Abstract
Squamous differentiation is the most common histological variation in urothelial carcinoma (UC). However, the clinical significance of squamous differentiation in upper urinary tract UC is unclear. To investigate the significance of squamous differentiation, hematoxylin and eosin stained slides from 140 patients with upper urinary tract UC who underwent nephroureterectomy were reviewed by a single pathologist and the presence of squamous differentiation was recorded. Squamous differentiation was observed in 23 out of 140 studied cases (16%). Squamous differentiation significantly correlated with several adverse prognostic factors including histological grade 3 tumors, presence of lymphovascular invasion, concomitant carcinoma in situ, advanced tumor stage, and occurrence of lymph node metastasis. The Kaplan-Meier and univariate Cox regression analyses revealed that the presence of squamous differentiation was significantly associated with shorter metastasis-free survival [log-rank P = 0.030; univariate hazard ratio (HR), 2.30; 95% confidence interval (CI), 1.06-4.99], cancer-specific survival (log-rank P = 0.0024; univariate HR 3.34; 95% CI, 1.47-7.85), and overall survival (log-rank P = 0.018; univariate HR 2.39; 95% CI, 1.13-5.06) after nephroureterectomy. However, in multivariate analyses, squamous differentiation was not significantly associated with patient outcomes. These findings suggest that squamous differentiation is associated with disease progression, but is not an independent predictor of a worse prognosis in patients with upper urinary tract UC.
Keywords: Cancer, histology, pathology, prognostic marker, renal pelvis, ureter
Introduction
Carcinoma of the upper urinary tract is a relatively uncommon urothelial malignancy [1]. The majority of upper urinary tract carcinomas are pure urothelial carcinomas (UCs). However, UC has a pronounced ability to appear in different histological variants which are reported in up to 40% of upper urinary tract UC cases [2]. Squamous differentiation is the most common histological variant of UC [2,3]. Whereas the prognostic significance of squamous differentiation in UC of the urinary bladder has been well established [4-14], the clinical impact of squamous differentiation in upper urinary tract UC is conflicting. We therefore examined the clinicopathological and prognostic significance of squamous differentiation in upper urinary tract UC.
Materials and methods
Patient population
A total of 140 patients with primary upper urinary tract UC who underwent nephroureterectomy at The University of Tokyo Hospital from 1996 to 2012 were included in this study. Patients with a history of bladder cancer were excluded because treatment for bladder cancer may affect the histology. No patient received neoadjuvant chemotherapy. All research protocols in the present study were approved by our institutional review board.
Histopathological evaluation
Hematoxylin and eosin-stained slides of all cases were systematically reviewed by a single pathologist (T. M.) without prior knowledge of clinical outcomes [15]. Tumor grade was defined according to the 1973 World Health Organization (WHO) grading system [16]. Tumors were staged according to the TNM classification system [1]. Presence of squamous differentiation, which is characterized by keratinization or intercellular bridges, was also recorded (Figure 1).
Figure 1.
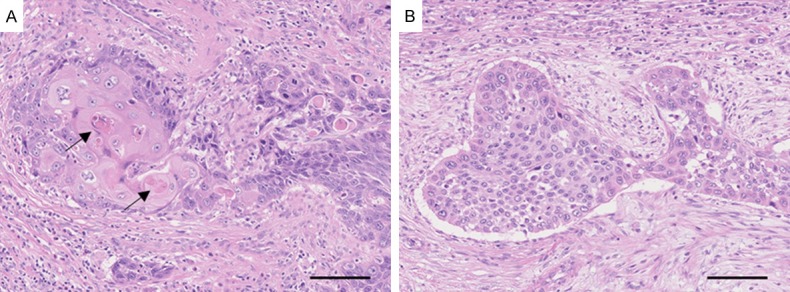
Representative photomicrographs of upper urinary tract urothelial carcinoma with (A) or without (B) squamous differentiation. (A) Invasive urothelial carcinoma with squamous differentiation. Arrows indicate keratinization sites. (B) Invasive urothelial carcinoma without squamous differentiation. Bars, 100 μm.
Statistical analysis
All statistical analyses were performed using SAS software (Version 9.3, SAS Institute, Cary, NC). All P values were two-sided. Differences were considered significant if P < 0.05. Categorical data were analyzed using χ2 test. The Kaplan-Meier method and log-rank test were used to analyze survival. Multivariate Cox proportional hazards regression models were used to control for confounding variables.
Results
Correlation between squamous differentiation and clinicopathological factors in upper urinary tract UC
Squamous differentiation was observed in 23 out of 140 patients studied (16%). The occurrence of squamous differentiation significantly correlated with the presence of histological grade 3 tumors, lymphovascular invasion, concomitant carcinoma in situ, advanced tumor stages, and lymph node metastasis (Table 1).
Table 1.
Correlation between the presence of squamous differentiation and clinicopathological features in patients with upper urinary tract urothelial carcinoma who underwent nephroureterectomy
| Squamous differentiation | ||||
|---|---|---|---|---|
|
|
||||
| Clinical or pathological features | Total N | Absent | Present | P value |
| All cases | 140 | 117 (84%) | 23 (16%) | |
| Sex | 0.083 | |||
| Male | 101 | 81 (80%) | 20 (20%) | |
| Female | 39 | 36 (92%) | 3 (8%) | |
| Age | 0.26 | |||
| < 70 | 76 | 66 (87%) | 10 (13%) | |
| ≥ 70 | 64 | 51 (80%) | 13 (20%) | |
| Side | 0.36 | |||
| Left | 73 | 63 (86%) | 10 (14%) | |
| Right | 67 | 54 (81%) | 13 (19%) | |
| Tumor location | 0.77 | |||
| Renal pelvis | 89 | 75 (84%) | 14 (16%) | |
| Ureter | 51 | 42 (82%) | 9 (18%) | |
| Tumor architecture | 0.63 | |||
| Papillary | 103 | 87 (84%) | 16 (16%) | |
| Sessile | 37 | 30 (81%) | 7 (19%) | |
| Grade | 0.014 | |||
| Grade 1 or 2 | 63 | 58 (92%) | 5 (8%) | |
| Grade 3 | 77 | 59 (77%) | 18 (23%) | |
| Lymphovascular invasion | 0.022 | |||
| Absent | 79 | 71 (90%) | 8 (10%) | |
| Present | 61 | 46 (75%) | 15 (25%) | |
| Concomitant carcinoma in situ | 0.04 | |||
| Absent | 76 | 68 (89%) | 8 (11%) | |
| Present | 64 | 49 (77%) | 15 (23%) | |
| Tumor stage | 0.0211 | |||
| pTa or pTis | 36 | 34 (94%) | 2 (6%) | |
| pT1 | 25 | 22 (88%) | 3 (12%) | |
| pT2 | 11 | 9 (82%) | 2 (18%) | |
| pT3 | 60 | 46 (77%) | 14 (23%) | |
| pT4 | 8 | 6 (75%) | 2 (25%) | |
| Lymph node metastasis | 0.0006 | |||
| Absent | 122 | 107 (88%) | 15 (12%) | |
| Present | 18 | 10 (56%) | 8 (44%) | |
| Adjuvant chemotherapy | 0.041 | |||
| No | 98 | 86 (88%) | 12 (12%) | |
| Yes | 42 | 31 (74%) | 11 (26%) | |
pTa-pT1 vs. pT2-pT4.
Squamous differentiation and clinical outcome of upper urinary tract UC
Among the 140 patients treated with nephroureterectomy, there were 23 patients with metastases, while 50 patients had bladder recurrences. There were 14 cancer-related deaths and 23 deaths of any cause during a median follow-up of 53 months (interquartile range, 25-87 months).
Kaplan-Meier analysis revealed that the presence of squamous differentiation was significantly associated with shorter metastasis-free, cancer-specific and overall survivals after nephroureterectomy (Figure 2A-C). On the other hand, squamous differentiation was not significantly associated with the bladder recurrence-free survival (Figure 2D).
Figure 2.
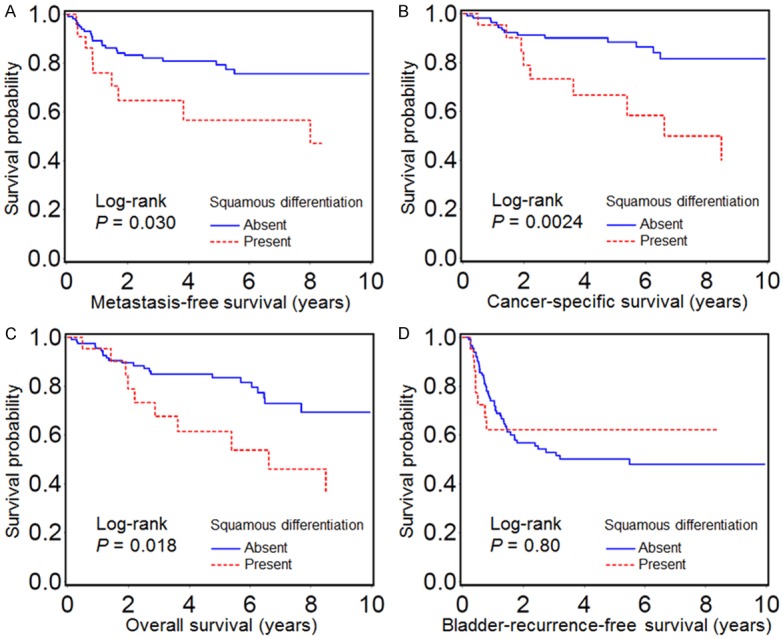
Kaplan-Meier analysis of metastasis-free survival (A), cancer-specific survival (B), overall survival (C), and bladder-recurrence-free survival (D) after nephroureterectomy with regards to the presence of squamous differentiation in upper urinary tract carcinoma.
The results of Cox proportional hazard regression analyses are shown in Table 2 (metastasis-free survival), Table 3 (cancer-specific survival), and Table 4 (overall survival). The presence of squamous differentiation was significantly associated with poorer patient outcomes in univariate analyses. However, in multivariate analyses adjusted for the disease stage and other clinicopathological factors, there were no significant links between squamous differentiation and patient outcomes (Tables 2, 3 and 4).
Table 2.
Squamous differentiation in upper urinary tract carcinoma and metastasis-free survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Squamous differentiation (present vs. absent) | 2.30 (1.06-4.99) | 0.035 | 1.00 (0.43-2.30) | 0.99 |
| Sex (female vs. male) | 1.00 (0.46-2.17) | 1 | - | - |
| Age (≥ 70 vs. < 70 years) | 2.03 (1.00-4.12) | 0.05 | - | - |
| Side (right vs. left) | 0.86 (0.43-1.72) | 0.66 | - | - |
| Tumor location (ureter vs. renal pelvis) | 1.90 (0.95-3.80) | 0.071 | - | - |
| Tumor architecture (sessile vs. papillary) | 1.57 (0.76-3.26) | 0.23 | - | - |
| Tumor grade (G3 vs. G1-2) | 4.47 (1.84-10.9) | 0.001 | - | - |
| Lymphovascular invasion (present vs. absent) | 6.73 (2.90-15.6) | < 0.0001 | - | - |
| Concomitant carcinoma in situ (present vs. absent) | 3.16 (1.49-6.68) | 0.0026 | - | - |
| Tumor stage (pT2-pT4 vs. pTa-pT1) | 9.84 (2.99-32.4) | 0.0002 | 6.77 (1.96-23.3) | 0.0025 |
| Lymph node metastasis (present vs. absent) | 6.37 (3.13-12.9) | < 0.0001 | 3.36 (1.54-7.34) | 0.0024 |
| Adjuvant chemotherapy (present vs. absent) | 2.87 (1.43-5.78) | 0.0031 | - | - |
The multivariate Cox regression models initially included gender, age at diagnosis, tumor side, tumor location, tumor architecture, tumor grade, lymphovascular invasion, concomitant carcinoma in situ, tumor stage, lymph node metastasis, and adjuvant chemotherapy. A backward elimination was performed with a threshold of P = 0.05 to select variables in the final model. CI, confidence interval; HR, hazard ratio.
Table 3.
Squamous differentiation in upper urinary tract carcinoma and cancer-specific survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Squamous differentiation (present vs. absent) | 3.34 (1.47-7.85) | 0.0043 | 1.21 (0.48-3.05) | 0.69 |
| Sex (female vs. male) | 0.98 (0.39-2.50) | 0.97 | - | - |
| Age (≥ 70 vs. < 70 years) | 2.78 (1.19-6.52) | 0.019 | - | - |
| Side (right vs. left) | 0.84 (0.37-1.91) | 0.67 | - | - |
| Tumor location (ureter vs. renal pelvis) | 1.50 (0.66-3.44) | 0.34 | - | - |
| Tumor architecture (sessile vs. papillary) | 2.15 (0.94-4.91) | 0.069 | - | - |
| Tumor grade (G3 vs. G1-2) | 6.97 (2.07-23.5) | 0.0018 | - | - |
| Lymphovascular invasion (present vs. absent) | 13.4 (3.96-45.6) | < 0.0001 | 8.86 (2.45-32.0) | 0.0009 |
| Concomitant carcinoma in situ (present vs. absent) | 3.30 (1.36-8.03) | 0.0085 | - | - |
| Tumor stage (pT2-pT4 vs. pTa-pT1) | 22.7 (3.05-169) | 0.0023 | - | - |
| Lymph node metastasis (present vs. absent) | 7.45 (3.26-17.0) | < 0.0001 | 3.09 (1.24-7.70) | 0.016 |
| Adjuvant chemotherapy (present vs. absent) | 3.63 (1.54-8.57) | 0.0033 | - | - |
The multivariate Cox regression models initially included gender, age at diagnosis, tumor side, tumor location, tumor architecture, tumor grade, lymphovascular invasion, concomitant carcinoma in situ, tumor stage, lymph node metastasis, and adjuvant chemotherapy. A backward elimination was performed with a threshold of P = 0.05 to select variables in the final model. CI, confidence interval; HR, hazard ratio.
Table 4.
Squamous differentiation in upper urinary tract carcinoma and overall survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Squamous differentiation (present vs. absent) | 2.39 (1.13-5.06) | 0.022 | 1.12 (0.52-2.54) | 0.72 |
| Sex (female vs. male) | 0.89 (0.40-1.98) | 0.78 | - | - |
| Age (≥ 70 vs. < 70 years) | 2.91 (1.43-5.92) | 0.0033 | 2.42 (1.16-5.05) | 0.019 |
| Side (right vs. left) | 1.14 (0.57-2.25) | 0.72 | - | - |
| Tumor location (ureter vs. renal pelvis) | 1.25 (0.62-2.53) | 0.53 | - | - |
| Tumor architecture (sessile vs. papillary) | 1.94 (0.96-3.90) | 0.064 | - | - |
| Tumor grade (G3 vs. G1–2) | 3.84 (1.66-8.88) | 0.0017 | 2.62 (1.07-6.40) | 0.035 |
| Lymphovascular invasion (present vs. absent) | 5.23 (2.41-11.4) | < 0.0001 | 3.34 (1.45-7.68) | 0.0046 |
| Concomitant carcinoma in situ (present vs. absent) | 2.77 (1.33-5.74) | 0.0064 | - | - |
| Tumor stage (pT2–pT4 vs. pTa–pT1) | 5.74 (2.21-14.9) | 0.0003 | - | - |
| Lymph node metastasis (present vs. absent) | 4.93 (2.40-10.1) | < 0.0001 | - | - |
| Adjuvant chemotherapy (present vs. absent) | 1.77 (0.89-3.51) | 0.10 | - | - |
The multivariate Cox regression models initially included gender, age at diagnosis, tumor side, tumor location, tumor architecture, tumor grade, lymphovascular invasion, concomitant carcinoma in situ, tumor stage, lymph node metastasis, and adjuvant chemotherapy. A backward elimination was performed with a threshold of P = 0.05 to select variables in the final model. CI, confidence interval; HR, hazard ratio.
Discussion
Here, we report our findings regarding squamous differentiation in upper urinary tract UC in relation to clinicopathological features and clinical outcome. We found that the occurrence of squamous differentiation was significantly associated with several adverse prognostic factors and poorer prognosis in univariate analyses. However, squamous differentiation was not significantly associated with worse patient outcomes in multivariate analyses. These findings suggest that squamous differentiation is associated with disease progression, but is not an independent predictor of worse prognosis in patients with upper urinary tract UC.
Although there have been several previous studies that examined a prognostic significance of squamous differentiation in upper urinary tract UC [3,17-20], conclusions about the impact of squamous differentiation on patient prognosis were inconsistent between these reports. Squamous differentiation was found to correlate generally with advanced stage, higher grade tumors, and poor prognosis in univariate analyses [3,17-20]. In some of these studies [17,18,20], these findings also achieved statistical significance in multivariate analyses, whereas other reports [3,19] failed to confirm that. Inconsistent results may be attributable to several factors such as different patient population, method of histological examination, and covariates included in the multivariate model. For example, Lee et al. recently reported that squamous and/or glandular differentiation of upper urinary tract UC independently predicted poor patient outcome [18]. However, this study classified squamous and glandular differentiation into the same group, therefore, the exact prognostic effect of solely squamous differentiation remained unknown.
In the present study, slides of all cases were systematically reviewed by the same pathologist to eliminate the effect of inter-observer variability. This was in contrast to the majority of previous studies in which the presence of squamous differentiation was just retrieved from the pathology reports. Cases of glandular differentiation were not merged into the squamous differentiation group, thus the exact prognostic effect of squamous differentiation could be specifically evaluated. Furthermore, the postoperative follow-up of each patient was recorded in detail, and sufficient prognostic covariates were included in the multivariate model. Although we could not show an independent effect of squamous differentiation on patient outcomes, we believe that it is still important to report the negative findings to avoid “publication bias”. The phenomenon of publication bias happens because studies with null findings have a higher likelihood of being unwritten and unpublished compared to those with significant results [21]. To elucidate the prognostic significance of squamous differentiation in upper urinary tract UC further, larger data sets, preferably in a prospective setting, are warranted.
Prognostic significance of squamous differentiation in UC of the urinary bladder has been well studied. A number of studies reported a significant correlation between squamous differentiation and higher grade and stage of tumors [4-6,9,10,14]. In addition, squamous differentiation was associated with poorer patient outcome in univariate analyses in several studies [7-13]. However, all studies but one [9] failed to achieve statistical significance in multivariate analyses [7,8,10-13]. Thus, squamous differentiation in UC of the urinary bladder is not an independent prognostic factor. Our present findings obtained in patients with UC of the upper urinary tract are in line with these previous reports on squamous differentiation in UC of the urinary bladder.
In conclusion, squamous differentiation in upper urinary tract UC was associated with several adverse prognostic factors including higher grade and stage of tumors. However, it was not an independent predictor of patient outcomes.
Acknowledgements
This work was supported in part by a research grant from the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care (to T. M.) and a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (to T. M.). We are grateful to Kei Sakuma and Harumi Yamamura for their excellent technical support.
Disclosure of conflict of interest
None.
References
- 1.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Bohle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Montiel D, Wakely PE, Hes O, Michal M, Suster S. High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol. 2006;19:494–503. doi: 10.1038/modpathol.3800559. [DOI] [PubMed] [Google Scholar]
- 3.Rink M, Robinson BD, Green DA, Cha EK, Hansen J, Comploj E, Margulis V, Raman JD, Ng CK, Remzi M, Bensalah K, Kabbani W, Haitel A, Rioux-Leclercq N, Guo CC, Chun FK, Kikuchi E, Kassouf W, Sircar K, Sun M, Sonpavde G, Lotan Y, Pycha A, Karakiewicz PI, Scherr DS, Shariat SF. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol. 2012;188:398–404. doi: 10.1016/j.juro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Billis A, Schenka AA, Ramos CC, Carneiro LT, Araujo V. Squamous and/or glandular differentiation in urothelial carcinoma: prevalence and significance in transurethral resections of the bladder. Int Urol Nephrol. 2001;33:631–633. doi: 10.1023/a:1020597611645. [DOI] [PubMed] [Google Scholar]
- 5.Xylinas E, Rink M, Robinson BD, Lotan Y, Babjuk M, Brisuda A, Green DA, Kluth LA, Pycha A, Fradet Y, Faison T, Lee RK, Karakiewicz PI, Zerbib M, Scherr DS, Shariat SF. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy. Eur J Cancer. 2013;49:1889–1897. doi: 10.1016/j.ejca.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Mitra AP, Bartsch CC, Bartsch G Jr, Miranda G, Skinner EC, Daneshmand S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol. 2014;32:117–127. doi: 10.1016/j.urolonc.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Mazzucchelli L, Bacchi M, Studer UE, Markwalder R, Sonntag RW, Kraft R. Invasion depth is the most important prognostic factor for transitional-cell carcinoma in a prospective trial of radical cystectomy and adjuvant chemotherapy. Int J Cancer. 1994;57:15–20. doi: 10.1002/ijc.2910570104. [DOI] [PubMed] [Google Scholar]
- 8.Yang MH, Yen CC, Chen PM, Wang WS, Chang YH, Huang WJ, Fan FS, Chiou TJ, Liu JH, Chen KK. Prognostic-factors-based risk-stratification model for invasive urothelial carcinoma of the urinary bladder in Taiwan. Urology. 2002;59:232–238. doi: 10.1016/s0090-4295(01)01590-4. discussion 238-239. [DOI] [PubMed] [Google Scholar]
- 9.Antunes AA, Nesrallah LJ, Dall’Oglio MF, Maluf CE, Camara C, Leite KR, Srougi M. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol. 2007;33:339–345. doi: 10.1590/s1677-55382007000300006. discussion 346. [DOI] [PubMed] [Google Scholar]
- 10.Erdemir F, Tunc M, Ozcan F, Parlaktas BS, Uluocak N, Kilicaslan I, Gokce O. The effect of squamous and/or glandular differentiation on recurrence, progression and survival in urothelial carcinoma of bladder. Int Urol Nephrol. 2007;39:803–807. doi: 10.1007/s11255-006-9151-0. [DOI] [PubMed] [Google Scholar]
- 11.Frazier HA, Robertson JE, Dodge RK, Paulson DF. The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. 1993;71:3993–4001. doi: 10.1002/1097-0142(19930615)71:12<3993::aid-cncr2820711233>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Honma I, Masumori N, Sato E, Takayanagi A, Takahashi A, Itoh N, Tamagawa M, Sato MA, Tsukamoto T. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004;64:744–748. doi: 10.1016/j.urology.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Jozwicki W, Domaniewski J, Skok Z, Wolski Z, Domanowska E, Jozwicka G. Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis: a mapping study. Urology. 2005;66:1122–1126. doi: 10.1016/j.urology.2005.06.134. [DOI] [PubMed] [Google Scholar]
- 14.Kim SP, Frank I, Cheville JC, Thompson RH, Weight CJ, Thapa P, Boorjian SA. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol. 2012;188:405–409. doi: 10.1016/j.juro.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki Y, Sasaki T, Kawai T, Morikawa T, Matsusaka K, Kunita A, Kume H, Aoki I, Homma Y, Fukayama M. HER2 protein overexpression and gene amplification in upper urinary tract urothelial carcinoma-an analysis of 171 patients. Int J Clin Exp Pathol. 2014;7:699–708. [PMC free article] [PubMed] [Google Scholar]
- 16.van Rhijn BW, Musquera M, Liu L, Vis AN, Zuiverloon TC, van Leenders GJ, Kirkels WJ, Zwarthoff EC, Boeve ER, Jobsis AC, Bapat B, Jewett MA, Zlotta AR, van der Kwast TH. Molecular and clinical support for a four-tiered grading system for bladder cancer based on the WHO 1973 and 2004 classifications. Mod Pathol. 2015;28:695–705. doi: 10.1038/modpathol.2014.154. [DOI] [PubMed] [Google Scholar]
- 17.Jang NY, Kim IA, Byun SS, Lee SE, Kim JS. Patterns of failure and prognostic factors for locoregional recurrence after radical surgery in upper urinary tract transitional cell carcinoma: implications for adjuvant radiotherapy. Urol Int. 2013;90:202–206. doi: 10.1159/000343729. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Moon KC, Jeong CW, Kwak C, Kim HH, Ku JH. Impact of squamous and glandular differentiation on oncologic outcomes in upper and lower tract urothelial carcinoma. PLoS One. 2014;9:e107027. doi: 10.1371/journal.pone.0107027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langner C, Hutterer G, Chromecki T, Rehak P, Zigeuner R. Patterns of invasion and histological growth as prognostic indicators in urothelial carcinoma of the upper urinary tract. Virchows Arch. 2006;448:604–611. doi: 10.1007/s00428-006-0150-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim DS, Lee YH, Cho KS, Cho NH, Chung BH, Hong SJ. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology. 2010;75:328–332. doi: 10.1016/j.urology.2009.07.1350. [DOI] [PubMed] [Google Scholar]
- 21.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]


