Abstract
Estrogen and estrogen receptor (ER)-α and -β play a role in the development and progression of thyroid cancer. ERβ2 is one major splicing variant of ERβ. In this study, we investigated the clinical significance of ERβ2 protein expression in the papillary thyroid carcinoma (PTC) lesion. ERβ2 expression was immunohisto-chemically examined in formalin-fixed, paraffin-embedded thyroid tissues from 106 patients with PTC by Elivision™ plus two-step system as previously described. The relationships between ERβ2 expression and clinicopathological/biological factors were then analyzed. ERβ2 protein was expressed in all the PTC patients studied. It was positively associated with Ki-67 expression in female PTC patients with advanced reproductive age (>45 years, in low-estrogen status) and with VEGF expression in male PTC patients with reproductive age (18~45 years, in low-estrogen status) (P=0.005 and P=0.044, respectively). There was no association between ERβ2 expression and tumor size, extrathyroidal extension and tumor-node-metastasis stage in PTC patients. In addition, ERβ2 expression was lower in female patients of reproductive age (18~45 years, in relatively high-estrogen status) with lymph node metastasis than that in those patients without lymph node metastasis (P=0.035). The present results suggest that the expression of ERβ2 in PTC is associated with the progression of the disease. Its potential effect may vary with different estrogen status. Further study will assess the underlying molecular mechanisms of ERβ2 in PTC.
Keywords: Estrogen receptor, ERβ2, thyroid cancer, immunohistochemistry
Introduction
Thyroid cancer is the most common endocrine neoplasm, accounting for an estimated 62,450 new cases and 1,950 deaths in 2015 in the United States [1]. In recent years, the incidence of thyroid cancer has increased. Radiation exposure is a risk factor for thyroid cancer, while female sex hormones may also play a role in thyroid carcinogenesis because there is a strong female predominance in thyroid cancer. Histopathologically, thyroid cancer can be classified into several types, such as papillary, follicular, medullary and anaplastic/undifferentiated thyroid cancers. Among these types, papillary thyroid cancer (PTC) is the most common type of thyroid cancer, accounting for 80% of all thyroid cancers and often occurring in young females with excellent prognosis. Like most other cancers, the molecular pathogenesis of PTC remains to defined, although accumulated evidence indicates an important role of estrogen and estrogen receptor (ER) in the pathogenesis of PTC [2,3]. Thus, we need to better understand the etiology and pathogenesis of PTC in order to effectively control PTC in the clinic.
Towards this end, our research has primarily focused on the risk factors and PTC carcinogenesis, namely, estrogen and ER. Estrogen binds to ERs, which are in turn activated and bind to DNA and regulate expression of many different target genes. In vitro studies have indicated that estrogen may have direct actions in PTC cells via ER-dependent mechanisms to modulate tumor cell proliferation, migration and invasion [4-7]. Besides that, estrogens are also able to exert non-genomic events mediated by a novel transmembrane ER G protein-coupled receptor 30 (GPR30) [8]. ERs consist of two subtypes, ERα and ERβ, which show significant overall sequence homology. Both ERs are widely expressed in different types of tissues and cells with notable expression patterns. For example, ERα is predominantly expressed in female sex organs such as the breast, uterus and ovaries, especially during the reproductive years, while ERβ is widely expressed in many other tissues in both genders, but to a lesser degree in males than in females [9]. In this study, we mainly focused on ERβ, which includes five full-length subtypes (ERβ1-ERβ5) as a result of alternative splicing of the last coding exon. ERβ1 (the wild-type ERβ) is the only fully functional isoform [10]. ERβ2 (also known as ERβcx) does not form homodimers and has no innate activities by its own, but it has been reported to antagonize wild-type ERα and to enhance ERβ1 transcriptional activity through heterodimerization [10,11]. In our recent study, we found that ERβ1 and ERβ2 had differential expression patterns between PTC and nodular thyroid goiter [12]. Thus far, the clinical significance of ERβ2 expression has been widely studied in breast, prostate and colorectal cancers [13-15]. The data showed that ERβ2 expression was associated with the development and progression of these cancers. Specifically, ERβ2 protein significantly increased in ductal carcinoma in situ (DCIS) and invasive breast cancer compared to the adjacent normal mammary glands, suggesting a role of ERβ2 in breast carcinogenesis [13]. In contrast, colorectal cancer samples lacked expression of ERβ1 and ERβ2 proteins, indicating potentially protective effects of both ERβ1 and ERβ2 in the colorectal carcinogenesis [14]. ERβ2 expression was also decreased in endometrioid carcinoma, especially higher grade tumors as compared to proliferative endometrium [15]. All the evidence suggests ERβ2 may have dual functions in human carcinogenesis. It is still unclear if the dual functions are cancer type specific or influenced by some relative factors.
Of the biological markers, the expression of Ki-67, mutant p53 and VEGF has been the most actively investigated. These factors have well established actions in proliferation, malignant transformation and angiogenesis in estrogen-related tumor [16-19]. Ki-67 has recently emerged as an intermediate marker of long-term outcome in breast cancer [16]. The dominant oncogenic properties of mutant p53 have been recognized as its growth-promoting effects associated with tumor progression [17]. The interactions between mutant P53 and ERα/β have been suggested to play a potential role in mammary tissue homeostasis and cancer formation [18]. VEGF is a key regulator of developmental, physiological and pathological neovascularization, which is especially involved in tumor growth, aggressiveness and metastasis. It has been reported that estrogen could stimulate follicular thyroid cancer cell line (ML-1) to secret more VEGF likely as a result of ER signalling [19]. However, the associations between ERβ2 and these biological markers have not been reported in PTC.
Thus, the present study was undertaken to examine the expression of ERβ2 in PTC and to explore the association between ERβ2 expression and these clinicopathological/biological factors.
Materials and methods
Patients and tissue specimens
Thyroid tissue specimens were obtained from 106 Chinese PTC patients, consisting of 50 females of reproductive age (18~45 years old), 39 of advanced reproductive age (>45 years old), and 17 males of reproductive age (18~45 years old). All of these patients were admitted to our hospital for a standard thyroidectomy within 3 years between 2007 and 2010. Their diagnoses were confirmed by histopathological examination. None of these patients had a history of familial thyroid cancer or external irradiation in the neck region. Clinicopathological data, such as tumor size, presence of extra thyroidal extension (ETE), and lymph node metastasis (LNM), were retrieved from patients’ medical records. ETE was defined as invasion of adjacent organs or skeletal muscle outside the isthmus [20]. The cancer stage was defined according to the 7th edition of tumor, node and metastasis system classification by the American Joint Committee on Cancer [21]. The study protocol was performed according to the declaration of Helsinki and approved by the Medical Ethics Committee of China Medical University. Written and verbal informed consent was obtained from all participants.
Immunohistochemistry
Five sections per sample of PTC tissues were used to quantify protein expression for this study. The formalin-fixed and paraffin-embedded surgical specimens from PTC patients were cut into 4-μm thick sections and immunostained with antibodies against ERβ2 and other biological makers (Ki-67, mutant p53, and VEGF) using the Elivision™ plus two-step system. Briefly, the tissue sections were deparaffinized, rehydrated and subjected to microwave antigen retrieval in 10 mM citrate buffer (pH 6.0) for 20~25 min. Endogenous peroxidase was then blocked with 3% H2O2 for 10 min in room temperature and then washed in phosphate buffered saline (PBS). Next, the sections were incubated with a primary antibody against ERβ2 (Clone 57/3, Serotec; 1:400), Ki-67 (Clone SP6, Abcam; 1:100), mutant p53 (Clone N235K N239Y, Bioss; 1:350) or VEGF (Clone EP1176Y, Abcam; 1:100) at 4°C overnight. After washing with PBS, the sections were then incubated with the Elivision™ plus two-step system (Maixin Bio, China) and color reaction was performed with 3,3’-diamino-benzidine (DAB; Maixin Bio). Then, the sections were counterstained with hematoxylin, washed, dehydrated in ethanol, cleared in xylene, and mounted with coverslips. Appropriate positive and negative controls were used in each batch of staining experiments. Tissue sections from human breast cancer were used as positive controls for ERβ2. Negative control sections were incubated with normal mouse or rabbit IgG instead of a primary antibody.
Review and scoring of stained tissue sections
IHC staining for ERβ2, mutant p53 and VEGF were interpreted using the Allred score [22,23]. In brief, each entire section was evaluated under a light microscope. A proportion staining score (PS) was assigned, which represented the estimated proportion of positive-staining tumor cells as follows: 0, no staining; 1, <1⁄100; 2, 1⁄100 to 1⁄10; 3, 1⁄10 to 1⁄3; 4, 1⁄3 to 2⁄3; and 5, >2⁄3. Moreover, we also assigned an intensity score (IS) for each tissue section to represent the average intensity of positive tumor cells as follows: 0, no staining; 1, weak, 2, intermediate; and 3, strong. After, a total score (TS) was calculated from the sum of PS and IS (ranging from 0, 2-8). “Positive” staining was then defined as scores ≥3. IHC staining for Ki-67 was interpreted using the Ki-67 labeling index (Ki-67 LI), which was expressed as a percentage of immunoreactive tumor cells to the total counted tumor cells; at least 1,000 tumor cells were counted. Sections from the same patient were stained in multiple batches of staining and five random fields of view at 400× magnification per section were counted to confirm consistency of Allred score for individual patient tissue. A double-blind analysis was performed by two independent investigators (Dong W and Huang Y). If discrepancies occurred, a third investigator (Li J) evaluated the tissue sections and the consistent result was rendered.
Statistical analysis
Descriptive statistics were used according to distribution of the variables. The association between ERβ2 and biological makers was assessed using the Spearman correlation test. The association between ERβ2 and clinicopathological factors was assessed using the Mann-Whitney U test. All statistical analyses were performed by using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). A P<0.05 was considered statistically significant.
Results
Expression of ERβ2 protein in PTC tissue specimens
In this study, we first detected ERβ2 expression in 106 cases of PTC tissue specimens using immunohistochemistry. Our data showed that nuclear expression of ERβ2 protein occurred in 105 cases (99.06%), while one case (0.94%) showed both nuclear and cytoplasmic staining of ERβ2 protein. The staining score frequencies of ERβ2 in 106 cases of PTC tissue specimens were Score 3 (3 cases, 2.8%), Score 4 (9 cases, 8.5%), Score 5 (9 cases, 8.5%), Score 6 (30 cases, 28.3%), Score 7 (34 cases, 32.1%) and Score 8 (21 cases, 19.8%). Representative images were shown in Figure 1.
Figure 1.

Immunohistochemical staining of ERβ2 expression. PTC tissues show different staining patterns, i.e., nuclear staining of ERβ2 with different “Allred score” 3 (A), 4 (B), 5 (C), 6 (D), 7 (E) and 8 (F) or both nuclear and cytoplasmic staining of ERβ2 with Allred score 3 (G) and negative control of ERβ2 (H) (magnification ×400).
Association of ERβ2 expression with biological makers in PTC tissue specimens
Next, we examined the expression of Ki-67, mutant p53 and VEGF in PTC lesions and explored the association of ERβ2 expression with the above biological makers (Figure 2; Table 1). We found that ERβ2 expression was associated with Ki-67 expression in PTC tissues in advanced reproductive age female patients (r=0.443, P=0.005). ERβ2 expression was also associated with VEGF expression in PTC tissues of reproductive age male patients (r=0.495, P=0.044). These results indicated that high levels of ERβ2 expression in low-estrogen status patients were associated with PTC progression. However, there was no association of ERβ2 expression with mutant p53 expression.
Figure 2.
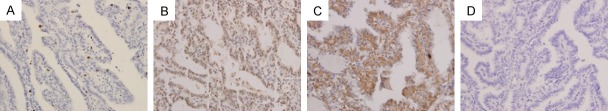
Immunohistochemical staining of Ki-67, mutant p53 and VEGF expression. PTC tissues show typical staining patterns of Ki-67 (A), mutant p53 (B) and VEGF (C) and negative control (D) in those samples (magnification ×400).
Table 1.
Associations between ERβ2 expression and other biological makers in PTC tissue specimens
| Female in reproductive age | Female in advanced reproductive age | Male in reproductive age | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Case | r | P | Case | r | P | Case | r | P | |
| Ki-67 | 50 | 0.164 | 0.256 | 39 | 0.443 | 0.005 | 17 | -0.141 | 0.590 |
| MTp53 | 50 | -0.090 | 0.533 | 39 | 0.227 | 0.165 | 17 | -0.427 | 0.087 |
| VEGF | 50 | -0.026 | 0.855 | 39 | 0.067 | 0.686 | 17 | 0.495 | 0.044 |
r, Spearman correlation coefficient; P value was assessed using Spearman correlation test.
Association of ERβ2 expression with clinicopathological factors from PTC patients
ERβ2 expression was not associated with tumor size, presence of ETE and TNM stage when stratified by age and gender in PTC. However, ERβ2 expression was significantly lower in reproductive age female patients with LNM than that in those patients without LNM (Z=-2.107, P=0.035) (Table 2). This indicated that high levels of ERβ2 expression in relatively high-estrogen status patients may inhibit the LNM of PTC.
Table 2.
Associations between ERβ2 expression and clinicopathological factors from PTC patients
| Female in reproductive age | Female in advanced reproductive age | Male in reproductive age | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| n | Total score | P | n | Total score | P | n | Total score | P | |
| Tumor size | |||||||||
| ≤2 cm | 25 | 6.6±1.3 | 0.793 | 16 | 6.2±1.7 | 0.812 | 5 | 5.6±1.7 | 0.456 |
| >2 cm | 25 | 6.7±1.2 | 23 | 6.5±0.9 | 12 | 6.1±1.0 | |||
| LNM | |||||||||
| - | 33 | 7.0±1.1 | 0.035 | 23 | 6.3±1.6 | 0.870 | 10 | 5.7±1.0 | 0.525 |
| + | 17 | 6.3±1.2 | 16 | 6.5±0.7 | 7 | 6.1±1.3 | |||
| ETE | |||||||||
| - | 22 | 6.7±1.3 | 0.529 | 23 | 6.3±1.3 | 0.388 | 6 | 6.0±1.1 | 0.915 |
| + | 28 | 6.5±1.1 | 16 | 6.6±1.3 | 11 | 5.9±1.5 | |||
| TNM stage | |||||||||
| I/II | 47 | 6.6±1.2 | 0.898 | 19 | 6.3±1.4 | 0.682 | 16 | 5.9±1.2 | 1.0 |
| III/IV | 3 | 6.7±1.5 | 20 | 6.5±1.1 | 1 | 6.0 | |||
LNM, lymph node metastases; ETE, extra thyroidal extension; TNM stage, tumor-node-metastasis stage; n, number of patients. Total score, Mean TS±SD. P value was assessed with Mann-Whitney U test.
Discussion
ERβ2 is one of the major splice variants of ERβ and has been known to play an important role in cancer development and progression [13-15]. This is the first study to explore the protein expression of ERβ2 and the associations with different biological markers (Ki-67, mutant p53 and VEGF) and clinicopathological factors in PTC. In this study, we detected the expression of ERβ2, Ki-67, mutant p53 and VEGF proteins in PTC tissue specimens. We found that ERβ2 expression was associated with Ki-67 expression in PTC tissues from female patients with an advanced reproductive age and with VEGF expression in PTC tissues from male patients with a reproductive age. Moreover, ERβ2 expression was significantly lower in reproductive age female patients with LNM than that in those patients without LNM. These data suggested that the roles of ERβ2 may be associated with the status of estrogen of the patients with PTC. It has been reported that ERβ2 does not form homodimers and has no innate activities of its own, but readily heterodimerizes with ERβ1 in the presence of physiological concentrations of E2 in a dose-dependent manner and enhances ERβ1 transcriptional activity [10]. ERβ1 protein expression was significantly correlated with negative lymph node status in breast cancer [24]. It is therefore tempting to speculate that ERβ2 may enhance ERβ1 transcriptional activity by more strongly induced heterodimer formation between ERβ2 and ERβ1 in higher estrogen status and therefore inhibit lymph node metastasis of PTC. In PTC ERβ2 could promote tumor growth of PTC in low-estrogen state patients while ERβ2 could inhibit LNM of PTC in relatively high-estrogen status patients.
The association of ERβ2 expression with tumor progression has been explored in a few of previous studies. For example, in a microarray analysis of 82 normal and malignant breast tissue specimens, the tumors with reduced ERβ2 expression had a high number of lymph node-positive breast cancers and distant metastasis at the time of diagnosis [25,26]. Our current study is consistent with their findings. However, Saji et al reported that there was no statistically significant association between the expression of ERβ2 and LNM in breast cancer [27]. Wong et al found that a higher ERβ2 protein expression was associated with the presence of LNM in colorectal carcinoma [14]. In addition, some studies also showed that expression of ERβ2 protein was not associated with tumor size and histological grade , although Dey P et al found that ERβ2 increased proliferation and up-regulated factors involved in bone metastasis in prostate cancer cells [27-30]. Sugiura H et al reported that ERβ2 protein expression was significantly correlated with low histological grade and better disease-free and overall survival, but Honma N didn’t find ERβ2 influence survival in breast cancer [24,31]. Chantzi NI et al found ERβ2 is associated with poor prognosis in ERα-negative breast cancer [32]. Leung YK et al found ERβ2 was associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion [33].
Recently, the studies have majored on immunohistochemical markers and evaluated the expressions of thyroid transcription factor-1, Ki-67, p63, p53 and VEGF among PTCs [34-36]. They have been considered to be the useful makers reflecting the biological behavior and prognosis of PTC. However, few studies have investigated the association of ERβ2 expression with the above biological markers in PTC. We are the first to report that ERβ2 positively correlated with Ki-67 and VEGF, but not mutant p53 in PTC. However, there were no significant associations between ERβ2 expression and Ki-67 in colorectal carcinoma and endometrial carcinoma [14,37].
We recognize that our cohort is relatively small (106 patients) and includes few patients with postoperative recurrence (4 patients) and postoperative death (0 patient) when compared with that of a breast cancer study by Shaaban et al [38]. ERβ2 expression as a prognosis factor, such as in breast cancer and prostate cancer, could allow us to follow up PTC patients longer [33,38].
In conclusion, our current study demonstrated that expression of ERβ2 protein was associated with PTC progression. The potential effect of ERβ2 protein may vary with different levels of estrogen in the patients. Thus, the biological and clinical significance of ERβ2 in PTC needs further study.
Acknowledgements
This work was supported by the Key Scientific and Technological Project from Liaoning Province (JH2) (No: 2008225007), Program for Liaoning Excellent Talents in University (LNET) (No: LJQ 2011080), Liaoning Baiqianwan Talents Program (No. 2014921033), the Liaoning Province PhD Start-up Fund (No. 20141042) and National Natural Science Foundation of China (No. 81402208).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014;21:T273–283. doi: 10.1530/ERC-14-0053. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed RA, Aboelnaga EM. Thyroid Cancer in Egypt: Histopathological Criteria, Correlation With Survival and Oestrogen Receptor Protein Expression. Pathol Oncol Res. 2015;21:793–802. doi: 10.1007/s12253-014-9892-5. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif. 2007;40:921–935. doi: 10.1111/j.1365-2184.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010;36:1067–1080. doi: 10.3892/ijo_00000588. [DOI] [PubMed] [Google Scholar]
- 6.Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, Tiwari RK. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong W, Zhang H, Li J, Guan H, He L, Wang Z, Shan Z, Teng W. Estrogen Induces Metastatic Potential of Papillary Thyroid Cancer Cells through Estrogen Receptor alpha and beta. Int J Endocrinol. 2013;2013:941568. doi: 10.1155/2013/941568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res. 2011;2011:875125. doi: 10.4061/2011/875125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Sukocheva OA, Hussey DJ, Watson DI. Estrogen, male dominance and esophageal adenocarcinoma: is there a link? World J Gastroenterol. 2012;18:393–400. doi: 10.3748/wjg.v18.i5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. J Mol Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 12.Dong W, Li J, Huang Y, Zhang H, Shan Z, Teng W. Differential expression patterns of estrogen receptor (ER)-beta splice variants between papillary thyroid cancer and nodular thyroid goiter. Med Sci Monit. 2012;18:BR351–355. doi: 10.12659/MSM.883344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esslimani-Sahla M, Kramar A, Simony-Lafontaine J, Warner M, Gustafsson JA, Rochefort H. Increased estrogen receptor betacx expression during mammary carcinogenesis. Clin Cancer Res. 2005;11:3170–3174. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- 14.Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol. 2005;207:53–60. doi: 10.1002/path.1807. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarty D, Srinivasan R, Ghosh S, Gopalan S, Rajwanshi A, Majumdar S. Estrogen receptor beta1 and the beta2/betacx isoforms in nonneoplastic endometrium and in endometrioid carcinoma. Int J Gynecol Cancer. 2007;17:905–913. doi: 10.1111/j.1525-1438.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheri A, Dowsett M. Developments in Ki67 and other biomarkers for treatment decision making in breast cancer. Ann Oncol. 2012;23(Suppl 10):x219–x227. doi: 10.1093/annonc/mds307. [DOI] [PubMed] [Google Scholar]
- 17.Menendez D, Inga A, Resnick MA. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc Natl Acad Sci U S A. 2010;107:1500–1505. doi: 10.1073/pnas.0909129107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konduri SD, Medisetty R, Liu W, Kaipparettu BA, Srivastava P, Brauch H, Fritz P, Swetzig WM, Gardner AE, Khan SA, Das GM. Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci U S A. 2010;107:15081–15086. doi: 10.1073/pnas.1009575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat A, Rajoria S, George A, Suriano R, Shanmugam A, Megwalu U, Prakash PB, Tiwari R, Schantz S. Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg. 2011;137:1146–1153. doi: 10.1001/archoto.2011.194. [DOI] [PubMed] [Google Scholar]
- 20.Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. 2010;17:386–391. doi: 10.1245/s10434-009-0832-7. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 22.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 23.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 24.Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, Iwase H, Yamashita H. Expression of estrogen receptor beta wild-type and its variant ERbetacx/beta2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007;37:820–828. doi: 10.1093/jjco/hym114. [DOI] [PubMed] [Google Scholar]
- 25.Ahr A, Karn T, Solbach C, Seiter T, Strebhardt K, Holtrich U, Kaufmann M. Identification of high risk breast-cancer patients by gene expression profiling. Lancet. 2002;359:131–132. doi: 10.1016/S0140-6736(02)07337-3. [DOI] [PubMed] [Google Scholar]
- 26.Ahr A, Holtrich U, Solbach C, Scharl A, Strebhardt K, Karn T, Kaufmann M. Molecular classification of breast cancer patients by gene expression profiling. J Pathol. 2001;195:312–320. doi: 10.1002/path.955. [DOI] [PubMed] [Google Scholar]
- 27.Saji S, Omoto Y, Shimizu C, Warner M, Hayashi Y, Horiguchi S, Watanabe T, Hayashi S, Gustafsson JA, Toi M. Expression of estrogen receptor (ER) (beta)cx protein in ER(alpha)-positive breast cancer: specific correlation with progesterone receptor. Cancer Res. 2002;62:4849–4853. [PubMed] [Google Scholar]
- 28.Vinayagam R, Sibson DR, Holcombe C, Aachi V, Davies MP. Association of oestrogen receptor beta 2 (ER beta 2/ER beta cx) with outcome of adjuvant endocrine treatment for primary breast cancer--a retrospective study. BMC Cancer. 2007;7:131. doi: 10.1186/1471-2407-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmieri C, Lam EW, Mansi J, MacDonald C, Shousha S, Madden P, Omoto Y, Sunters A, Warner M, Gustafsson JA, Coombes RC. The expression of ER beta cx in human breast cancer and the relationship to endocrine therapy and survival. Clin Cancer Res. 2004;10:2421–2428. doi: 10.1158/1078-0432.ccr-03-0215. [DOI] [PubMed] [Google Scholar]
- 30.Dey P, Jonsson P, Hartman J, Williams C, Strom A, Gustafsson JA. Estrogen receptors beta1 and beta2 have opposing roles in regulating proliferation and bone metastasis genes in the prostate cancer cell line PC3. Mol Endocrinol. 2012;26:1991–2003. doi: 10.1210/me.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 32.Chantzi NI, Tiniakos DG, Palaiologou M, Goutas N, Filippidis T, Vassilaros SD, Dhimolea E, Mitsiou DJ, Alexis MN. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J Cancer Res Clin Oncol. 2013;139:1489–1498. doi: 10.1007/s00432-013-1467-4. [DOI] [PubMed] [Google Scholar]
- 33.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17:675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan A, Etit D, Bayol U, Altinel D, Tan S. Comparison of proliferating cell nuclear antigen, thyroid transcription factor-1, Ki-67, p63, p53 and high-molecular weight cytokeratin expressions in papillary thyroid carcinoma, follicular carcinoma, and follicular adenoma. Ann Diagn Pathol. 2011;15:108–116. doi: 10.1016/j.anndiagpath.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Tallini G, Garcia-Rostan G, Herrero A, Zelterman D, Viale G, Bosari S, Carcangiu ML. Downregulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol. 1999;23:678–685. doi: 10.1097/00000478-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, Lloyd RV. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. Am J Surg Pathol. 2010;34:44–52. doi: 10.1097/PAS.0b013e3181c46677. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarty D, Gupta N, Goda JS, Srinivasan R, Patel FD, Dhaliwal L. Steroid receptors, HER2/neu and Ki-67, in endometrioid type of endometrial carcinoma: Correlation with conventional histomorphological features of prognosis. Acta Histochem. 2010;112:355–363. doi: 10.1016/j.acthis.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]


