investigate the expression of SATB2 in well-differentiated neuroendocrine tumors to explore its potential as a diagnostic marker for hindgut well-differentiated neuroendocrine tumors. Immunohistochemical staining with a monoclonal antibody to SATB2 was performed on full tissue blocks in 167 well-differentiated neuroendocrine tumors of various origins. The staining was semi-quantitatively scored as 0 (no tumor cell staining), 1+ (1-25%), 2+ (26-50%), 3+ (51-75%) and 4+ (76-100%). Positive SATB2 staining was seen in 17% foregut (14/84, 12/66 primary and 2/18 metastatic), 12% midgut (3/22, 3/18 primary and 0/7 metastatic), and 90% hindgut (52/58, 44/49 primary and 8/9 metastatic) well differentiated neuroendocrine tumors. Most hindgut well-differentiated neuroendocrine tumors (41/58) showed 4+ staining. The specificity of SATB2 for foregut, midgut and hindgut well-differentiated neuroendocrine tumors was 34%, 54% and 84%, respectively. Our results indicate that SATB2 is a sensitive marker for hindgut well-differentiated neuroendocrine tumors though it is not entirely specific. SATB2 should be included in the immunohistochemical panel in working out metastatic well-differentiated neuroendocrine tumor of an unknown origin.
Keywords: SATB2, well-differentiated neuroendocrine tumor, foregut, midgut, hindgut
Introduction
The incidence of neuroendocrine neoplasms has increased dramatically during the past 30 years [1]. Neuroendocrine neoplasms are divided into well-differentiated neuroendocrine tumors (WDNETs) and poorly differentiated neuroendocrine carcinomas (PDNECs). PDNECs are highly aggressive and treated similarly with platinum-based chemotherapy regardless of their origin, whereas WDNETs exhibit a wide range of biological behavior and are treated differently according to their primary sites [2].
Most WDNETs present with locoregional disease but as many as 20% of them present with distant metastasis [1]. This number is even higher for WDNETs from certain organs such as pancreas and ileojejunum [1]. Even though non-surgical approaches (chemotherapy and/or radiation) are the main treatment for metastatic WDNETs, surgical resection of both primary and metastatic tumors are still beneficial in some of these patients because cytoreduction can reduce acute complication in primary site, minimize endocrine symptoms and decrease the requirement of somatostatin analogs [3,4]. In addition, chemotherapy and targeted therapy protocols are different for metastatic well differentiated neuroendocrine tumors of different origins [2,5]. Therefore determination of the primary origins of metastatic WDNETs has some therapeutic implications.
Determination of the origin of metastatic WDNETs is usually achieved with imaging studies including CT and somatostatic receptor imaging (Octroscan). However, these traditional imaging techniques had a less than 50% successful rate in determining the primary site of metastatic WDNETs of unknown origin [6-8]. Approximately 9-19% of WDNETs still present with unknown origins and such tumors account for approximately 5% of malignancies of unknown origin [2,9,10]. Expensive PET/CT tracer may be more sensitive but it is not available in many areas. Therefore, in some instances determination of the origin of metastatic WDNETs still rely on pathology. However, WDNETs from different sites have similar and overlapping morphology, and it is often difficult to determine their origin just based on morphology. In these situations, immunohistochemical markers are often needed to facilitate to determine the primary site of a metastatic WDNET.
Recently, special AT-rich sequence binding protein-2 (SATB2) has been identified as a marker with a highly selective expression pattern in the lower gastrointestinal tract mucosa [11]. About 85- 93% colorectal adenocarcinomas were immunohistochemically positive for SATB2 [11,12]. During our clinical practice, we have observed that SATB2 labels all epithelial cells in lower gastrointestinal mucosa, implicating that SATB2 labels both non-neuroendocrine epithelial cells and neuroendocrine cells. Mucosal neuroendocrine cells give rise to neuroendocrine tumors. In this study, with immunohistochemical staining we investigated SATB2 expression in 167 well-differentiated neuroendocrine tumors from various sites to explore the possible utility of SATB2 as a diagnostic marker for lower gastrointestinal WDNETs especially in the metastatic setting.
Materials and methods
Case selection
The study was approved by the Institutional Ethnic Committees (Institutional Review Boards). The surgical pathology files of Peking University Cancer Hospital, the Chinese PLA General Hospital, the Second Affiliated Hospital of Guangzhou Medical College, Hubei Cancer Hospital, and the second Affiliated Hospital of Jilin University were searched for WDNETs/carcinoids. The diagnosis of all WDNETs was confirmed with immunohistochemical staining of chromogranin and synaptophysin. Ki-67 proliferation index was also performed for all gastrointestinal and pancreatic neuroendocrine neoplasms for grading purpose. The grades of gastrointestinal and pancreatic neuroendocrine neoplasms were assigned according to the WHO 2010 criteria (G1: mitotic count < 2 per 10 high power fields (HPFs) and/or ≤ 2% Ki-67 index; G2: mitotic count 2-20 per 10 HPFs and/or 3-20% Ki67 index; G3 mitotic count > 20 per 10 HPFs and/or > 20% Ki67 index [13]. G1 and G2 gastrointestinal and pancreatic neuroendocrine neoplasms are classified as WDNETs whereas G3 tumors are PDNECs. In thymus and lung, carcinoid and atypical carcinoid are classified as WDNETs, and small cell carcinoma and large cell neuroendocrine carcinoma are classified as PDNECs.
One hundred sixty-seven (167) WDNETs were collected including 133 primary and 34 metastatic ones (Tables 1, 2, 3 and 4). These tumors were assigned to foregut (N = 84), midgut (N = 25) and hindgut (N = 58) origins according to previous criteria [14]. The foregut WDNETs included those from lung (11 carcinoids, 2 atypical carcinoids; all primary), thymus (6 carcinoids, 2 atypical carcinoids; all primary), pancreas (13 G1, 14 G2; 13 primary and 14 metastatic), gallbladder (1 G1, primary), esophagus (N = 0), stomach (14 G1, 8 G2; 19 primary and 3 metastatic) and the proximal half of the duodenum (8 G1, 5 G2; 12 primary and 1 metastatic). WDNETs from the distal half of duodenum (N = 0), jejunum (2 G1, 1 G2; 2 primary and 1 metastatic), ileum (11 G1, 2 G2; 8 primary and 5 metastatic), cecum (1 G2, primary), appendix (4 G1, all primary), ascending colon (3 G1, 1 G2; 3 primary and 1 metastatic), and proximal 1/3 transverse colon (N = 0) are classified as midgut WDNETs. Hindgut WDNETs are those from distal 2/3 transverse colon (N = 0), descending colon (N = 0), sigmoid colon (1 G1, primary) and rectum (45 G1, 12 G2; primary 48, metastatic 9).
Table 1.
Immunohistochemical staining result of SATB2 in 84 foregut well differentiated neuroendocrine tumors
| Site of Origin | Primary | Metastatic | Total |
|---|---|---|---|
| Stomach (N = 22) | 2/19 (10%) | 0/3 (0%) | 2/22 (9%) |
| Duodenal (proximal half) (N = 13) | 1/12 (8%) | 0/1 (0%) | 1/13 (8%) |
| Pancreas (N = 27) | 2/13 (15%) | 2/14 (14%) | 4/27 (15%) |
| Gallbladder (N = 1) | 0/1 (0%) | 0/0 | 0/1 (0%) |
| Lung (N = 13) | 3/13 (23%) | 0/0 | 3/13 (23%) |
| Thymus (N = 8) | 4/8 (50%) | 0/0 | 4/8 (50%) |
| Total (N = 84) | 12/66 (18%) | 2/18 (11%) | 14/84 (17%) |
Table 2.
Immunohistochemical staining result of SATB2 in 25 midgut well differentiated neuroendocrine tumors
| Site of Origin | Primary | Metastatic | Total |
|---|---|---|---|
| Ileum (N = 13) | 1/8 (13%) | 0/5 | 1/13 (8%) |
| Jejunum (N = 3) | 0/2 | 0/1 | 0/3 |
| Appendix (N = 4) | 1/4 (25%) | 0/0 | 1/4 (25%) |
| Cecum and ascending colon (N = 5) | 1/4 | 0/1 | 1/5 (20%) |
| Total (N = 25) | 3/18 (17%) | 0/7 (0%) | 3/25 (12%) |
Table 3.
Immunohistochemical staining result of SATB2 in 58 hindgut well differentiated neuroendocrine tumors
| Site of Origin | Primary | Metastatic | Total |
|---|---|---|---|
| Sigmoid colon (N = 1) | 1/1 (100%) | 0/0 | 1/1 (100%) |
| Rectum (N = 57) | 43/48 (90%) | 8/9 (89%) | 51/57 (89%) |
| Total (N = 58) | 44/49 (90%) | 8/9 (89%) | 52/58 (90%) |
Table 4.
Comparison of SATB2 staining in foregut, midgut, and hindgut well differentiated neuroendocrine tumors
| Site of Origin | Percentage of tumors positive for SATB2 staining | P value |
|---|---|---|
| Foregut (N = 84) | 17% (14/84) | P < 0.001 between foregut and hindgut |
| Midgut (N = 25) | 12% (3/25 ) | P < 0.001 between midgut and hindgut |
| Hindgut (N = 58) | 90% (52/58) | P = 0.76 between foregut and midgut |
Immunohistochemistry
One formalin fixed paraffin embedded tissue block from each case was used to generate 4 um unstained slides for immunohistochemical staining with a monoclonal antibody to SATB2 (clone EPNCIR130A, dilution 1:100, OriGene Technologies, Maryland, USA). Appropriate positive and negative controls were included for each batch of immunohistochemical staining. Only nuclear staining was considered positive. The immunohistochemical staining was scored semi-quantitatively as 0 (no tumor cell staining), 1+ (1-25% tumor cells stained), 2+ (26-50%), 3+ (51-75%), and 4+ (76-100%).
Statistic analysis
Fisher exact test was used to compare the SATB2 staining among foregut, midgut and hindgut WDNETs. A P value < 0.05 was considered statistically significant. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated with the following formats: sensitivity = number of true positives/(number of true positives + number of false negatives), specificity = number of true negatives/(number of true negatives and number of false positives), PPV = number of true positives/(number of true positives + number of false positives), and NPV = number of true negatives/(number of true negatives and number of false negatives).
Results
SATB2 staining in foregut well differentiated neuroendocrine tumors (N = 84)
Among the 84 foregut WDNETs, 14 (17%) showed positive SATB2 staining including 12/66 (18%) primary and 2/18 (11%) metastatic tumors (Table 1). Primary tumors and metastatic ones did not show difference in SATB2 staining (P = 0.7238).
Among the 12 (of 66) primary tumors showing positive SATB2 staining, 8 showed staining in no more than 50% tumor cells (1+ in 6/12, 2+ in 2/12) and 4 showed staining in more than 50% tumor cells (3+ in 2/12, 4+ in 2/12). The two metastatic tumors (both from pancreas) showed 2+ and 3+ staining, respectively.
SATB2 staining in midgut well differentiated neuroendocrine tumors (N = 25)
Positive SATB2 staining was seen in 3/25 (12%) midgut WDNETs including 3/18 (17%) primary and 0/7 (0%) metastatic ones (Table 2). The percentage of tumor cells showing SATB2 staining was no more than 50% in all 3 cases (1+ in 1, 2+ in 2).
SATB2 staining in hindgut well differentiated neuroendocrine tumors (N = 58)
Among the 58 hindgut WDNETs, positive SATB2 staining was seen in 52 (89%) tumors including 44/49 (90%) primary (Figure 1) and 8/9 (89%) metastatic tumors (Figure 2; Table 3). The staining pattern distribution in 44 SATB2-positive primary tumors was 1+ in 4 (4/44, 9%), 2+ in 4 (4/44, 9%), 3+ in 2 (2/44, 5%), 4+ in 34 (34/44, 77%). Among the 8 (of 9) SATB2-positive metastatic hindgut WDNETs, 7 (88%) showed 4+ staining and 1 (12%) showed 3+ staining. Overall 41 of 58 (71%) hindgut WDNETs showed 4+ staining.
Figure 1.
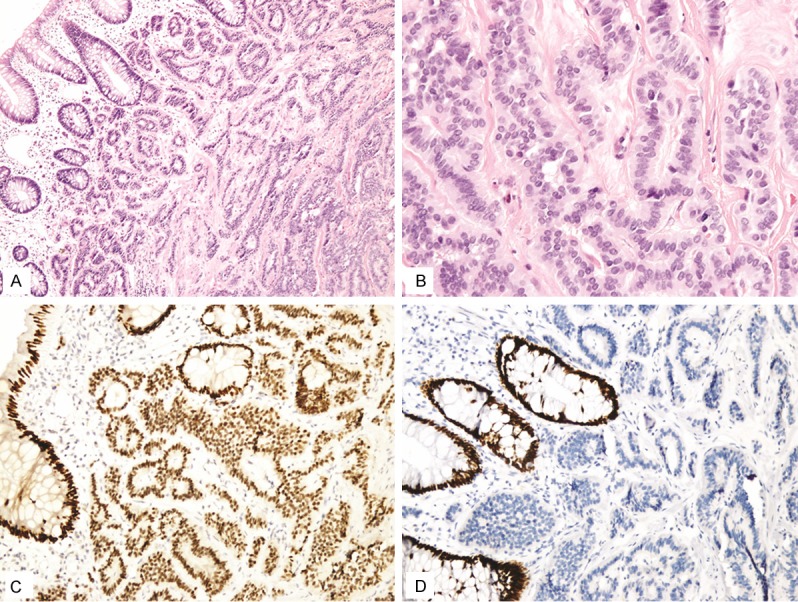
In this study 90% of rectal well differentiated neuroendocrine tumors (A, B) showed positive staining for SATB2 with most of them showing diffuse staining (C). The normal rectal mucosal epithelium showed diffuse SATB2 staining (C). This tumor was negative for CDX2 (D). Normal rectal mucosal epithelium was positive for CDX2.
Figure 2.
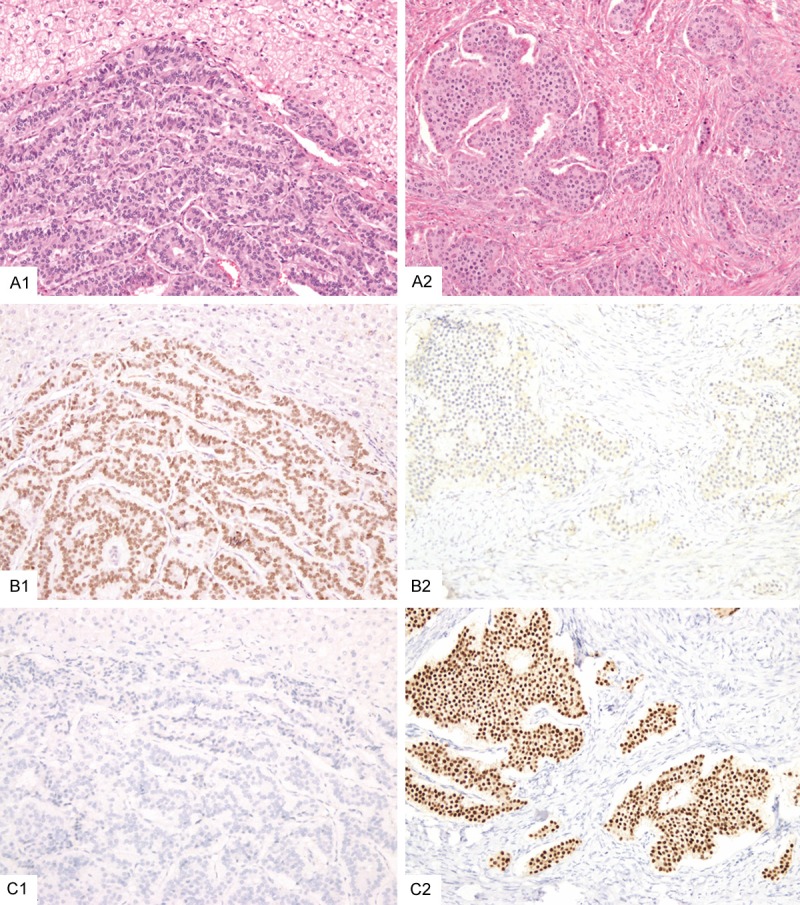
This rectal well differentiated neuroendocrine tumor that metastasized to the liver (A1) was positive for SATB2 (B1) but negative for CDX2 (C1). In contrast, an ileal well differentiated neuroendocrine tumor that metastasized to one ovary (A2) showed negative staining for SATB2 (B2) but was positive for CDX2 (C2).
Sensitivity and specificity of SATB2 for well-differentiated neuroendocrine tumors from foregut, midgut and hindgut
In this study, the sensitivity of SATB2 for labeling foregut, midgut and hindgut WDNETs was 17%, 12% and 90%, respectively (P < 0.001 between foregut and hindgut, P < 0.001 between midgut and hindgut, P = 0.76 between foregut and midgut) (Table 4). The specificity of SATB2 for foregut, midgut and hindgut WDNETs was 34%, 54% and 84%, respectively (Table 5). The positive predictive value of SATB2 for foregut, midgut and hindgut WDNETs was 20%, 4%, and 75%, respectively (Table 5). The negative predictive value of SATB2 for foregut, midgut and hindgut WDNETs was 29%, 78%, and 94%, respectively (Table 5).
Table 5.
Sensitivity, specificity, positive predictive value and negative predictive value of SATB2 for 167 well differentiated neuroendocrine tumors
| Foregut WDNETs | Midgut WDNETs | Hindgut WDNETs | |
|---|---|---|---|
| Sensitivity | 17% (14/84) | 12% (3/25) | 90% (52/58) |
| Specificity | 34% (28/83) | 54% (76/142) | 84% (92/109) |
| Positive predictive value | 20% (14/69) | 4% (3/69) | 75% (51/69) |
| Negative predictive value | 29% (28/98) | 78% (76/98) | 94% (92/98) |
Discussion
SATB2 is a DNA-binding protein that interacts with transcription factors to regulate craniofactial development, cortical neuron differentiation, immunoglobulin mu gene expression, skeletal development and osteoblastic differentiation [15-18]. In a previous study [11], Magnusson et al first showed that SATB2 was a sensitive marker (85% sensitivity) for colorectal adenocarcinomas, which was later confirmed by another study (93% sensitivity) [12]. In addition, SATB2 was also a sensitive osteoblastic marker [19,20].
In this study, we investigated the expression of SATB2 in a large series of 167 WDNETs. We found that SATB2 staining was seen in 17% foregut, 12% midgut and 90% hindgut WDNETs. Among those 52 (of 58) SATB2-positive hindgut WDNETs, most of them (44/52 or 85%) showed staining in more than 50% tumor cells and 80% (41/52) labeled more than 90% tumor cells (4 + staining). Our study indicates that among WDNETs, SATB2 is preferentially expressed in hindgut WDNETs and is therefore a sensitive marker for hindgut WDNETs. The high sensitivity of SATB2 for hindgut WDNETs holds not only for primary tumors (44/49 or 90%) but also for metastatic ones (8/9 or 89%).
Previous studies have identified several immunohistochemical markers for determining the origins of metastatic WDNETs. These markers included thyroid transcription factor 1 (TTF1), caudal type homeobox 2 (CDX2), neuroendocrine secretory protein-55 (NESP-55), (PDX1), paired box 8 (PAX8), and Islet1 [2,21-48]. TTF1 is a relatively specific marker for pulmonary carcinoid/atypical carcinoids (the overall sensitivity is 32%) [2,21,22,24,25,31,32,35]. CDX2 is considered a sensitive marker for midgut WDNETs (86-92% sensitivity) but some foregut (3% to 33%) and hindgut (29-43%) WDNETs also show immunoreactivity [2,23-32,35,36]. NESP55 is relatively specific for pancreatic WDNETs but with low sensitivity (50% sensitivity) [2,31-34]. PDX1 mainly labeled gastroduodenopancreatic WDNETs (46-60% sensitivity) but it also labeled a variable percentage of lung (3/39 or 8%), appendiceal (7/17 or 41%) and rectal (2/14 or 14%) WDNETs [2,31,35-39]. PAX8 and Islet1 mainly labeled foregut (61-68% and 80-89% duodenopancreatic WDNETs, respectively) and hindgut WDNETs (56% and 88% rectal WDNETs, respectively) [2,39-48]. When compared to these previously reported markers, SATB2 showed similar sensitivity to Islet1 for rectal WDNETs (89% versus 88%, P = 1.0) and was more sensitive than PAX8 for these tumors (89% versus 56%, P < 0.01). The specificity of SATB2, Islet1 and PAX8 for rectal WDNETs was 84%, 55%, and 63%, respectively (P < 0.01 between SATB2 versus Islet1, P < 0.01 between SATB2 and PAX8) (specificity for Islet1 and PAX8 was calculated based on the data from literature as summarized in Table 6). Therefore even though SATB2 and Islet1 show similar sensitivity for rectal WDNETs, SATB2 is more specific than Islet1 for these tumors. SATB2 is both more sensitive and specific than PAX8 for rectal WDNETs. Another marker for rectal WDNETs that is not usually used but reported in the early literature is prostatic acid phosphatase (PASP) [49-53]. The reported positive rate of PSAP in large series of rectal WDNETs (carcinoids) was ranging from 67-82% [49-53], not as sensitive as SATB2. Although SATB2 is not specific for hindgut WDNETs, it has a high negative predictive value (94%) i.e. a WDNET negative for SATB2 is unlikely from the hindgut region.
Table 6.
Comparison of SATB2 to other well differentiated neuroendocrine tumor markers in the literature
| Markers | Foregut Origin | Midgut origin | Hindgut Origin | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Lung | Stomach | Pancreas | Duodenum | Jejunoilecum | Appendix | Cecum | Colon | Rectum | ||
| TTF1 | 32% (189/588) | 0.03% (4/928) | [2,21-22,24-25,31-32,35] | |||||||
| CDX2 | 3% (8/233) | 15% (12/80) | 16% (81/499) | 33% (17/52) | 90% (306/339) | 92% (72/78) | 86% (6/7) | 43% (10/23) | 29% (32/112) | [2,23-32,35-36] |
| NESP55 | 10% (2/20) | 0% (0/9) | 50% (49/99) | 0% (0/5) | 0% (0/57) | 0 (0/11) | NA | NA | 7% (1/16) | [2,31-34] |
| PDX1 | 8% (3/39) | 60% (3/5) | 56% (124/220) | 46% (18/39) | 0% (0/49) | 41% (7/17) | 0% (0/3) | 0% (0/2) | 14% (2/14) | [2,35-39] |
| PAX8 | 6% (7/117) | 14% (5/37) | 68% (145/213) | 61% (11/18) | 0% (0/152) | 17% (5/30) | NA | 0% (0/1) | 56% (28/50) | [2,42,44-48] |
| Islet1 | 11% (11/101) | 0% (0/22) | 80% (256/322) | 89% (25/28) | 1% (2/137) | 15% (7/46) | 0% (0/1) | 23% (3/13) | 88% (22/25) | [2,39-43] |
| SATB2 | 23% (3/13) | 9% (2/22) | 15% (4/27) | 8% (1/13) | 6% (1/16) | 25% (1/4) | 0% (0/1) | 40% (2/5: ascending 1/4, sigmoid 1/1) | 89% (51/57) | This study |
The proportions of WDNETs arising foregut, midgut and hindgut origins are highly variable among different racial groups. In the USA SEER data, in Caucasians foregut WDNETs were most common (45%) followed by midgut (25%) and hindgut ones (12-16%) whereas in Asians/Pacific islanders, the most common WDNETs were from hindgut (approximately 41-45%, more than 90% hindgut WDNETs were rectal) followed by foregut (34%) and midgut (7%) [1]. In east Asian countries such as China, Korea and Japan, hindgut WDNETs (vast majority are rectal) account for 50-70% of gastrointestinal WDNETs and only 10% of gastrointestinal WDNETs are of midgut origin [54,59]. The distant metastasis rate of rectal WDNETs is related to the tumor size, ranging from 5.5% in less than 1.0 cm tumors to 4-30% in 1.0-1.9 cm tumors to 70-80% in tumors 2.0 cm or larger [54-59]. Given the large number of population in East Asian countries, the number of patients with metastatic rectal WDNETs is not small [54-59]. Even in USA, in a recent study 13% (11/85) rectal WDNETs were metastatic at presentation and additional 5% (4/85) had metastasis on follow-up [60]. Therefore even though rectal WDNETs have a lower metastasis rate than that of midgut and pancreatic WDNETs, the number of metastatic rectal WDNETs is actually not that small. Most metastasis occurred in the liver but rectal WDNETs can metastasize to lung and pancreas, creating significant difficulty in differentiating them from primary pulmonary and pancreatic WDNETs [54-60]. In such situations, use of immunohistochemical markers is critical to make such distinction. Sensitive hindgut WDNET markers such as SATB2 are particularly useful in this aspect. Such diagnostic utility is even more appreciated in eastern Asian countries given the large patient population and high proportion of rectal WDNETs.
It should be pointed out that even though SATB2 is a sensitive marker for hindgut WDNETs, it is not specific. As a matter of fact, except TTF1 (TTF1 is relatively specific for pulmonary WDNETs and thyroidal medullary carcinoma), none of the currently used WDNET markers is specific for WDNET from any particular site. Therefore a panel of immunohistochemical markers is often needed in determining the primary site of a metastatic WDNET. Bellizzi [2] recommended an initial panel to include TTF1, CDX2 and PAX8 (or Islet1). We would recommend to add SATB2 to this panel to increase sensitivity and specificity for working out metastatic WDNETs of unknown origin.
Obviously SATB2 should be always used in conjunction with tumor morphology. SATB2 was highly expressed in colorectal adenocarcinomas and occasionally in some other types of carcinomas [11,12]. Therefore SATB2 is not useful to distinguish WDNET from adenocarcinoma in the hindgut region. Their distinction relies on other parameters (morphology, mitotic figures/Ki67 index, and neuroendocrine markers chromogranin and synaptophysin). In addition, we have found that SATB2 is also expressed in approximately one third of PDNECs of various organs (data not published). Therefore SATB2 is not useful to distinguish WDNETs from PDNECs.
One limitation of our study is that the number of metastatic WDNETs is relatively small (foregut 18, midgut 7 and hindgut 9). Further studies are needed to include more WDNETs from various sites particularly metastatic ones to test whether the high sensitivity of SATB2 for metastatic hindgut WDNETs can be maintained.
In summary, we investigated SATB2 expression in a large series of WDNETs. Our results indicate that SATB2 is a sensitive marker for hindgut WDNETs though it is not specific. SATB2 should be included in the immunohistochemical panel for working out metastatic WDNETs from an unknown origin.
Acknowledgements
This work was supported by the Discretional Fund from Peking University Cancer Hospital & Institute (12-01 to DC) and Support Fund from Beijing Health and Family Planning Commission (to DC for Academic Pathology Leadership Program), The Clinical Science Fund of the Chinese PLA General Hospital (No. 2012-FC-TSYS-3050 to JY), and Beijing Municipal Science and Technology Commission NOVA Program Fund (No. 2012B033 to ZL).
Disclosure of conflict of interest
None.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, EVans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20:285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 3.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am. 2003;12:231–42. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 4.Akerström G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:87–109. doi: 10.1016/j.beem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, Anlauf M, Wiedenmann B, Salazar R, Barcelona Consensus Conference Participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 6.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 2010;37:67–77. doi: 10.1007/s00259-009-1205-y. [DOI] [PubMed] [Google Scholar]
- 7.Savelli G, Lucignani G, Seregni E, Marchiano A, Serafini G, Aliberti G, Villano C, Maccauro M, Bombardieri E. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumours. Nucl Med Commun. 2004;25:445–9. doi: 10.1097/00006231-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Schreiter NF, Bartels AM, Froeling V, Steffen I, Pape UF, Beck A, Hamm B, Brenner W, Rottgen R. Searching for primaries in patients with neuroendocrine tumors (NET) of unknown primary and clinically suspected NET: Evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA octreotide SPECT/CT. Radiol Oncol. 2014;48:339–47. doi: 10.2478/raon-2014-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirshbom PM, Kherani AR, Onaitis MW, Yanawa M. Carcinoids of unknown origins: comparative analysis with foregut, midgut and hindgut carcinoids. Surgery. 1998;124:1063–170. doi: 10.1067/msy.1998.93105. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez A, Llanos-Muñoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jiménez-Fonseca P, Teulé A, Sastre-Valera J, Benavent-Viñuelas M, Monleon A, Salazar R. Incidence, pattern of care and prognostic factors for outcome of gastroenteropancreatic tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21:1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A, Wester K, Gry M, Bjartell A, Gallagher WM, Rexhepaj E, Kilpinen S, Kallioniemi OP, Belt E, Goos J, Meijer G, Birgisson H, Glimelius B, Borrebaeck CA, Navani S, Uhlén M, O’Connor DP, Jirström K, Pontén F. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. Am J Surg Pathol. 2011;35:937–48. doi: 10.1097/PAS.0b013e31821c3dae. [DOI] [PubMed] [Google Scholar]
- 12.Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: Results of a pathology-based clinical prospective study. Am J Clin Pathol. 2014;141:630–8. doi: 10.1309/AJCPWW2URZ9JKQJU. [DOI] [PubMed] [Google Scholar]
- 13.Rindi G, Klimstra DS, Arnold R. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 13–14. [Google Scholar]
- 14.Williams ED, Sandler M. The Classification of carcinoid tumours. Lancet. 1963;1:238–9. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 15.FitzPatrick DR, Carr IM, McLaren L, Leek JP, Wightman P, Williamson K, Gautier P, McGill N, Hayward C, Firth H, Markham AF, Fantes JA, Bonthron DT. Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet. 2003;12:2491–501. doi: 10.1093/hmg/ddg248. [DOI] [PubMed] [Google Scholar]
- 16.Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Fariñas I, Karsenty G, Grosschedl R. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–86. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Dobreva G, Dambacher J, Grosschedl R. SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression. Genes Dev. 2003;17:3048–61. doi: 10.1101/gad.1153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-Alonso J, Aguado T, Wu CS, Palazuelos J, Hofmann C, Garcez P, Guillemot F, Lu HC, Lutz B, Guzmán M, Galve-Roperh I. The CB1 Cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J Neurosci. 2012;32:16651–16665. doi: 10.1523/JNEUROSCI.0681-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63:36–49. doi: 10.1111/his.12138. [DOI] [PubMed] [Google Scholar]
- 20.Ordonez NG. SATB2 is a novel marker of osteoblastic differentiation and colorectal adenocarcinoma. Adv Anat Pathol. 2014;21:63–7. doi: 10.1097/PAP.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 21.Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13:238–42. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]
- 22.Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24:1217–23. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Suh E, Traber PG. Intestine-specific gene transcription. Annu Rev Physiol. 1996;58:275–97. doi: 10.1146/annurev.ph.58.030196.001423. [DOI] [PubMed] [Google Scholar]
- 24.Saqi A, Alexis D, Remotti F. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394–404. doi: 10.1309/UKN6-PVRK-XHG4-22DA. [DOI] [PubMed] [Google Scholar]
- 25.Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407–14. doi: 10.1097/01.pai.0000210416.53493.0f. [DOI] [PubMed] [Google Scholar]
- 26.Barbareschi M, Roldo C, Zamboni G, Capelli P, Cavazza A, Macri E, Cangi MG, Chilosi M, Doglioni C. CDX-2 homeobox gene product expression in neuroendocrine tumors: its role as a marker of intestinal neuroendocrine tumors. Am J Surg Pathol. 2004;28:1169–76. doi: 10.1097/01.pas.0000131531.75602.b9. [DOI] [PubMed] [Google Scholar]
- 27.Erickson L, Papouchado B, Dimashkieh H, Zhang S, Nakamura N, Lloyd RV. Cdx2 as a marker for neuroendocrine tumors of unknown primary sites. Endocr Pathol. 2004;15:247–52. doi: 10.1385/ep:15:3:247. [DOI] [PubMed] [Google Scholar]
- 28.Jaffee IM, Rahmani M, Singhal MG, Younes M. Expression of the intestinal transcription factor CDX2 in carcinoid tumors is a marker of midgut origin. Arch Pathol Lab Med. 2006;130:1522–6. doi: 10.5858/2006-130-1522-EOTITF. [DOI] [PubMed] [Google Scholar]
- 29.Kaimaktchiev V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M, Sauter G, Corless CL. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17:1392–9. doi: 10.1038/modpathol.3800205. [DOI] [PubMed] [Google Scholar]
- 30.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33:626–32. doi: 10.1097/PAS.0b013e31818d7d8b. [DOI] [PubMed] [Google Scholar]
- 32.Denby KS, Briones AJ, Bourne PA, Spaulding BO, Lu D, Fischer-Colbrie R, Qu Z, Wang HL, Xu H. IMP3, NESP55, TTF-1 and CDX2 Serve as an Immunohistochemical Panel in the Distinction Among Small-cell Carcinoma, Gastrointestinal Carcinoid, and Pancreatic Endocrine Tumor Metastasized to the Liver. Appl Immunohistochem Mol Morphol. 2012;20:573–579. doi: 10.1097/PAI.0b013e3182494009. [DOI] [PubMed] [Google Scholar]
- 33.Jakobsen AM, Ahlman H, Kölby L, Abrahamsson J, Fischer-Colbrie R, Nilsson O. NESP55, a novel chromogranin-like peptide, is expressed in endocrine tumors of the pancreas and adrenal medulla but not in ileal carcinoids. Br J Cancer. 2003;88:1746–1754. doi: 10.1038/sj.bjc.6600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava A, Padilla O, Fischer-Colbrie R, Tischler AS, Dayal Y. Neuroendocrine secretory protein-55 (NESP-55) expression discriminates pancreatic endocrine tumors and pheochromocytomas from gastrointestinal and pulmonary carcinoids. Am J Surg Pathol. 2004;28:1371–1378. doi: 10.1097/01.pas.0000135527.96318.20. [DOI] [PubMed] [Google Scholar]
- 35.Chan ES, Alexander J, Swanson PE, Jain D, Yeh MM. PDX-1, CDX-2, TTF-1 and CK7: a reliable immunohistochemical panel for pancreatic neuroendocrine neoplasms. Am J Surg Pathol. 2012;36:737–743. doi: 10.1097/PAS.0b013e31824aba59. [DOI] [PubMed] [Google Scholar]
- 36.Hermann G, Konukiewitz B, Schmidt A, Perren A, Koppel G. Hormonally defined pancreatic and duodenal neuroendocrine tumors differ in their transcription factors signatures: expression of ISL1, PDX1, NGN3 and CDX2. Virchows Arch. 2011;459:147–154. doi: 10.1007/s00428-011-1118-6. [DOI] [PubMed] [Google Scholar]
- 37.Fendrich V, Remerth R, Waldmann J, Maschuw K, Langer P, Bartsch DK, Slater EP, Ramaswamy A, Rothmund M. Sonic hedgehog and pancreatic-duodenal homeobox 1 expression distinguishes between pancreatic and duodenal gastrinomas. Endocr Relat Cancer. 2009;16:613–622. doi: 10.1677/ERC-08-0204. [DOI] [PubMed] [Google Scholar]
- 38.Park JY, Hong SM, Klimstra DS, Goggins MG, Maitra A, Hruban RH. PDX1 expression in pancreatic precursors and neoplasms. Appl Immunohistochem Mol Morphol. 2011;19:444–449. doi: 10.1097/PAI.0b013e318206d958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermann G, Konukiewitz B, Schmidt A, Perren A, Koppel G. Hormonally defined pancreatic and duodenal neuroendocrine tumors differ in their transcription factors signatures: expression of ISL1, PDX1, NGN3 and CDX2. Virchows Arch. 2011;459:147–154. doi: 10.1007/s00428-011-1118-6. [DOI] [PubMed] [Google Scholar]
- 40.Agaimy A, Erlenbach-Wunsch K, Konukiewitz B, Schmitt AM, Rieker RJ, Vieth M, Kiesewetter F, Hartmann A, Zamboni G, Perren A, Klöppel G. Islet1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neoplasms of extrapancreatic origin. Mod Pathol. 2013;26:995–1003. doi: 10.1038/modpathol.2013.40. [DOI] [PubMed] [Google Scholar]
- 41.Graham RP, Shrestha B, Caron BL, Smyrk TC, Grogg KL, Lloyd RV, Zhang L. Islet-1 is a sensitive but not entirely specific marker for pancreatic neuroendocrine neoplasms and their metastases. Am J Surg Pathol. 2013;37:399–405. doi: 10.1097/PAS.0b013e31826f042c. [DOI] [PubMed] [Google Scholar]
- 42.Koo J, Mertens RB, Mirocha JM, Wang HL, Dhall D. Value of Islet1 and PAX8 in identifying metastatic neuroendocrine tumors of pancreatic origin. Mod Pathol. 2012;25:893–901. doi: 10.1038/modpathol.2012.34. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt AM, Riniker F, Anlauf M, Schmid S, Soltermann A, Moch H, Heitz PU, Klöppel G, Komminoth P, Perren A. Islet1 expression is a reliable marker for pancreatic endocrine tumors and their metastasis. Am J Surg Pathol. 2008;32:420–425. doi: 10.1097/PAS.0b013e318158a397. [DOI] [PubMed] [Google Scholar]
- 44.Laury AR, Sangoi AR, Rai RK. A comprehensive analysis of PAX8 in human epeithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 45.Long KB. Srisvastava A, Hirsch MS, Hornick JL. PAX8 expression in well differentiated endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol. 2010;34:723–729. doi: 10.1097/PAS.0b013e3181da0a20. [DOI] [PubMed] [Google Scholar]
- 46.Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, Truong LD. PAX8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–764. doi: 10.1038/modpathol.2011.3. [DOI] [PubMed] [Google Scholar]
- 47.Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK, Pai RK. PAX8 expression reliably distinguishes pancreatic well differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol. 2011;24:412–424. doi: 10.1038/modpathol.2010.176. [DOI] [PubMed] [Google Scholar]
- 48.Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. App Immnohistochem Mol Morphol. 2011;19:293–299. doi: 10.1097/PAI.0b013e3182025f66. [DOI] [PubMed] [Google Scholar]
- 49.Sobin LH, Hjermstad BM, Sesterhenn IA, Helwig EB. Prostatic acid phosphatase activity in carcinoid tumors. Cancer. 1986;58:136–8. doi: 10.1002/1097-0142(19860701)58:1<136::aid-cncr2820580124>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Kimura N, Sasano N. Prostate-specific acid phosphatase in carcinoid tumors. Virchows Arch A Pathol Anat Histopathol. 1986;410:247–251. doi: 10.1007/BF00710831. [DOI] [PubMed] [Google Scholar]
- 51.Federspiel BH, Burke AP, Sobin LH, Shekitka KM. Rectal and colonic carcinoids. A clinicopathologic study of 84 cases. Cancer. 1990;65:135–40. doi: 10.1002/1097-0142(19900101)65:1<135::aid-cncr2820650127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 52.Azumi N, Traweek ST, Battifora H. Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies. Am J Surg Pathol. 1991;15:785–90. doi: 10.1097/00000478-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Burke AP, Thomas RM, Elsayed AM, Sobin LH. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer. 1997;79:1086–93. [PubMed] [Google Scholar]
- 54.Ito T, Sasano H, Tanako M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Nakamura K, Igarashi H, Jensen RT, Wiedenmann B, Imamura M. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–243. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 55.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 56.Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park do Y, Lee JH, Chang H, Jung ES, Kim HK, Jin SY, Choi JH, Gu MJ, Kim S, Kang MS, Cho CH, Park MI, Kang YK, Kim YW, Yoon SO, Bae HI, Joo M, Moon WS, Kang DY, Chang SJ. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157–65. doi: 10.4143/crt.2012.44.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colonoscopy Study group of Korean Society of Coloproctology. Clinical characteristics of colorectal carcinoid tumors. J Korean Soc Coloproctol. 2011;27:17–20. doi: 10.3393/jksc.2011.27.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin HH, Lin JK, Jiang JK, Lin CC, Lan YT, Yang SH, Wang HS, Chen WS, Lin TC, Liang WY, Chang SC. Clinicopathological analysis of colorectal carcinoid tumors and patient outcomes. World J Surg Oncol. 2014;12:366. doi: 10.1186/1477-7819-12-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XH, Lu XL, Wu N, Liu B, Wang FY, Zhang RS, Zhou XJ. Clinicopathological features of colorectal neuroendocrine neoplasms and prognostic significance of WHO staging system. Zhonghua Bing Li Xue Za Zhi. 2013;42:191–6. doi: 10.3760/cma.j.issn.0529-5807.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Kwaan MR, Goldberg JE, Beday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008;143:471–5. doi: 10.1001/archsurg.143.5.471. [DOI] [PubMed] [Google Scholar]


