Abstract
Background: Legg-Calvé-Perthes Disease (Perthes’ disease) is a childhood hip disorder initiated by ischemic necrosis of the growing femoral head. So far, the etiology and pathogenesis of Perthes’ disease is poorly understood. Materials and methods: Avascular osteonecrosis rat model was established to mimic the pathophysiological changes of femoral head necrosis. The chondrocytes of newborn Sprague-Dawley rats were isolated and cultured in hypoxic and normoxic condition. The expression characteristic of the hypoxia-inducible factor-1 alpha (HIF-1α) was evaluated both in vivo and in vitro models. Vascular endothelial growth factor (VEGF) and apoptotic genes in chondrocytes treated with normoxia and hypoxia were also studied. Results: HIF-1α expression increased greatly after ischemic operation and kept at relative high level in the arthromeningitis stage and declined in the stages of osteonecrosis and reconstruction. The HIF-1α mRNA levels of chondrocytes incubated at hypoxia were significantly higher than the cells treated with normoxia at 24 and 72 hours. Hypoxia inhibited VEGF expression; chondrocytes could oppose this inhibition manifested by the increasing of VEGF mRNA level after 72 hours hypoxia. The expression of apoptotic genes, Casp3, Casp8 and Casp9, elevated in chondrocytes after hypoxia with time differences. Conclusion: Hypoxia might be an etiological factor for femoral head necrosis, HIF-1α, VEGF as well as apoptotic genes participated the pathophysiological process of ischemic osteonecrosis.
Keywords: Legg-Calvé-Perthes Disease, ischemic osteonecrosis, hypoxia-inducible factor-1 alpha (HIF-1α), vascular endothelial growth factor (VEGF), apoptotic genes
Introduction
Legg-Calvé-Perthes Disease (Perthes’ disease) is a childhood hip disorder initiated by ischemic necrosis of the growing femoral head [1,2]. The name originated from American, French and German orthopedic surgeons: Arthur Legg; Jacques Calvé and Georg Perthes [3]. The annual incidence of Perthes’ disease among children under age 15 years ranged from 0.2 per 100,000 to 19.1 per 100,000 [3]. The clinical pathophysiological procedure underwent 3 to 5 years including stages of arthromeningitis; osteonecrosis and collapse; disintegrating and reconstruction [4]. Unfortunately, current clinical treatment on Perthes’ disease is limited and the outcome is poor [5].
The etiology of Perthes’ disease is not known, children with Perthes’ disease have delayed bone age, disproportionate growth, and a mildly shortened stature [6]. Perthes’ disease may be idiopathic, or it may result from a slipped capital femoral epiphysis, trauma, steroid use, sickle-cell crisis, toxic sinusitis, or congenital dislocation of the hip [7]. In short, current clinicians believe that different etiological factors lead to the disruption of blood flow to the femoral head, then all patients share similar pathophysiological procedure of ischemic necrosis of the femoral head, and the outcome varied by individual prognostic factors [8].
The clinical research on Perthes’ disease was impeded by its occult onset, complex changes in disease course, and the rare and dispersive case [9]. A large number of evidences suggested that disruption of blood flow to the femoral head including arterial occlusion is the main risk factor for Perthes’s disease [10]. Also, animal experiments have demonstrated that femoral neck blood supply damaged by operation will lead femoral head necrosis in goat, rat and pig [11]. Thus, animal models were employed widely in Perthe’s disease research instead of clinical patients.
Osteogenesis and angiogenesis are two important and tightly coupled physiological processes during bone development and regeneration [12]. The hypoxia-inducible factor-1 alpha (HIF-1α) pathway has been identified as a key component in this process which participates and controls the mesenchymal cells in the developing stroma and angiogenic signals to recruit new blood vessels into bone [13]. HIF-1 is a DNA binding protein complex contains at least two basic helix-loop-helix PAS-domain (bHLH-PAS) proteins, HIF-1α and aryl hydrocarbon nuclear receptor translocator (ARNT) [14]. Current knowledge show that HIF-1α is the dominant host responser to various hypoxic physiological conditions and will be activated and accumulates rapidly in the host cells [15]. Studies demonstrated that overexpression of HIF-1 in mature osteoblasts profoundly increases angiogenesis and osteogenesis via the action of vascular endothelial growth factor (VEGF) on the endothelial cells [16-18]. Study in porcine ischemic femoral heads model showed that HIF-1α activates Sox9 expression and enhances Sox9-mediated transcriptional activity which suggested a chondroprotective role of HIF-1α [19]. Chondrocytes in the superficial layer of the acute ischemic epiphyseal cartilage showed HIF-1alpha activation and VEGF up-regulation with subsequent revascularization occurring in the cartilage [20].
Above literatures suggested temporal and spatial correlations between HIF-1α and the physiological process of Perthes’ disease, while, the repertoire in the chondrocytes of ischemic femoral head remain to be further evaluated. In this study, femoral head necrosis rat model was established and the possible roles of HIF-1α, VEGF as well as apoptosis were assessed primarily.
Materials and methods
Ethical issue
All animal experiments in this report were conducted in accordance with internationally recognized guidelines for animal experiments (Animal Research: Reporting in vivo Experiments guidelines) and were approved by the Animal Ethics Committee of Children’s Hospital of Fudan University.
Animal experiment
6-week-old Sprague Dawley rats were used in animal experiments. Of vascular deprivation-induced femoral head necrosis group, the blood supply for femoral head was damaged by vasotomy around the neck of femur as reported previously [21]. For the control, sham operation group, right hip was exposed by operation and blood supply for femoral head was not damaged. There are 5 rats in each group, both the experimental and control groups included 6 groups, the rats in these groups will be followed up on day 1, 5, 10, 15, 30 and 60, respectively. The pathophysiological changes in femoral head will be checked by the naked eye, H&E staining and immunohistochemistry.
Histopathology and immunohistochemistry
Arthromeningitis, osteonecrosis and collapse, disintegrating and reconstruction in femoral head were verified by histopathology. Hematoxylin and eosin (H&E) staining was used. The femoral heads were removed and inflated with 4% paraformaldehyde. Then the tissues were embedded in paraffin wax and cut into sections (5 μm thick), which were stained using a standard H&E protocol. To study the level of HIF-1α in the femoral heads of mice, slides were dewaxed, rehydrated and processed for antigen retrieval. Endogenous peroxidase was quenched with 0.03% hydrogen peroxide for 30 min, and nonspecific reaction was blocked with 10% skimmed milk for 30 min at room temperature, sections were then incubated with antibody against HIF-1α (Abcam, Cambridge, MA), at 4°C for 2 hours. After washing with phosphate buffered saline buffer for 3 times, the slides were incubated with horseradish peroxidase-labeled secondary antibody (VECTOR LABORATORIES, Burlingame, CA) at room temperature for 20 min. the HIF-1α expression and localization were visualized by diaminobenzidine (VECTOR LABORATORIES, Burlingame, CA) reaction for 5 min.
Primary cell culture and hypoxia treatment
To study the pathophysiological changes and the role of chondrocyte in hypoxia, the chondrocytes of newborn Sprague Dawley rats were isolated and cultured following the protocol of Gosset et al [22]. Cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) with high glucose, supplemented with 10% fetal bovine serum, 100 U of penicillin, and 100 µg of streptomycin per ml of medium. For hypoxic treatment, cells were exposed to 10% CO2 and 0.5% O2 balanced with nitrogen in a Sanyo three gas incubator (Sanyo, Japan).
Gene expression analysis
To study the gene expression profile in pathophysiological procedure of Perthes’ disease model, real-time reverse transcript PCR analysis was used. Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed to cDNA using the Two-Step RT-PCR Clone Kit (Biovisualab, Shanghai, China) according to the manufacturer’s protocol. The genes and their corresponding primers were: ACTB: (Forward: 5’CGTAAAGACCTCTATGCCAACA3’; Reverse: 5’GGAGGAGCAATGATCTTGATCT3’). Cycs: (Forward: 5’CACAGATGCCAACAAGAACAAAGG3’; Reverse: 5’AGGGATGTACTTTTTGGGATTTTCC3’). Casp9: (Forward: 5’GCGACATGATCGAGGATATTCAGC3’; Reverse: 5’TGCCTCCCTCGAGTCTCAAGATC3’). Casp8: (Forward: 5’ACGTCTGGGCAACGAAGAACTG3’; Reverse: 5’GCACCAGGACATCATTGATGGAC3’). Casp3: (Forward: 5’TGTATGCTTACTCTACCGCACCCG3’; Reverse: 5’GCGCAAAGTGACTGGATGAACC3’). HIF-1: (Forward: 5’AGCTTCTGTTATGAGGCTCACCATC3’; Reverse: 5’TCTTCAATGTCAAGATCACCAGCAC3’). VEGF: (Forward: 5’AAATCCTGGAGCGTTCACTGTGAG3’; Reverse: 5’AACATTTACACGTCTGCGGATCTTG3’).
Quantitative PCR was performed using a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA) and the GREAT Real-Time SYBR PCR Kit (Biovisualab, Shanghai, China) following the manufacturer’s instructions. The relative mRNA expression level was calculated using the 2-ΔΔCT method with the CT values normalized using β-actin as an internal control [23,24].
Statistical analyses
An analysis of variance (ANOVA) was used to determine the differences between all groups. Pairs of groups were compared using Student’s t-test. P-values < 0.05 were considered to indicate statistical significance.
Results
Avascular osteonecrosis rat model mimicked the pathophysiological changes of femoral head necrosis
As showed in Figure 1, the femoral head of rat displayed pathophysiological changes of femoral head necrosis, on day 5 and day 10, cartilage layer is hypertrophic and loose which represented the arthromeningitis; on day 15 and day 30, femoral head showed osteonecrosis and collapse; on day 60, femoral head displayed disintegrating and reconstruction (Figure 1, upper), in conclusion, histopa thological assessment showed that avascular osteonecrosis rat model mimicked the pathophysiological course of femoral head necrosis.
Figure 1.
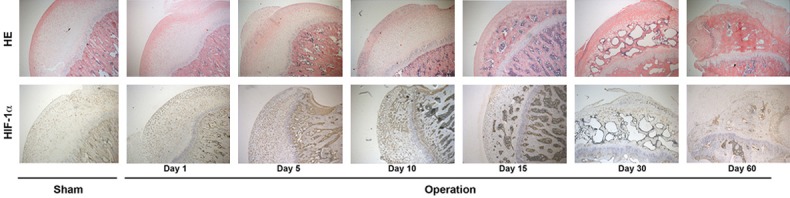
Histopathology assessment on caput femoris. Upper, Hematoxylin and eosin (H&E) staining was performed on the avascular osteonecrosis rat model mimicked the pathophysiological changes of femoral head necrosis; Lower, immunohistochemical staining using anti-HIF-1α antibody.
HIF-1α expression in the femoral head following ischemic injury
Since HIF-1α is the dominant response factor to ischemic injury, HIF-1α expression in the femoral head following ischemic injury was evaluated. As showed in Figure 1, the expression of HIF-1α increased greatly after operation and kept at relative high expression level from day 1 to day 10, which suggests that HIF-1α expression was activated in the pathophysiological process of arthromeningitis (Figure 1, bottom). On day 15 and day 30, immunohistochemical assessment showed that the HIF-1α expression fall back to relative low level, which suggests that HIF-1α expression declined in the pathophysiological process of osteonecrosis and collapse (Figure 1, bottom). As expected, on day 60, HIF-1α expression also kept at low level in the pathophysiological process of disintegrating and reconstruction (Figure 1, lower).
HIF-1α expression in the chondrocytes
Above in vivo data showed the expression characteristics of HIF-1α, which is HIF-1α expression elevated in femoral head at early stage of ischemic injury. Current biotechnology is hard to know the cellular and molecular pathophysiological change in detail, while, early cellular and molecular pathophysiological change is important to the development and prognosis of pathophysiology. To learn the HIF-1α expression in chondrocytes at early stage of hypoxia, the chondrocytes were isolated. The chondrocytes were then cultured in hypoxic and normoxic incubators, HIF-1α mRNA level was determined. As showed in Table 1, the 2-ΔΔCT of HIF-1α in normoxic and hypoxic conditions at 0, 8, 24 and 72 hours were 1.00 (0.83~1.21), 0.39 (0.31~0.49), 0.55 (0.46~0.66) and 0.06 (0.05~0.07); and 0.43 (0.36~0.52), 0.75 (0.63~0.89), 8.36 (5.23~13.37) and 6.09 (5.11~7.25), respectively. The HIF-1α mRNA levels of chondrocytes incubated at hypoxia were significantly higher than the cells treated with normoxia at 24 and 72 hours (Figure 2A).
Table 1.
Expression of VEGF and HIF-1
| Time course (hours) | VEGF | HIF-1α | ||
|---|---|---|---|---|
|
|
|
|||
| Normoxia | Hypoxia | Normoxia | Hypoxia | |
| 0 | 1.00 (0.82~1.22)* | 0.00 (0.00~0.00) | 1.00 (0.83~1.21) | 0.43 (0.36~0.52) |
| 8 | 0.15 (0.13~0.19) | 0.06 (0.05~0.08) | 0.39 (0.31~0.49) | 0.75 (0.63~0.89) |
| 24 | 0.94 (0.80~1.11)* | 0.16 (0.13~0.19) | 0.55 (0.46~0.66) | 8.36 (5.23~13.37)* |
| 72 | 0.05 (0.04~0.06) | 0.20 (0.17~0.24)* | 0.06 (0.05~0.07) | 6.09 (5.11~7.25)* |
The genes expression levels were calcaulted by the 2-ΔΔCT method. Data were presented as 2-ΔΔCT and range.
Indicated significance (p < 0.05) when compared with normoxia or hypoxia.
VEGFA, vascular endothelial growth factor A; HIF-1, hypoxia-inducible factor-1.
Figure 2.
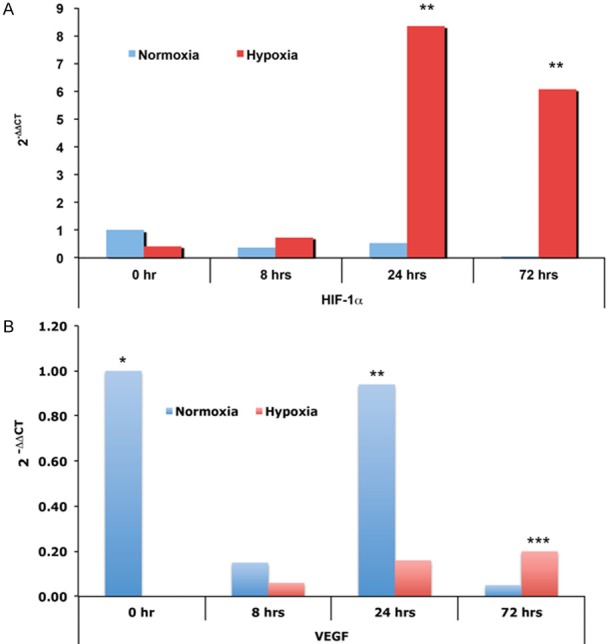
Expression of HIF-1α and VEGF. Chondrocytes were isolated from newborn rat, cultured in either normoxic or hypoxic incubators. The expression of HIF-1α and VEGF were checked using qRT-PCR. A. Expression of HIF-1α, *indicated significance (P < 0.05) when compared with control, the 2-ΔΔCT and ranges were showed in Table 1. B. Expression of VEGF, *indicated significance (P < 0.05) when compared with normoxia or hypoxia groups, the 2-ΔΔCT and ranges were showed in Table 1.
VEGF expression in the chondrocytes
Since angiogenesis and osteogenesis are tightly coupled during bone development and regeneration and VEGF is a well-characterized proangiogenic factor [12], the expression of VEGF was then studied by real-time RT-PCR analysis, as showed in Table 1, the 2-ΔΔCT of VEGF in normoxic and hypoxic conditions at 0, 8, 24 and 72 hours were 1.00 (0.82~1.22), 0.15 (0.13~0.19), 0.94 (0.80~1.11) and 0.05 (0.04~0.06); and 0.00 (0.00~0.00), 0.06 (0.05~0.08), 0.16 (0.13~0.19) and 0.20 (0.17~0.24), respectively. The VEGF mRNA levels were difficult to understand, chondrocytes in normoxic condition showed irregular VEGF mRNA levels, VEGF mRNA levels of chondrocytes in hypoxic condition showed increasing tendency (Table 1; Figure 2B). At the initial stage (0 hour) and 24 hours incubation, VEGF levels of normoxia are significantly higher in the chondrocytes incubated in normoxic condition than that in hypoxic condition (Table 1; Figure 2B). At 72 hours, VEGF level increased and is significantly higher in the chondrocytes incubated in hypoxic condition than that in normoxic condition (Table 1; Figure 2B).
Apoptotic genes expression
The apoptosis was presumed to participate in the ischemic injury in many diseases, to study whether and how the apoptosis involved in ischemic osteonecrosis, the expression of CYCS, Casp3, Casp8 and Casp9 in chondrocytes in hypoxic and normoxic conditions were also studied. As showed in Table 2, all genes displayed expression fluctuation along with time course, although CYCS gene displayed expression difference between normoxia and hypoxia at all time points, there is no significance was observed. Of Casp3 and Casp8, the mRNA levels in the chondrocytes incubated in hypoxic condition were significantly higher than that in normoxic condition at 24 and 72 hours, which might suggest that Casp3 and Casp8 involved the early response of ischemic osteonecrosis (Table 2). Of Casp9, its mRNA level was significantly lower in hypoxic chondrocytes than that in normoxic chondrocytes at 0 hour, conversely, its mRNA level was significantly higher in hypoxic chondrocytes than that in normoxic chondrocytes at 72 hour (Table 2).
Table 2.
Expression of apoptotic genes
| Time course (hours) | Condition | Genes | |||
|---|---|---|---|---|---|
|
| |||||
| CYCS | Casp3 | Casp8 | Casp9 | ||
| 0 | Normoxia | 1.00 (0.58~1.73) | 1.00 (0.83~1.20) | 1.00 (0.83~1.22) | 1.00 (0.82~1.22) |
| Hypoxia | 0.22 (0.17~0.27) | 0.15 (0.12~0.17) | 0.33 (0.27~0.40) | 0.03 (0.03~0.04)* | |
| 8 | Normoxia | 0.36 (0.31~0.42) | 0.29 (0.25~0.34) | 0.35 (0.28~0.44) | 0.22 (0.19~0.26) |
| Hypoxia | 0.20 (0.17~0.23) | 0.46 (0.39~0.54) | 0.21 (0.18~0.24) | 0.03 (0.03~0.04) | |
| 24 | Normoxia | 0.38 (0.31~0.46) | 1.12 (0.95~1.31) | 0.32 (0.27~0.37) | 0.62 (0.53~0.74) |
| Hypoxia | 0.41 (0.36~0.47) | 6.15 (4.08~9.26)* | 4.43 (3.92~5.00)* | 0.63 (0.52~0.76) | |
| 72 | Normoxia | 0.25 (0.18~0.36) | 0.29 (0.25~0.34) | 0.31 (0.26~0.38) | 0.10 (0.08~0.13) |
| Hypoxia | 1.02 (0.86~1.21) | 2.47 (2.14~2.85)* | 3.10 (2.52~3.81)* | 1.42 (1.16~1.75)* | |
The genes expression levels were calcaulted by the 2-ΔΔCT method. Data were presented as 2-ΔΔCT and range.
Indicated significance (p < 0.05) when compared with normoxia or hypoxia.
CYCS, Cytochrome C, Somatic; Casp, caspase.
Discussion
Perthes’ disease is a big challenge to children’s health, while, the etiology and pathogenesis remain unclear [25], however, some studies insight into the pathogenesis of a femoral head deformity after ischemic necrosis have been gained [25]. In this study, we established an avascular osteonecrosis model in rat, histopathological assessment showed that rat model represented the pathophysiological course of femoral head necrosis, the rat model showed arthromeningitis, osteonecrosis and collapse, disintegrating and reconstruction within 60 days observation. In vivo data showed that HIF-1α expression elevated in femoral head at early stage of ischemic injury and kept at low level in the pathophysiological process of disintegrating and reconstruction. Although HIF-1α is known to be elevated during hypoxia through a regulated proteolytic process [12], the dynamic change of HIF-1α in whole pathophysiological process of avascular osteonecrosis model is rare. Our in vitro experiments showed HIF-1α expression in chondrocytes elevated significantly 24 hours after hypoxic treatment, and HIF-1α level kept higher compare to normoxic incubated chondrocytes up to 72 hours. Although HIF-1α level elevated in culture cells in hypoxic treatment was also reported by other researches [26,27], there are two main differences between our study and other reports, previous experiments are mainly performed in cell line strains, in our study, the chondrocytes were primarily isolated from newborn rat; pre-existing reports showed that the HIF-1α will increased quickly within hour when triggered by hypoxia [26-28], our data showed that the HIF-1α increased a bit of a lag which might represented the characteristic of isolated chondrocytes. The VEGF could be released by hypertrophic chondrocytes and plays a critical role in angiogenesis [29], our data showed that chondrocytes did produce VEGF, while hypoxia restrained the expression of VEGF and the restraint was broken through to a certain extent along with the hypoxic time increasing, which suggests that ischemic injury also inhibits VEGF producing in chondrocytes and hence contributed to the pathogenesis of ischemic femoral head necrosis. Further apoptosis relative genes analysis showed that Casp3, Casp8 and Casp9 participated in the hypoxic injury of chondrocytes.
Hydroxylation of HIF-1α requires molecular oxygen, and the hydroxylation is inhibited by hypoxia [12]. Under the condition of hypoxia, the HIF-1α accumulates in the cytoplasm and then translocates to the nucleus, where it dimerizes with the HIF-1β. The dimer of HIF-1α and HIF-1β then binds to a highly conserved hypoxia-response element within promoters of a cluster of hypoxia-responsive genes [30]. Genes containing functional hypoxia-response element encode proteins involved in angiogenesis including VEGF and endothelin-1 [31]. In this study, our immunohistochemical staining using anti-HIF-1α antibody showed that the HIF-1α concentrated in the nucleus of the chondrocytes in cartilage (Figure 1), which suggested that HIF-1α pathway was activated and played its function in early stage of avascular osteonecrosis. Also, our following VEGF data showed that hypoxia inhibited VEGF expression, chondrocytes could oppose this inhibition manifested by the increasing of VEGF mRNA level after 72 hours hypoxia.
Many studies demonstrated that HIF-1α protein keeps as very lower level in cells at normoxia condition [12,18-20], our in vivo data also confirmed this phenomenon, HIF-1α protein is relative lower in the tissue without ischemic injury and in the stages of osteonecrosis and reconstruction in rat with avascular osteonecrosis.
This is a primary report focused on the HIF-1α, VEGF and apoptotic genes in mimicking femoral head necrosis, the expressions of VEGF and apoptotic genes were only examined in mRNA level, no protein data of these genes were provided. We are devoting ourselves to complete current research. In conclusion, our primary data suggested that hypoxia might be a main etiological factor for ischemic femoral head necrosis and HIF-1α, VEGF as well as apoptotic genes participated in the pathophysiological process of ischemic osteonecrosis temporally and spatially.
Disclosure of conflict of interest
None.
References
- 1.Kim HK. Legg-Calvé-Perthes disease. J Am Acad Orthop Surg. 2010;18:676–86. doi: 10.5435/00124635-201011000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Molloy MK, MacMahon B. Incidence of Legg-Perthes disease (osteochondritis deformans) N Engl J Med. 1966;275:988–90. doi: 10.1056/NEJM196611032751804. [DOI] [PubMed] [Google Scholar]
- 3.Perry DC, Machin DM, Pope D, Bruce CE, Dangerfield P, Platt MJ, Hall AJ. Racial and geographic factors in the incidence of Legg-Calvé-Perthes’ disease: a systematic review. Am J Epidemiol. 2012;175:159–66. doi: 10.1093/aje/kwr293. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Kamimura M, Mukaiyama K, Ikegami S, Uchiyama S, Kato H. A Case with Atypical Clinical Course Diagnosed as Osteoarthritis, Osteonecrosis, Subchondral Insufficiency Fracture, or Rapidly Destructive Coxopathy. Open Rheumatol J. 2014;8:20–23. doi: 10.2174/1874312901408010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph B. Management of Perthes’ disease. Indian J Orthop. 2015;49:10–16. doi: 10.4103/0019-5413.143906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45:87–97. doi: 10.1016/j.ocl.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JC, Lam TP, Ng BK. Prognosis and prognostic factors of Legg-Calve-Perthes disease. J Pediatr Orthop. 2011;31:S147–51. doi: 10.1097/BPO.0b013e318223b470. [DOI] [PubMed] [Google Scholar]
- 8.Copeliovitch L. Femoral varus osteotomy in Legg-Calve-Perthes disease. J Pediatr Orthop. 2011;31:S189–91. doi: 10.1097/BPO.0b013e318223b55c. [DOI] [PubMed] [Google Scholar]
- 9.Froberg L, Christensen F, Pedersen NW, Overgaard S. Long-term follow-up of a patient cohort with Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2011;20:273–7. doi: 10.1097/BPB.0b013e3283474268. [DOI] [PubMed] [Google Scholar]
- 10.Kim HK, Herring JA. Pathophysiology, classifications, and natural history of Perthes disease. Orthop Clin North Am. 2011;42:285–95. doi: 10.1016/j.ocl.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40:345–54. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 12.Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322–6. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito T, Kawaguchi H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage. 2010;18:1552–6. doi: 10.1016/j.joca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–28. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudas LJ, Fu L, Minton DR, Mongan NP, Nanus DM. The role of HIF1α in renal cell carcinoma tumorigenesis. J Mol Med (Berl) 2014;92:825–36. doi: 10.1007/s00109-014-1180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686–91. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–26. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towler DA. Vascular biology and bone formation: hints from HIF. J Clin Invest. 2007;117:1477–80. doi: 10.1172/JCI32518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Yang F, Cornelia R, Tang W, Swisher S, Kim H. Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone. 2011;48:507–13. doi: 10.1016/j.bone.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Bian H, Aya-ay J, Garces A, Morgan EF, Gilbert SR. Hypoxia and HIF-1alpha expression in the epiphyseal cartilage following ischemic injury to the immature femoral head. Bone. 2009;45:280–8. doi: 10.1016/j.bone.2009.03.665. [DOI] [PubMed] [Google Scholar]
- 21.Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. Int J Exp Pathol. 1998;79:173–81. doi: 10.1046/j.1365-2613.1998.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–60. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Kim HK. Legg-Calve-Perthes disease: etiology, pathogenesis, and biology. J Pediatr Orthop. 2011;31:S141–6. doi: 10.1097/BPO.0b013e318223b4bd. [DOI] [PubMed] [Google Scholar]
- 26.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 27.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92:2260–8. [PubMed] [Google Scholar]
- 28.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D’Amore PA, Olsen BR. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 30.Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science. 2002;295:807–8. doi: 10.1126/science.1069825. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]


