Abstract
The differential diagnosis between atypical leiomyoma and leiomyosarcoma may be hard based on morphological criterion at times. It would be helpful to find out biomarkers that can be used to distinguish them. The aim of the study was to investigate the diagnostic value of progesterone receptor (PR), p16, p53 and pHH3 expression in a series of uterine smooth muscle tumors. Immunohistochemical expression of PR, p16, p53 and pHH3 was investigated on 32 atypical leiomyomas, 15 leiomyosarcomas and 15 usual leomyomas. The difference in expression was compared between atypical leiomyoma and other groups. The expression of PR, p16, and pHH3 was found significantly different between atypical leiomyomas and leiomyosarcomas, but lack of significant difference between atypical leiomyomas and usual leiomyomas. There was no significant difference with regard to p53 distribution among these uterine smooth muscle tumors. High p16, pHH3 expression and low PR expression preferred the diagnosis of leiomyosarcoma. The panel of antibodies used in this study is a useful complementary analysis in the assessment of problematic uterine smooth muscle tumors.
Keywords: Atypical leiomyoma, immunohistochemistry, p16, p53, PR, pHH3
Introduction
Smooth muscle tumors are the most common neoplasms of the uterus, in most instances, smooth muscle tumors of the uterus can be reliably diagnosed as either benign (leiomyoma) or malignant (leiomyosarcoma, LMS) by application of a combination of microscopic features including the presence of coagulative necrosis, the degree of cytologic atypia and the mitotic activity. However, a small subset of cases that do not fulfill these standard features of benign and malignant may have diagnostic problems. This situation causes concern especially for those atypical smooth muscle tumors demonstrating moderate to severe cytologic atypia but devoid of tumor cell necrosis or elevated mitotic activity. Due to the relatively rare case numbers and variations in subjective identifications of atypia, the diagnosis of atypical leiomyoma remains the most challenging diagnostic dilemma for pathologists. In this respect, there was a constant concern for the application of immunohistological markers capable to identify these problematic uterine smooth muscle neoplasms. The role of steroid receptor and cell cycle regulatory protein had been widely studied by different researchers [1-4]. However, due to the different criteria for immunohistochemistry analysis as well as diverse smooth muscle tumor classification, the results of these studies are conflicting.
The aim of this study was to investigate whether there are differences in the expression of a panel of steroid receptor and cell cycle regulatory protein markers including progesterone receptor (PR), p16, p53 and pHH3 in a series uterine smooth muscle tumors and whether the different expressions have diagnostic value in the assessment of problematic cases. In addition, in atypical leiomyoma cases with known clinical follow-up, we explored whether the expression of certain markers was associated with patients’ survival.
Materials and methods
Case selection and histologic evaluation
We retrospectively searched the surgical pathology files from the Women’s Hospital School of Medicine Zhejiang University from January 1997 to December 2009 for atypical leiomyoms and a total of 32 cases were collected for this study. This study was approved by the Ethics Committee of the Women’s Hospital School of Medicine Zhejiang University. In comparison, 15 usual leiomyomas and 15 LMSs were also included. All available slides were reviewed by 2 pathologists. In this study according to Bell et al’ s criterion [5], tumors with one of the following sets of criteria were grouped as atypical leiomyoma: (1) “atypical leiomyoma with limited experience”, focal or multifocal moderate-to-severe atypia, mitotic index <10 /10HPFs and no tumor cell necrosis; (2) “atypical leiomyoma with low risk of recurrence”, diffuse moderate-to-severe atypia, mitotic index <10/10HPFs, and no tumor cell necrosis; LMS was confirmed if the tumor demonstrated at least two of the following features: moderate to severe atypia, tumor cell necrosis, mitotic index ≥10/10HPFs. Only tumors composed of spindle cell were include; tumors with epithelioid or myxiod differentiations were excluded for different histologic criteria. Clinical information and follow-up were obtained from the medical records including patient’s age at diagnosis, chief complaints, surgical procedure performed tumor size, date of recurrence and date of death.
Immunohistochemistry
For immunohistochemical analysis, a panel of monoclonal antibodies was used against PR (clone PgR636; Dako; 1:50), p16 (clone DC-648; Santa Cruz; 1:100), p53 (clone DO-7; Dako; 1:400) and pHH3 (polyclonal; Upstate Biotechology; 1:100). Immunohistochemical staining was performed by the 2-step En vision method according to the manufacturer’s instructions and visualized with 3,3’-diaminobenzidine tetrachloride (DAB, Glostrup, Denmark). The omission of primary body was used as the negative control. For p16, moderate to strong nuclear staining or combination of nuclear and cytopalsmic staining was considered positive. For p53, PR and pHH3 only moderate to strong nuclear staining was evaluated. Cases were scored as 0 (negative or occasional positive cells), 1+ (<50% cells positive), 2+ (50%-75% cells positive), 3+ (>75% cells positive). Due to different staining properties, the assessment of the degree of immunohistochemical staining was varied. For p16 immunostains, 0, 1+ and 2+ were regarded as low expression, while 3+ was regarded as high expression. For p53 and PR immunostains, 0 and 1+ were regarded as low expression, while 2+ and 3+ were regarded as high expression. The pHH3 expressions were calculated in areas of mitoses “hot spot” and the number of positive cells per 10 high-power fields (HPF) were recorded.
Statistical analysis
Fisher exact tests were used to compare the frequency distributions of different antibody expressions between the various uterine smooth muscle tumors. P values of less than 0.05 were considered statistically significant. The analysis was performed with SPSS 18.0.
Result
Clinical and pathological findings of atypical leiomyoma
Patients with atypical leiomyoma ranged in age from 28-61 (mean 47) years. Presenting symptoms were unspecific in these tumors including pelvic mass [11], irregular menstruation [9], menorrhagia [5] and anemia [4]. In 3 patients, the tumor was asymptomatic and discovered incidentally. Nine patients underwent a myomectomy, the remainder had simple hysterectomy. None of the patients received chemotherapy or radiation therapy. The tumors ranged in size from 2.8 to 14 cm (mean 6.4 cm). All had well-circumscribed margins. The cut surface was white and whorled in 24 cases and part yellow in other 8 cases. On microscopic evaluation, all tumors demonstrated moderated to severe nuclear atypia that was focal [5], multifocal [10] or diffuse [17]. Mitotic counts ranged from 1-5/10HPF by the highest count method. Infarct-type necrosis was found in 6 cases.
Follow information was available in 25 atypical leiomyoma cases. Twenty-three patients behaved in a benign fashion with no evidence of recurrence after a 54-170 months follow-up. Two atypical leiomyoma patients who underwent myomectomy had recurrence in the uterus at 65 months and 84 months. Both cases showed diffuse, moderate to severe ctyologic atypia without coagulative necrosis. Mitotic activity was 2/10HPF in one case, and 4/10HPF in another.
Immunohistochemistry expression
The results of PR, p16 and p53 immunohistochemical staining were shown in Table 1. All usual leiomyomas showed high PR expression and high expression in atypical leiomyomas (Figure 1B) was found in 28 (87.5%) cases. The difference between this two groups was not significant (P=0.291). LMS showed the least PR expression (Figure 2B), and only 4 (26.7%) cases had high PR expression. The difference between LMS and atypical leiomyoma was highly significant (P=0.000). On the contrary, the expression of p16 was most prominent in the malignant side of the spectrum (LMS) with 7 (46.7%) cases strongly stained this antibody (Figure 2A). High expression of p16 was seen in just 4 atypical leiomyomas (Figure 1A) but none in ordinary leiomyoma. The p16 expression differences between LMS and atypical leiomyoma was significant (P=0.023) but lack of significant difference between atypical leiomyoma and ordinary leiomyoma (P=0.355). The high expression of p53 was 7 (46.7%) in LMS (Figure 2C), 10 (31.3%) in atypical leiomyoma (Figure 1C) and 2 (13.3%) in ordinary leiomyoma. For comparing atypical leiomyoma to LMS or to ordinary leiomyoma, there were no different (P=0.344, P=0.288). There was also significant difference with regard to PR distribution and p16 distribution between the leiomyosarcoma and the combined groups (atypical leiomyoma and cellular leiomyoma) (P=0.000, P=0.002).
Table 1.
PR, p16 and p53 expression in uterine smooth muscle tumors (cases)
| Tumor type | PR | p16 | p53 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| High | Low | High | Low | High | Low | |||||||
|
| ||||||||||||
| 3+ | 2+ | + | 0 | 3+ | 2+ | + | 0 | 3+ | 2+ | + | 0 | |
| Leiomyoma (15) | 15 | 0 | 0 | 0 | 0 | 2 | 5 | 8 | 0 | 2 | 4 | 9 |
| Atypical myoma (32) | 17 | 11 | 3 | 1 | 4 | 12 | 10 | 6 | 2 | 8 | 13 | 9 |
| LMS (15) | 2 | 2 | 3 | 8 | 7 | 3 | 4 | 1 | 3 | 4 | 6 | 2 |
Figure 1.
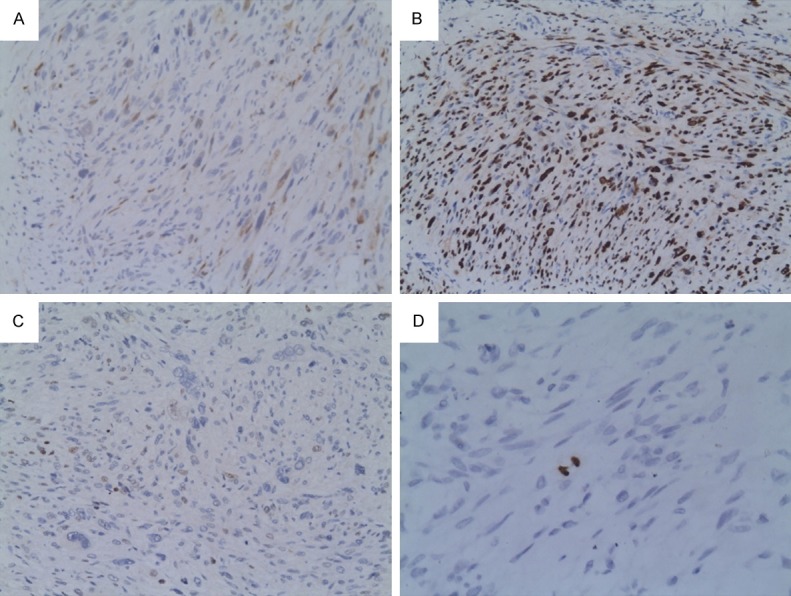
Immunostaining in atypical leiomyoma. A. Week nuclear and cytopalsmic staining in p16 (×200). B. Strong nuclear positivity in PR (×200). C. Scattered nuclear positivity in p53. D. One bipolar-mitosis labeled with pHH3 (×400).
Figure 2.
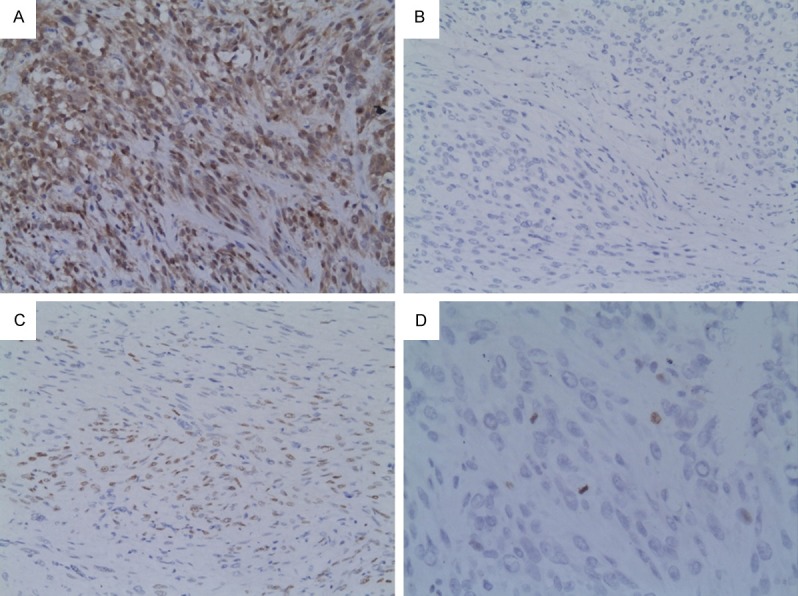
Immunostaining in leiomyosarcoma. A. High nuclear and cytopalsmic staining in p16 (×200). B. Scattered nuclear positivity in PR (×200). C. Low nuclear positivity in p53. D. Two mitosis and one cellular nuclei labeled with pHH3 (×400).
The pHH3 expression in uterine smooth muscle tumors is summarized in Table 2. Briefly, most of usual leiomyoma cases had low pHH3 expression, except one which expressed 7 pHH3 cells/10HPF. The positive cells varied from 1 to 10 in most of atypical leiomyoma cases (Figure 1D), however, two atypical leiomyomas also exhibited more positive pHH3 cells. In LMS, the majority of cases showed more than 10 positive cells per 10HPF (Figure 2D). The differences in pHH3 expression were significant between atypical leiomyoma and LMS (P=0.000) but not shown between usual leiomyoma and atypical leiomyoma (P=0.648).
Table 2.
pHH3 expression in uterine smooth muscle tumors (cases)
| Tumor type | pHH3 | |||
|---|---|---|---|---|
|
| ||||
| High | Low | |||
|
| ||||
| >10 | 6-10 | 1-5 | 0 | |
| Leiomyoma (15) | 0 | 1 | 8 | 6 |
| Atypical myoma (32) | 2 | 3 | 25 | 2 |
| LMS (15) | 10 | 2 | 2 | 1 |
The immune profile of the two recurrent cases was shown in Table 3. Both cases showed low p53, pHH3 expression and high PR expression except p16, which was diffusely positive in one tumor. However, the other case also showed low p16 expression.
Table 3.
Immunohistochemical expression in recurrent cases
| Recurrent case | p16 | p53 | PR | pHH3 |
|---|---|---|---|---|
| 1 | 3+ | + | 2+ | 5 |
| 2 | + | 2+ | 2+ | 3 |
Discussion
An accurate diagnosis for leiomyoma is crucial for clinical management. However, due to the overlapping features between malignant and benign, the differential diagnosis may be hard based on morphological criterion alone. This is particular true in atypical leiomyomas which had obvious nuclear atypia but low mitotic counts and no coagulative tumor cell necrosis. Therefore, it is necessary to find out biomarkers that can differentiate benign and malignant smooth muscle tumors. Various biomarkers including PR, p16, p53 and pHH3 have been investigated in different papers [1-4]. Yet, the current study is the first to study these promising markers together and compare the expression with their malignant counterparts. We compared the expression of these biomarkers in 62 cases of benign and malignant uterine smooth muscle neoplasm, with focus on clinical utility of these markers in differentiating atypical leiomyoma from LMS.
Of all the biomarkers that had been researched in this field, p16 protein was most intensively studied. p16 protein is a cyclin-dependent kinase inhibitor that controls the cellular crossing from G1 to S phase. Increased expression of p16 protein has been found in a number of neoplasms, including cervical epithelial neoplasm [6], where the upregulation of the expression can be regarded as a surrogate of the HPV infection. For uterine smooth muscle neoplasms, there is general agreement that p16 is more frequently and more strongly expressed in LMS compared to usual leiomyoma. Similar to previous reports [7,8], in our series, 46.7% LMS cases had high p16 expression, in contrast with none of usual leiomyoma cases showed high p16 expression. The difference between them was significant. However, the role of p16 in distinguishing LMS from atypical leiomyoma is contradictory. In the study by Ünver et al [9]. consisting of 14 cases of atypical leiomyoma and 21 cases of LMS, 14/21 cases of atypical leiomyoma showed p16 expression with a staining percentage of >50%. Only 2/14 cases of atypical leiomyoma showed expression of p16 with a staining percentage from 1%-25%. This difference was statistically significant. In another research, while 63% LMSs (10/16) demonstrated strong staining with p16, 9/44 cases of atypical leiomyoma also showed strong staining, so the author believed the difference was less dramatic than those previous reports [10]. However, in their research, the cytoplasmic staining for p16 was also scored. If just assessing the nuclear staining, only seven percent of atypical leiomyoma (3/44) had p16 expression. In other studies that showed variable degree of positive p16 staining in uterine smooth muscle neoplasm, the cutoff for p16 positivity is relatively lower (1-33%) [7,8]. Based on our finding, the use of a higher threshold value (ie 75%) and strong nuclear staining of p16 can improve the specificity in distinguishing LMS from atypical leiomyoma.
PR immunoexpression was present in the nucleus of tumor cells. In our experiment, for leiomyomas and atypical leiomyomas, intense and diffuse reactions were present in general all cases. However, in LMS cases, a prominent reduction in PR expression was found. Most researchers also had similar results with us. Hewedi et al. [11] stated that all 16 leiomyomas and 6 atypical leiomyomas were stained intensely for PR. Conversely, LMS showed reduced PR expression with only 1/15cases had strong staining. In another study conducted by Stanescu et al [12], the authors have investigated 16 cellular leiomyomas, 5 atypical leiomyomas and 6 leiomyosarcomas. Diffuse and intense PR immunoexpression was present in 82-100% of the tumor cells in the cellular leiomyomas, 75-90% for atypical leiomyomas and 5-12% for LMSs.
p53, another cell cycle control protein, function as a negative regulator of cell growth. The expression of p53 in uterine smooth muscle tumors is contradictory in different researchers. Stanescu et al. [12] observed that p53 had the highest expression in atypical leiomyomas and LMS, respectively 38.3±3.5 and 43.6±5.4, contrasted with 16.4±2.6 in cellular leiomyoma. However, in Hewedi’s study [11], both leiomyomas and atypical leiomyomas were either entirely negative or weakly stained for p53, while all LMSs were strongly stained with p53. On the other hand, Mills et al. [10] found the p53 expression was highly variable, with strong staining observed in 25% (4/16) of LMS, 7% (3/43) in atypical leiomyoma but none in leiomyoma. The author believed this antibody failed to specifically identify aggressive tumor type from others. Recently, Zhang et al. [13] examined p53 mutations by PCR and found 24% (9/38) of LMS cases had p53 mutations in exons 5-9, 12% (5/42) of ALM cases had p53 mutations in exons [4-7], but no p53 mutations were found in LM. They believed that the molecular alterations in atypical leiomyoma tend to be more closely related to LMS rather than usual leiomyoma, although atypical leiomyoma behaves in a benign fashion clinically. In our search, although the p53 staining was more frequent in atypical leiomyoma and LMS than leiomyoma, the difference was not significant. This variation across different researchers may be due to the heterogeneity of the studies groups, the small number of cases and different threshold for positive values. However, the results still contest both the sensitivity and specificity of p53 as a marker for leomyosarcoma differential diagnosis.
More recently, there have been attempts to test the application of pHH3 in uterine smooth muscle tumors. The novel proliferation marker expresses only during the late G2 phase and mitosis. Veras et al. [14] investigated the pHH3 expression difference in 12 cases of uterine smooth muscle tumors, including 6 atypical leiomyomas and 6 LMSs. In their research, 5/6 LMSs showed 6-61 pHH3 positive cells per 10HPF except that one case with extensive coagulative tumor cell necrosis had no pHH3 expression. Five of the six atypical leiomyomas showed 0-6 pHH3 positive cells/10HPF, despite one case with 16 pHH3 positive cells/10HPF. Similarly, Mill et al [10] showed more than 5/10HPF pHH3 expression was found in 6 (46%) LMSs, while just 1 (3%) atypical leiomyomas showed pHH3 expression at this level. In accordance with previous research, in our research 12/15 LMS cases showed pHH3 expression >5/10HPF, while only 5/32 atypical leiomyomas could achieve this level. On H&E sections of these atypical leiomyomas, some mitotic figures were likely interpreted as apoptotic bodies, while some pykontic nuclei-like structures could be counted as mitotic figures. The pHH3 expresses in cells thus providing a more strict assessment of the proliferation.
In this article, we have investigated the immunohistochemical expression of PR, p16, p53 and pHH3 in a series of uterine smooth neoplasms. Significant difference was found between LMS and atypical leiomyoma, in PR, p16 and pHH3, but not in p53. Our results suggested that these markers can be used to distinguish atypical leiomyoma from LMS. On the other hand, in keeping with the excellent prognosis in atypical leiomyoma patients previously reported [15-17], the immunoprofile of atypical leiomyoma for PR, p16 and pHH3 in our study was much closer to leiomyomas than LMSs. Although atypical cells in atypical leiomyoma have been attributed to abnormal cellular proliferation [18,19], the current data implied that the expression of steroid receptor (PR) and cell cycle regulatory protein markers (p16, pHH3) in atypical leiomyomas was not different from leiomyomas. For the two recurrent cases, the immune profile was similar with other atypical cases, although p16 was high in one case, however, our findings still cannot confirm the prognostic value of p16 expression.
In conclusion, a combination of high p16, pHH3 expression and low PR expression preferred the diagnosis of LMS. The panel of antibodies used in this study is a useful complementary analysis for histopathological differential diagnosis of atypical leiomyomas from LMS.
Acknowledgements
This work was supported by a grant from Health and Family planning Commission of Zhejiang Province (2014KYB140), Zhejiang, P.R. China awarded to YL.
Disclosure of conflict of interest
None.
References
- 1.Lee CH, Turbin DA, Sung YC, Espinosa I, Montgomery K, van de Rijn M, Gilks CB. A panel of antibodies to determine site of origin and malignancy in smooth muscle tumors. Mod Pathol. 2009;22:1519–31. doi: 10.1038/modpathol.2009.122. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2008;27:326–32. doi: 10.1097/PGP.0b013e31815ea7f5. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50:851–8. doi: 10.1111/j.1365-2559.2007.02699.x. [DOI] [PubMed] [Google Scholar]
- 4.Gökaslan H, Türkeri L, Kavak ZN, Eren F, Sişmanoğlu A, Ilvan S, Durmuşoğlu F. Differential diagnosis of smooth muscle tumors utilizing p53, pTEN and Ki-67 expression with estrogen and progesterone receptors. Gynecol Obstet Invest. 2005;59:36–40. doi: 10.1159/000080933. [DOI] [PubMed] [Google Scholar]
- 5.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–58. [PubMed] [Google Scholar]
- 6.Kalof AN, Cooper K. p16INK4a immunoexpression: surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–4. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32:98–102. doi: 10.1097/PAS.0b013e3181574d1e. [DOI] [PubMed] [Google Scholar]
- 8.Gannon BR, Manduch M, Childs TJ. Differential Immunoreactivity of p16 in leiomyosarcomas and leiomyoma variants. Int J Gynecol Pathol. 2008;27:68–73. doi: 10.1097/pgp.0b013e3180ca954f. [DOI] [PubMed] [Google Scholar]
- 9.Ünver NU, Acikalin MF, Öner Ü, Ciftci E, Ozalp SS, Colak E. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Arch Gynecol Obstet. 2011;284:483–90. doi: 10.1007/s00404-010-1690-z. [DOI] [PubMed] [Google Scholar]
- 10.Mills AM, Ly A, Balzer BL, Hendrickson MR, Kempson RL, McKenney JK, Longacre TA. Cell cycle regulatory markers in uterine atypical leiomyoma and leiomyosarcoma: immunohistochemical study of 68 cases with clinical follow-up. Am J Surg Pathol. 2013;37:634–42. doi: 10.1097/PAS.0b013e318287779c. [DOI] [PubMed] [Google Scholar]
- 11.Hewedi IH, Radwan NA, Shash LS. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. Diagn Pathol. 2012;5:7–1. doi: 10.1186/1746-1596-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stănescu AD, Nistor E, Sajin M, Stepan AE. Immunohistochemical analysis in the diagnosis of uterine myometrial smooth muscle tumors. Rom J Morphol Embryol. 2014;55(Suppl):1129–36. [PubMed] [Google Scholar]
- 13.Zhang Q, Ubago J, Li L, Guo H, Liu Y, Qiang W, Kim JJ, Kong B, Wei JJ. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer. 2014;120:3165–77. doi: 10.1002/cncr.28900. [DOI] [PubMed] [Google Scholar]
- 14.Veras E, Malpica A, Deavers MT, Silva EG. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int J Gynecol Pathol. 2009;28:316–21. doi: 10.1097/PGP.0b013e318193df97. [DOI] [PubMed] [Google Scholar]
- 15.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33:992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- 16.Dall’Asta A, Gizzo S, Musarò A, Quaranta M, Noventa M, Migliavacca C, Sozzi G, Monica M, Mautone D, Berretta R. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014;7:8136–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Ly A, Mills AM, McKenney JK, Balzer BL, Kempson RL, Hendrickson MR, Longacre TA. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37:643–9. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Mittal K. MIB-1 (Ki-67), estrogen receptor, progesterone receptor, and p53 expression in atypical cells in uterine symplastic leiomyomas. Int J Gynecol Pathol. 2010;29:51–4. doi: 10.1097/PGP.0b013e3181b0259b. [DOI] [PubMed] [Google Scholar]
- 19.Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997;21:1261–70. doi: 10.1097/00000478-199711000-00001. [DOI] [PubMed] [Google Scholar]


