Abstract
Objectives: This study aimed to evaluate key features of bone marrow trephine biopsy (BMT) involvement of lymphoma in Northern China. Methods: 950 cases were assessed for the occurrence of bone marrow involvement and architectural features including volume percentage, involvement pattern (diffuse, nodular, focal, para trabecular, or interstitial), and presence/absence of background changes (granuloma, stromal fibrosis or necrosis). Correlations with bone marrow aspirate (BMA) and flow cytometry (FCM) findings were made in a subset of paired cases (359 BMA and 364 FCM). Results: 153 (16.1%) cases involved BMT. The most frequent type was mantle cell lymphoma (28/153, 18.3%). Architectural features were similar to previous studies except that diffuse large B-cell lymphoma (DLBCL) preferred focal pattern (16/22 cases, 72.7%) most of all. BMA and BMT agreed in 84.1% of cases (302 of 359: 277 both negative, 25 both positive), while FCM and BMT agreed in 80.8% of cases (294 of 364: 242 both negative, 52 both positive). Both varied widely among different subgroups. Conclusions: Occurrence of BMT involvement by lymphoma in Northern China is relatively low. The volume percentage, distribution patterns and background changes may be useful pointers towards a particular lymphoma type in Chinese. FCM is more sensitive and reliable than BMA in China.
Keywords: Lymphoma, bone marrow, involvement, pathology, Chinese
Introduction
Despite the usage of flow cytometry (FCM) and bone marrow aspirate (BMA) methods in lymphoma staging, pathological detection with bone marrow trephine biopsy (BMT) remains the “gold standard” [1]. The BMT findings also have significant implications for clinical prognosis and therapy planning. Previous studies have reported that bone marrow (BM) involvement occurs in about 40% of all lymphoma cases in Western countries [2]. Chinese lymphoma epidemic spectrums are quite different from those in the Western world, with more patients suffering from mature T-cell and/or NK-cell lymphomas [3,4]. This may cause differences in the occurrence rate of lymphomas involving BM in Chinese patients. However, very few comprehensive studies focused on this subject have been reported [5].
As our hospital is one of the biggest lymphoma therapeutic centers in China, lymphoma patients come from most parts of Northern China. The aim of this study was to retrospectively assess the pathological data of BMT involvement in lymphoma cases in Northern China. In this study, the occurrence rates and patterns of BM involvement in different types of lymphoma were calculated and analyzed to improve our overall knowledge of the incidence rate for each type of lymphoma. In accordance with methods reported in the literature, parameters that may be useful for pathological diagnosis of the involvement of BM in lymphomas include: the frequency of BM involvement in each lymphoma type; the tumor volume percentage in BMT; the distribution patterns of lymphoma cells in BM. Finally, where available, BMT results were compared with both BMA and FCM findings, and statistical analysis was used to investigate differences between data sets. We believe that our relatively comprehensive study will provide an important reference on the overview of BM involvement in lymphoma for patients in Northern China.
Materials and methods
Sequential BMT cores from routine work were collected over a 5-year period from November 2008 to June 2014. BMT slides were reviewed by a minimum of two pathologists to confirm the involvement of BM and its pattern. Background changes including granulomas, stromal fibrosis and necrosis were also recorded. Inclusion criteria were as follows: (1) disease manifested primarily as solid mass but not leukemia; (2) cases must have been evaluated primarily before treatment; (3) immunophenotypic confirmation of lymphoma should also be available when it was deemed necessary. In total, 950 cases with a known diagnosis of lymphoma in whom bone marrow evaluation was performed met the inclusion criteria .In order to facilitate data analysis and further discussion, all cases involving BM were divided into the following five subgroups according to cell origin, morphological features, clinical behavior, and tendency of BM involvement: lymphoblastic lymphocyte lymphomas (LBLs), large- and medium-sized B-cell lymphomas (LMBLs, composed of diffuse large B-cell lymphoma DLBCL, Burkitt lymphoma BL, plasma blastic cell lymphoma, high-grade follicular lymphoma FL, and B-cell lymphoma, unclassifiable with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma or with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma), small B-cell lymphomas (SBLs, including mantle cell lymphoma MCL, small lymphocytic lymphoma/chronic lymphocytic leukemia, low-grade FL, marginal zone cell lymphoma MZL, lymphoplasmacytic lymphoma, and plasma cell myeloma), mature T and NK cell lymphomas (MTNKs), and Hodgkin lymphomas (HLs).
Semi-quantitative assessment of the level of BM involvement was made based on the percentage of lymphoma cells to the hematopoietic cells in BM: obscure (≤10%), obvious (10%-50%), and prominent (>50%).
According to previous studies [6,7], patterns of involvement were classified as diffuse, nodular, focal, paratrabecular, interstitial and mixed. Each patterns were defined as follows: (1) diffuse was regarded as most parts (>80%) of the observed area (more than 2 mm in diameters) in BM showing effacement owing to a dense infiltration of neoplastic lymphocytes; (2) nodular was defined as well circumscribed aggregates of lymphoid cells with relatively sharp line in the BM, usually with a round to oval shape; (3) focal referred to some irregular aggregates of lymphoma cells showing a lower volume percentage (<80%) than those characterized as a diffuse pattern; (4) paratrabecular was defined as lymphoid aggregates with broad bases aligned alongside bony trabeculae without intervening fat cells; (5) interstitial referred to lymphoma cells infiltrating between fat cells without distorting the normal architecture of hematopoietic cells and fat. This pattern also included cases with an intrasinusoidal pattern of infiltration, which is thought to be a specific pattern by some experts, yet hard to distinguish from the interstitial pattern and was proven to be non-specific by Arber [6]; (6) A mixed pattern referred to coexistence of two or more of the patterns described above, and each of the patterns represented 10% or more of the tumor cells involved.
Finally, it was possible to evaluate 360 paired cases with BMT and BMA tests performed at the same time, and 365 paired cases including the above 360 cases were compared using data from BMT and FCM tests. Paired fourfold table Chi-squared test was performed to analyze their difference.
Results
Occurrence of bone marrow involvement and frequencies in involved cases for each type of lymphoma
The 950 patients mostly came from Northern China. 153 of 950 (16.1%) specimens were found to be involved by lymphoma. For all of the 153 cases, the patients aged from 14-82 years (median age 55 years, and mean age 52.1 years), including 91 males and 62 females (with a male: Female ratio of 1.5:1).
The occurrence rate of BM involvement was 36.0% (18/50) for LBLs, 44.8% (90/201) for SBLs, 6.7% (28/416) for LMBLs, 8.40% (11/131) for MTNKs, and 4.4% (6/138) for HLs. None of the 14 cutaneous or subcutaneous mature T-cell lymphomas involved BM (0/14).
The frequency of involved cases in different groups were 58.4% (SBLs), 18.2% (LMBLs), 11.7% (LBLs), 7.1% (MTNKs), and 3.9% (HLs), with MCL ranking the first (18.2%) among all lymphoma types. Details for exact occurrence rates of BM involvement and frequency in involved cases for each lymphoma type were presented in Table 1.
Table 1.
Occurrence and Percentage of involvement for each type of lymphoma studied
| Main Groups | Lymphoma type | % occurrence (Involved/all) | Frequency in Involved cases | |
|---|---|---|---|---|
| lymphoblastic lymphoma | Subtotal | 36.0% (18/50) | 11.8% | |
| B-cell lymphoblastic lymphoma | 42.9% (3/7) | 2.0% | ||
| T-cell lymphoblastic lymphoma | 34.9% (15/43) | 9.8% | ||
| LMBL | Subtotal | 6.7% (28/416) | 18.3% | |
| diffuse Large B cell lymphoma -NOS | 6.4% (22/346) | 14.4% | ||
| primary mediastinal B cell lymphoma | 0.0% (0/8) | 0.0% | ||
| Burkitt lymphoma | 0.0% (0/7) | 0.0% | ||
| Plasma blastic lymphoma | 0.0% (0/2) | 0.0% | ||
| follicular lymphoma grade3 | 25.0% (4/16) | 2.6% | ||
| BCL-U | DLBCL-BL | 10.5% (2/19) | 1.3% | |
| DLBCL-CHL | 0.0% (0/1) | 0.0% | ||
| SBL with DLBCL transformation | FL-DLBCL | 0.0% (0/1) | 0.0% | |
| MALT-DLBCL | 0.0% (0/16) | 0.0% | ||
| SBL | Subtotal | 44.8% (90/201) | 58.8% | |
| follicular lymphoma grade1 | 41.7% (10/24) | 6.5% | ||
| follicular lymphoma grade2 | 46.2% (12/26) | 7.8% | ||
| marginal zone cell lymphoma -Extra nodal | 9.1% (4/44) | 2.6% | ||
| marginal zone cell lymphoma -Nodal | 58.8% (10/17) | 6.5% | ||
| marginal zone cell lymphoma -spleen | 100.0% (3/3) | 2.0% | ||
| mantle cell lymphoma | 49.1% (28/57) | 18.3% | ||
| SLL/CLL | 78.6% (22/28) | 14.4% | ||
| Plasmacytoma | 0.00% (0/1) | 0.0% | ||
| Lymphoplasmacytic lymphoma | 100.0% (1/1) | 0.7% | ||
| Mature T and NK cell lymphomas | Subtotal | 8.4% (11/131) | 7.2% | |
| NK/T cell lymphoma | 8.5% (5/59) | 3.3% | ||
| angioimmunoblastic T-cell lymphoma | 10.3% (3/29) | 2.0% | ||
| anaplastic large cell lymphoma -ALK(+) | 6.3% (1/16) | 0.7% | ||
| anaplastic large cell lymphoma -ALK(-) | 0.00% (0/13) | 0.0% | ||
| peripheral T-cell lymphoma -NOS | 14.3% (2/14) | 1.3% | ||
| HL | Subtotal | 4.4% (6/138) | 3.9% | |
| CHL | 3.7% (5/136) | 3.3% | ||
| NLPHL | 50.0% (1/2) | 0.7% | ||
| Cutaneous and subcutaneous PTCL | 0.00% (0/14) | 0.0% | ||
| Total Number | 16.1% (153/950) | 100% | ||
Abbreviations: BCL-U, B cell lymphoma, Unclassifiable; DLBCL-BL with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; CHL, classical Hodgkin lymphoma; DLBCL-CHL with features intermediate between diffuse large B cell lymphoma and classical Hodgkin lymphoma; DLBCL, diffuse Large B cell lymphoma; FL, follicular lymphoma; LMBL: large and medium sized B-cell lymphomasSBL, small B cell lymphoma group; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia; Subtotal, subgroup total number.
Volume percentage of BM involved by lymphomas
The volume percentage of BM involvement ranged from 1% to 95% with a mean percentage of 40.1% and a median percentage of .0%. Volume percentages were calculated separately according to different lymphoma types and were assessed further by semi-quantitative analysis. Full details see Table 2.
Table 2.
Volume percentage of involved lymphoma ingredients in bone marrows
| Main classification | Specific Lymphoma type | Grade of volume percentage | ||
|---|---|---|---|---|
|
| ||||
| Obscure (Volume≤10%) | Obvious (Volume 10-50%) | Prominent (Volume≥50%) | ||
| LBL | LBL-B | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| LBL-T | 8 (53.3%) | 2 (13.3%) | 5 (33.3%) | |
| LMBL | DLBCL-NOS | 9 (40.9%) | 9 (40.9%) | 4 (18.2%) |
| DLBCL-BL | 0 (0.00%) | 0 (0.00%) | 2 (100.0%) | |
| FL3 | 1 (33.3%) | 2 (66.7%) | 0 (0.0%) | |
| SBL | FL1 | 2 (20.0%) | 7 (70.0%) | 1 (10.0%) |
| FL2 | 3 (23.1%) | 5 (38.5%) | 5 (38.5%) | |
| MCL | 4 (14.3%) | 10 (35.7%) | 14 (50.0%) | |
| MZL-EN | 0 (0.0%) | 4 (100.0%) | 0 (0.0%) | |
| MZL-N | 2 (20.0%) | 5 (50.0%) | 3 (30.0%) | |
| MZL-S | 0 (0.0%) | 1 (33.3%) | 2 (66.7%) | |
| SLL/CLL | 3 (13.6%) | 9 (40.9%) | 10 (45.5%) | |
| LPL | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | |
| Mature T and NK cell lymphomas | NK/T | 2 (40.0%) | 3 (60.0%) | 0 (0.0%) |
| PTCL-NOS | 0 (0.0%) | 1 (50.0%) | 1 (50.0%) | |
| AITL | 0 (0.0%) | 3 (100.0%) | 0 (0.0%) | |
| ALCL-ALK (+) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) | |
| HL | NLPHL | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| CHL | 1 (20.0%) | 2 (40.0%) | 2 (40.0%) | |
| TOTAL (%) | 36 (23.53%) | 65 (42.5%) | 52 (3.40%) | |
Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; DLBCL-BL, B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; CHL, classical Hodgkin lymphoma; DLBCL, Diffuse Large B cell lymphoma; FL, Follicular lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; LMBL, Large and medium sized B cell lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone cell lymphoma; PTCL, peripheral T-cell lymphoma; SBL, small B cell lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia.
Patterns of BM involvement in different lymphoma types
82 lymphoma cases infiltrating BM were found to have a single primary pattern and the other 71 were in a mixed pattern. MCL (5 single primary & 4 mixed), T-LBL (4 single primary & 2 mixed), and SLL/CLL (4 single primary) were the three types of lymphoma that were most likely to involve in a diffuse pattern. Nodular pattern was seldom seen as single primary, and usually accompanied with other patterns where FL (7 cases) and MCL (4 cases) were found the most. Focal pattern was often seen in DLBCL (8 single primary and 8 mixed). Para trabecular localization was the most prevalent pattern in FL (6 single primary and 14 mixed), especially in low-grade cases (n=5). A single primary interstitial pattern was found mostly in MCL (n=5) and T-cell LBL (n=6). The other 71 cases of bone involvement presented a mixed pattern. The classical distribution patterns are shown in Figure 1A-G. Further details of lymphoma types and their infiltrating patterns in BM were showed in Table 3.
Figure 1.
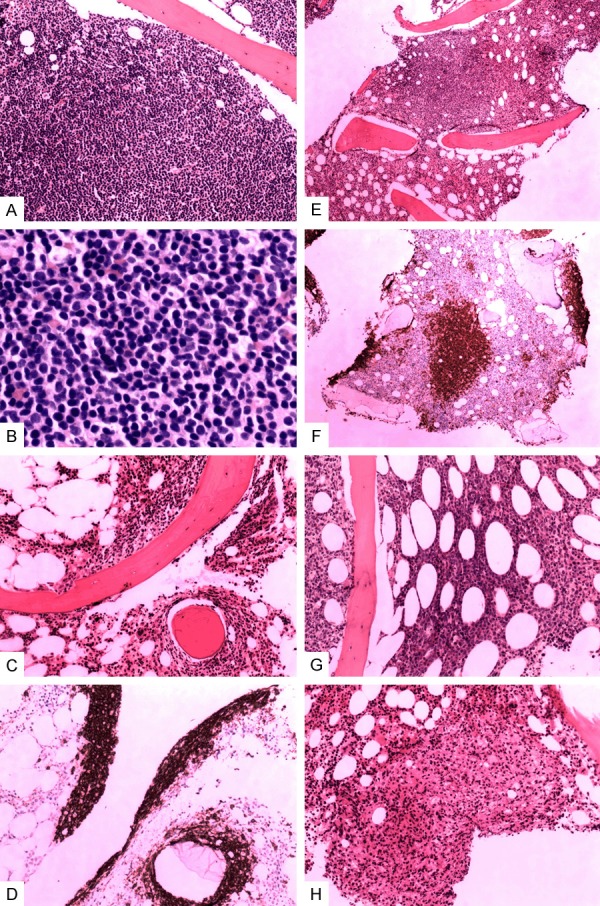
Patterns of lymphoma involvement in bone marrow. Notes: Tumor cells in diffuse pattern were shown in an example of chronic lymphocytic leukemia/small lymphocytic lymphoma in (A) without residual primary marrow structures, a view of infiltrating tumor cells in higher power field could be seen in (B). Paratrabecular pattern showed aggregates of lymphocytes adjacent to bony trabeculae, as shown from a case with follicular lymphoma of grade 1 in (C), this pattern was highlighted by immunohistochemistry (IHC) staining of CD20 antigen in (D). Aggregates of lymphoma with well defined border are shown in (E) from a case of marginal zone lymphoma and was called “nodular pattern”, this was also highlighted by IHC for CD20 in (F). Interstitial disease infiltrated between fat cells, as seen in a diffuse large B cell lymphoma in (G). Focal pattern was seen in a peripheral T cell lymphoma, it was characterized for clusters of irregularly outlined tumor cells seen in (H).
Table 3.
Distribution patterns in involved Bone marrows
| Lymphoma type | Primary Pattern | Mixed Pattern subtypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Diffuse | Nodular | Focal | ParaT | Interstitial | Mixed (Total number) | Mixed Diffuse | Mixed Nodular | Mixed Focal | Mixed ParaT | Mixed Interstitial | |
| LBL | |||||||||||
| LBL-B | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| LBL-T | 4 | 0 | 1 | 0 | 6 | 4 | 2 | 0 | 2 | 1 | 3 |
| LMBL | |||||||||||
| DLBCL-NOS | 1 | 1 | 8 | 1 | 1 | 10 | 0 | 2 | 8 | 1 | 10 |
| FL3 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 2 |
| BCL-U, DLBCL-BL | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| SBL | |||||||||||
| FL, grade1 | 0 | 1 | 0 | 3 | 0 | 6 | 0 | 3 | 1 | 6 | 2 |
| FL, grade2 | 1 | 0 | 1 | 2 | 0 | 9 | 0 | 4 | 3 | 7 | 5 |
| MCL | 5 | 2 | 3 | 1 | 5 | 12 | 4 | 4 | 7 | 1 | 9 |
| MZL-EN | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 2 |
| MZL-N | 2 | 2 | 0 | 0 | 0 | 6 | 0 | 2 | 4 | 3 | 6 |
| MZL-S | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 2 | 1 | 2 |
| SLL/CLL | 4 | 4 | 4 | 1 | 0 | 9 | 0 | 3 | 7 | 1 | 8 |
| LPL | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mature T and NK cell lymphomas | |||||||||||
| NK/T | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 2 |
| AITL | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 2 |
| ALCL-ALK (+) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PTCL-NOS | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 2 |
| HL | |||||||||||
| NLPHL | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHL | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 21 | 12 | 24 | 9 | 16 | 71 | 6 | 20 | 43 | 23 | 56 |
Notes: Those cases with a single pattern were listed by number of cases, all subtypes of the mixed pattern are included so the total exceeds the actually number of cases. Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, Anaplastic large cell lymphoma; BCL-U, DLBCL-BL, B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; CHL, classical Hodgkin lymphoma, DLBCL, Diffuse Large B cell lymphoma; FL, Follicular lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; LMBL, Large and medium sized B cell lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone cell lymphoma; Para T, Para trabecular; PTCL, peripheral T-cell lymphoma; SBL, small B cell lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia.
The involvement of BM in HLs was always accompanied by background cells (Figure 2A, 2B); with the involvement pattern of diffuse in 3 cases, focal in 2 cases, and nodular in 1 case. We noted that all MTNKs affected the BM in either focal or interstitial patterns (either single or mixed).
Figure 2.
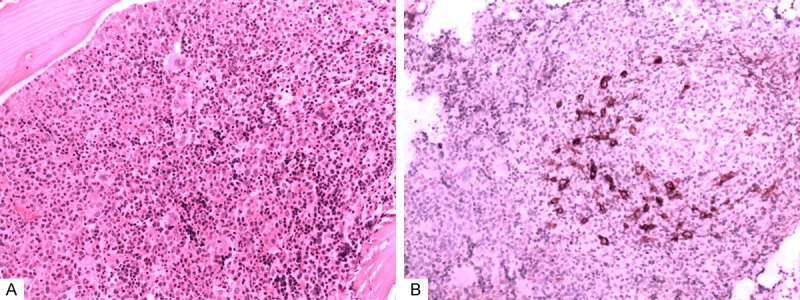
The involving ingredients of a Hodgkin Lymphoma. Notes: The tumor cells of HL always accompanied by mixed background cells as was shown in Figure 2A, and the disseminated tumor cells were outlined by immunohistochemical staining of CD30 (Figure 2B).
BM background changes
The background changes of 115 involved BM included 5 cases of granulomas, 23 cases of fibrosis, and 16 cases of necrosis. BM granulomas were most commonly seen in 3 cases of HLs, 1 case of DLBCL, and 1 case of FL (grade 2). Obvious fibrosis was seen in 5 cases of HLs, 4 cases of LMBLs (3 cases of DLBCL and 1 case of DLBCL-BL), 9 cases of SBLs (3 cases of low grade FL, 3 cases of SLL/CLL, 2 cases of MCL, 1 case of MZL), 5 cases of MTNKs, including 3 cases of NK/T cell lymphoma(NK/T), 1 case of angioimmunoblastic T-cell lymphoma, and 1 case of ALK-positive anaplastic large cell lymphoma, and none in LBLs. BM necrosis was most commonly seen in more invasive PTCLs (2 cases of NK/T, 2 cases of PTCL-NOS, and 1 case of ALK-positive anaplastic large cell lymphoma) and 3 cases of DLBCL, but it was also seen in SBLs (3 cases of SLL/CLL, 1 case of nodular MZL, 1 case of low-grade FL) and 1 case of T-LBL.
Compared with other groups, HLs showed the most variable background changes (2 cases of granulomas, 4 cases of fibrosis, and 2 cases of necrosis). And most MTNKs had fibrosis and necrosis (5 cases of fibrosis and 5 cases of necrosis). Full details were presented in Table 4.
Table 4.
Secondary changes in involved Bone marrows
| Type | Granulomas (%) | Fibrosis (%) | Necrosis (%) |
|---|---|---|---|
| LBL-T | 0 (0.00%) | 0 (0.00%) | 1 (6.30%) |
| DLBCL-NOS | 1 (20.00%) | 3 (13.00%) | 2 (12.50%) |
| BCL-U, DLBCL-BL | 0 (0.00%) | 1 (4.30%) | 0 (0.00%) |
| FL1 | 0 (0.00%) | 1 (4.30%) | 0 (0.00%) |
| FL2 | 1 (20.00%) | 2 (8.70%) | 1 (6.30%) |
| MCL | 0 (0.00%) | 2 (8.70%) | 0 (0.00%) |
| MZL-EN | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| MZL-N | 0 (0.00%) | 1 (4.30%) | 1 (6.30%) |
| MZL-S | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| SLL/CLL | 0 (0.00%) | 3 (13.00%) | 3 (18.90%) |
| NK/T | 0 (0.00%) | 3 (13.00%) | 2 (12.50%) |
| AITL | 0 (0.00%) | 1 (4.30%) | 0 (0.00%) |
| ALCL-ALK (+) | 0 (0.00%) | 1 (4.30%) | 1 (6.30%) |
| PTCL-NOS | 0 (0.00%) | 0 (0.00%) | 2 (12.50%) |
| NLPHL | 1 (20.00%) | 1 (4.30%) | 0 (0.00%) |
| CHL | 2 (40.00%) | 4 (17.40%) | 2 (12.50%) |
| Total | 5 | 23 | 16 |
Abbreviations: AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; BCL-U, DLBLC-BL, B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; CHL, classical Hodgkin lymphoma, DLBCL, Diffuse Large B cell lymphoma; FL, Follicular lymphoma; HL, Hodgkin lymphoma; LBL, lymphoblastic lymphoma; LMBL, Large and medium sized B cell lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone cell lymphoma; PTCL, peripheral T cell lymphoma; SBL, small B cell lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia.
Bone marrow aspirates and flow cytometry concordance
Of the 359 cases with both BMT and BMA data, 74 (20.6%) cases were positive by BMT and 33 cases (9.2%) were positive by BMA ,BMA and BMT agreed in 84.12% of cases (302 of 359: 277 both negative, 25 both positive). Statistically significant differences (P<0.01) could be seen between the results of BMT and BMA.
Among all 364 cases where the BMT and FCM tests were performed at the same time, 75 (20.6%) cases of BM involvement were discovered by BMT, and 99 cases (27.2%) by FCM. FCM and BMT agreed in 80.8% of cases (294 of 364: 242 both negative, 52 both positive). FCM was found to be more sensitive. Significant differences (P<0.01) existed when compared with BMT.
Details for the three detection methods of BM involvement for different lymphoma types were presented in Table 5. Main groups except HLs (HLs was not evaluated as we only had data on 1 case of HL) were analyzed, and detail data associated with concordance rate of each group presented in Table 6.
Table 5.
Comparison of positive findings between BMT and BMA or FCM
| Lymphoma types | BMT | FCM | BMA |
|---|---|---|---|
|
| |||
| Occurrence (Involved/Assessed) | Occurrence (Involved/Assessed) | Occurrence (Involved/Assessed) | |
| LBL | 38.10% (8/21) | 61.90% (13/21) | 38.10% (8/21) |
| LMBL | 8.70% (16/183) | 19.70% (36/183) | 3.90% (7/181) |
| SBL | 50.00% (44/88) | 52.30% (46/88) | 18.40%(16/87) |
| PTCL | 9.90% (7/71) | 5.60% (4/71) | 2.90% (2/69) |
| HL | 0.00% (0/1) | 0.00% (0/1) | 0.00% (0/1) |
| Total | 20.55% (74/364) | 27.10% (99/364) | 9.20% (33/359) |
Abbreviations: HL, Hodgkin lymphoma group; LBL, lymphoblastic lymphoma group; LMBL, Large and medium B cell lymphoma group; PTCL, peripheral T-cell lymphoma group; SBL, small B cell lymphoma group.
Table 6.
Comparison of detection results between BMT&BMA or BMT&FCM in different groups of lymphoma for main statistical indexes
| BMT VS. BMA | BMT VS. FCM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| NO | PPA | NPA | TCR | SD (P value) | NO | PPA | NPA | TCR | SD (P value) | |
| SBL | 88 | 30.23 | 58.33 | 62.50 | 0.000 | 89 | 79.55 | 75.56 | 77.53 | 0.824 |
| LBL | 21 | 62.50 | 76.92 | 71.43 | 1.000 | 21 | 100.00 | 61.54 | 76.19 | 0.063 |
| LMBL | 181 | 31.25 | 98.79 | 92.88 | 0.022 | 183 | 50.00 | 83.23 | 80.32 | 0.001 |
| PTCL | 63 | 28.57 | 100.00 | 92.06 | 0.063 | 65 | 14.29 | 94.83 | 86.15 | 0.000 |
| Total | 360 | 33.78 | 97.20 | 84.17 | 0.000 | 365 | 69.33 | 83.79 | 80.82 | 0.006 |
Abbreviations: BMA, bone marrow cell aspirate; BMT, bone marrow trephine biopsy; FCM, flow cytometry; HL, Hodgkin lymphoma group; LBL, lymphoblastic lymphoma group; LMBL, Large and medium sized B cell lymphoma group; NPA, negative percent agreement; PPA, positive percent agreement; PTCL, peripheral T cell lymphoma; SD, significant difference; SBL, small B cell lymphoma group; TCR, total concordance rate.
Discussion
Several conclusions can be drawn from our analysis of relatively large data sets on BM involvement of lymphoma in Northern China. In general, BM involvement can be found in all age groups and there were no obvious gender differences.
Although for some types of lymphoma it was difficult to evaluate the accuracy of the involvement rate from a relatively limited number of cases, we can still learn from those types with higher number of cases (defined as more than 20 cases to assure accuracy), of which SLL/CLL ranked the first in the rate of BM involvement (79%). It was in agreement with previous studies [6,7] and in accordance with its nomenclature as either lymphoma or leukemia.
The total occurrence calculated from our BMT specimens was only about 16%, which was much lower than the 40% incidence rate of previous reports from the series of western countries [2]. This difference might be resulted from different spectrums of lymphoma type between China and west countries. Groups which are more inclined to involve BM including SBLs and LBLs (the BM involvement occurrence was about 45% and 36%) yet accounted for less than 20% and only 5% of all 950 cases. Moreover, these 3 groups had lower involvement occurrence like LMBLs (less than 7.0%), MTNKs (only 8.0%), HLs (only 4.3%), they each contributed about 40%, 15.0% and 14.3% of all biopsies in our studies.
What is more, none of the cutaneous or subcutaneous mature T-cell lymphomas involved BM, and cases of indolent B-cell lymphoma (FL and mucosa associated lymphoid tissue lymphoma) with large and invasive B-cell lymphoma transformation were identified, but none were found to involve BM. This might be related to loss of homeostatic chemokine (like CXCR4) in DLBCL [8-10]. The BM involvement rate of HL was low but equivalent to what reported previously from a larger series (5.2%) [11]. The data above collectively indicate a low level of BM involvement by lymphoma in our lymphoma series.
MTNKs were less well-documented owing to its low incidence [6,7], take NK/T lymphoma for example, it was the most common MTNKs in China [3,4], in our series occurrence of NK/T type involving BM was similar to papers from Korea and Hong Kong, which reported that BM involvement in less than 10% of patients with T/NK cell lymphoma at initial diagnosis [12,13], The precursors of T/NK-cell maturated in the thymus [14]; unlike SBLs, they do not express homing signals, and their clinical invasive behavior causes a short interval from onset to being diagnosed, which might be another important reason for their low occurrence rate in BM infiltration. However, Tong’s [5]report from Zhejiang Province was relatively high (23% for NK/T, and 40% for all MTNKs), more comprehensive analysis on BMT of MTNK cases is needed to get more accurate conclusion about its total involvement rate in China.
As expected, in involved biopsies, the frequency of SBLs was higher than the more invasive LMBLs (more than three times). This observation was in accordance with the western series in which the incidences of SBLs and LMBLs were 72.1%-72.5% and 12.5%-18.2%, respectively [6,7]. The frequency was much higher than Arber’s study for LBLs (0.7%) but similar for MTNKs (6.7%) [6]. The HLs frequency was also similar to what reported by Sovani et al. (5.7%) [7]. An overview of these frequencies will help us to predict the outcome of BMT for any special lymphoma subtype.
It was suggested that both involved volume percentage and distribution pattern could help us in future pathological diagnosis and evaluations. The volume percentage of lymphoma cell involved in BM was variable. SBLs group contained largest number of cases with prominent and obvious infiltrations. The volume percentage of MTNKs and HLs were usually obvious or prominent, such made it easy to be discovered.
The most common types with minimal infiltration were DLBCL and T-LBL. In such circumstances, further Immunohistochemistry staining was essential.
Although most lymphoma types can involve in any pattern without absolute prevalence, preference on certain specific form of distribution were still shown in some lymphoma types. MCL, T-LBL, and SLL/CLL were found most likely to involve diffusely, and DLBCL were seen preference on focal pattern which was quite different from reports of Arber and Sovani (they found diffuse pattern most) [6,7]. “Focal pattern” was not defined as a separate pattern previously, and can be seen in 2/3 of all DLBCL cases as a single pattern and 4/5 of the remained DLBCL cases with a mixed pattern. It could be predicted that this focal pattern called by us used to be classified as “diffuse pattern” in previous studies [6,7]. Para trabecular was the most prevalent pattern in low-grade FL, occurring in nearly 4/5 of cases (including either single or mixed patterns). The same phenomenon was observed previously (about 76%-90%) [6,7]. The nodular pattern was almost only found in SBLs and interstitial pattern was found in nearly all kinds of non-Hodgkin lymphomas, but they were seldom found as a single primary pattern. The involvement of HL cells was accompanied by “background” cells and were recorded as single primary patterns (diffuse, focal, or nodular) without exception. Similar observations were seen in other studies [15,16].
Most MTNKs were found to infiltrate the BM in a focal pattern, either with single primary or mixed patterns; this was inconsistent with their invasive behaviors, yet similar phenomenon was reported previously [1].
Secondary (background) changes were seen in most types of lymphoma involved cases, Obvious fibrosis could be seen without significant prevalence of any types, and it could remind us of lymphoma involvement in BM. Necrosis was most commonly seen in invasive lymphoma types such as MTNKs and DLBCLs, which accorded with their primary tumor morphologies and previous observations [6,7]. As BM granulomas were most commonly seen in HLs, it might be considered as a histological indication for HL involvement in BMT findings. However, it was not an absolutely specific morphological feature [17].
BMA might be the least sensitive method for detection owing to the reticulum interfering with successful aspiration and aspiration contamination by blood [18]. Our PPA for BMA was much lower than previously assessed in all lymphomas (65-71%) [7,19]. In most parts of China, BMA were done in other clinical labs rather than pathology department, this might be the reason. When it was sorted by groups, significant differences were found only in SBLs and LMBLs.
It appeared that FCM was more sensitive than BMT, so it was important to perform BMT and FCM simultaneously. The total concordance rate between BMT and FCM was almost the same as reported data (88.7%) [20]. When analyzed by groups, significant differences were found in LMBLs and MTNKs, However, further studies are still needed for confirmation.
The total BM involvement rate of lymphoma of the Northern China is relatively low (16.2%). SLL/CLL type was most likely to involve the BM, The occurrence of BM involvement by MTNKs was relatively low.
Among the BM-involved cases, MCL ranked the first in frequency. Volume percentage, distribution patterns and background changes were useful pointers towards a particular lymphoma subtype.
Positive correlation between aspiration and biopsy was low, and FCM appeared to be more sensitive, therefore, neither FCM nor BMA could take the part of BMT for BM evaluation of lymphoma especially in China.
Disclosure of conflict of interest
None.
References
- 1.Zhang QY, Foucar K. Bone marrow involvement by Hodgkin and non-Hodgkin lymphomas. Hematol Oncol Clin North Am. 2009;23:873–902. doi: 10.1016/j.hoc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Lambertenghi-Deliliers G, Annaloro C, Soligo D, Oriani A, Pozzoli E, Quirici N, Luksch R, Polli EE. Incidence and histological features of bone marrow involvement in malignant lymphomas. Ann Hematol. 1992;65:61–65. doi: 10.1007/BF01698130. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, Sun L, Wei L, Li M, Liu C, Zheng J, Liu W, Li G, Chen J. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138:429–434. doi: 10.1309/AJCP7YLTQPUSDQ5C. [DOI] [PubMed] [Google Scholar]
- 4.Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, Bi CF, Zuo Z, Wang XQ, Huang J, Dai L, Liu WP. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77. doi: 10.1186/1746-1596-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong H, Ren Y, Qian W, Xiao F, Mai W, Meng H, Jin J. Clinicopathological study on peripheral T-cell non-Hodgkin lymphoma with bone marrow involvement: a retrospective analysis from China. Int J Hematol. 2009;90:303–310. doi: 10.1007/s12185-009-0390-6. [DOI] [PubMed] [Google Scholar]
- 6.Arber DA, George TI. Bone marrow biopsy involvement by non-Hodgkin’s lymphoma: frequency of lymphoma types, patterns, blood involvement, and discordance with other sites in 450 specimens. Am J Surg Pathol. 2005;29:1549–1557. doi: 10.1097/01.pas.0000182405.65041.8b. [DOI] [PubMed] [Google Scholar]
- 7.Sovani V, Harvey C, Haynes AP, McMillan AK, Clark DM, O’Connor SR. Bone marrow trephine biopsy involvement by lymphoma: review of histopathological features in 511 specimens and correlation with diagnostic biopsy, aspirate and peripheral blood findings. J Clin Pathol. 2014;67:389–395. doi: 10.1136/jclinpath-2013-201520. [DOI] [PubMed] [Google Scholar]
- 8.Robertson LE, Redman JR, Butler JJ, Osborne BM, Velasquez WS, McLaughlin P, Swan F, Rodriguez MA, Hagemeister FB, Fuller LM. Discordant bone marrow involvement in diffuse large-cell lymphoma: a distinct clinical-pathologic entity associated with a continuous risk of relapse. J. Clin. Oncol. 1991;9:236–242. doi: 10.1200/JCO.1991.9.2.236. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham-Schmid C, Schaider H, Neumeister P. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol. 2013;26:182–194. doi: 10.1038/modpathol.2012.134. [DOI] [PubMed] [Google Scholar]
- 10.Trentin L, Cabrelle A, Facco M, Carollo D, Miorin M, Tosoni A, Pizzo P, Binotto G, Nicolardi L, Zambello R, Adami F, Agostini C, Semenzato G. Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood. 2004;6:502–508. doi: 10.1182/blood-2003-09-3103. [DOI] [PubMed] [Google Scholar]
- 11.Howell SJ, Grey M, Chang J, Morgenstern GR, Cowan RA, Deakin DP, Radford JA. The value of bone marrow examination in the staging of Hodgkin’s lymphoma: a review of 955 cases seen in a regional cancer centre. Br J Haematol. 2002;119:408–411. doi: 10.1046/j.1365-2141.2002.03842.x. [DOI] [PubMed] [Google Scholar]
- 12.Sung CO, Ko YH. Bone marrow is involved in <10% of patients with nasal-type NK/T cell lymphoma at initial diagnosis. J Korean Med Sci. 2004;19:229–233. doi: 10.3346/jkms.2004.19.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong KF, Chan JK, Cheung MM, So JC. Bone marrow involvement by nasal NK cell lymphoma at diagnosis is uncommon. Am J Clin Pathol. 2001;115:266–270. doi: 10.1309/E5PR-6A9R-Q02N-8QVW. [DOI] [PubMed] [Google Scholar]
- 14.Hale JS, Fink PJ. Back to the thymus: peripheral T cells come home. Immunol Cell Biol. 2009;87:58–64. doi: 10.1038/icb.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele J, Zirbes TK, Kvasnicka HM, Fischer R. Focal lymphoid aggregates(nodules) in bone marrow biopsies: differentiation between benign hyperplasia and malignant lymphoma--a practical guideline. J Clin Pathol. 1999;52:294–300. doi: 10.1136/jcp.52.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco V, Tripodo C, Rizzo A, Stella M, Florena AM. Bone marrow biopsy in Hodgkin’s lymphoma. Eur J Haematol. 2004;73:149–155. doi: 10.1111/j.1600-0609.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Colan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin’s lymphoma: the clinical significance of morphologic discordance between lymph node and bone marrow nebraska lymphoma study group. J. Clin. Oncol. 1990;8:1163–1172. doi: 10.1200/JCO.1990.8.7.1163. [DOI] [PubMed] [Google Scholar]
- 18.Sah SP, Matutes E, Wotherspoon AC, Morilla R, Catovsky D. A comparison of flow cytometry, bone marrow biopsy, and bone marrow aspirates in the detection of lymphoid infiltration in B cell disorders. J Clin Pathol. 2003;56:129–132. doi: 10.1136/jcp.56.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Bolkainy TMN, Abo Deif WS, Gouda HM, Mokhtar NM. Evaluation of bone marrow in 143 lymphomas: the relative frequency and pattern of involvement, secondary myelopathies, pitfalls and diagnostic validity. J Egypt Natl Canc Inst. 2008;20:17–30. [PubMed] [Google Scholar]
- 20.Mazur G, Halon A, Wrobel T, Jelen M, Kuliczkowski K. Contribution of flow cytometric immunophenotyping and bone marrow trephine biopsy in the detection of lymphoid bone marrow infiltration in non-Hodgkin’s lymphomas. Neoplasma. 2014;51:159–163. [PubMed] [Google Scholar]


