Abstract
Recent evidence suggested a positive correlation between environmental estrogens (EEs) and high incidence of abnormalities in male urogenital system. EEs are known to cause the abnormalities of testes development and testicular descent. Diethylstilbestrol (DES) is a nonsteroidal synthetic estrogen that disrupts the morphology and proliferation of gubernacular cells, and its nongenomic effects on gubernaculum testis cells may be mediated by G protein-coupled estrogen receptor (GPER). In this study, we detected the expression of GPER in mouse gubernacular testis and investigated the effects of DES on the expression of GPER in gubernaculum testis cells. RT-PCR analysis revealed that GPER mRNA was expressed in the gubernaculum. GPER protein was detected in the parenchymal cells of the gubernaculum early in development. Furthermore, we demonstrate that GPER inhibitor G15 relieved DES-induced inhibition of GPER expression in gubernaculum testis cell, but ER inhibitor ICI 182780 had the converse effects on DES-induced inhibition of GPER expression in these cells. These data suggest that the effects of DES on mouse gubernaculum testis cells are mediated at least partially by the regulation of GPER expression.
Keywords: GPER, DES, EEs, gubernaculums testis
Introduction
The relationship between environmental pollution and adverse trends in male reproductive health is still one serious problem worldwide, especially the environmental estrogens (EEs) [1]. Increasing incidence of testicular cancer, low semen quality, high incidence of undescended testis and hypospadias have been postulated as the symptoms of one underlying entity, testicular dysgenesis syndrome (TDS) [2,3]. Therefore, it is urgent to explore the effect of EEs on male productive system, but the underlying mechanism remains largely elusive [4,5].
Testicular descent indicates the migration of the testis from the abdominal cavity to the scrotum and is essential for proper functioning of the testis. Testicular descent is an integral part of the male differentiation process. The process of the normal testicular descent is generally subdivided into two phases: tansabdominal and inguinoscrotal migration phases. The migration involves the regression of the cranial suspensory ligament and the development of the gubernaculums. Several studies indicate that hormonal effects on the gubernaculum may mediate testicular descent [6-8]. It is known that estrogen exerts its effects through ER. Mouse testis gubernaculum showed growth retardation, atrophy, and even cryptorchid symptoms in ERα knockout mice (ERKO) [9]. However, recent data suggest that G protein-coupled estrogen receptor (GPER) is implicated in the nongenomic effects of EEs [10]. EEs were shown to activate GPER-cAMP/protein kinase A (PKA)-CREB signaling pathway in seminoma cells [11].
Diethylstilbestrol (DES) is a nonsteroidal synthetic estrogen, and exposure to DES leads to high rate of reproductive abnormalities in males, such as hypospadia, testicular hypoplasia, epididymal cysts and cryptorchidism [12]. Recently, we isolated mouse gubernaculum testis cells and showed that DES impaired the morphology as well as the proliferation and contractility of gubernaculum testis cells [13], and the nongenomic effects may be mediated by GPER-protein kinase A-ERK-CREB signaling pathway [14]. Therefore, we propose that GPER is another important estrogen receptor to mediate nongenomic effects. In this study, we detected the expression of GPER in mouse gubernacular testis and investigated the effects of DES on the expression of GPER in gubernaculum testis cells.
Materials and methods
Mouse gubernaculums testis
Kunming mice were maintained at the Animal Research Laboratory in the Medical College of Shantou University. These mice were kept under controlled conditions at a fixed temperature and under a 12-h night/dark cycle, and with free access to water and laboratory chow. After mating, gestational mice were housed individually. The day of vaginal plug was designated day 0 (GD0) and the day of parturition was designated day 0 (PD0). Thereafter, the litter males and mother were kept together until the young were 3-5 days old. Mice were killed by decapitation in different stages (GD17, GD19, PD0, PD3, PD7, PD14, PD21) and gubernaculum tissues were collected for following experiments.
Primary cell culture and treatment
Primary gubernaculums testis cells were isolated from mice as described previously [13]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% charcoal dextran-treated fetal bovine serum (FBS) under 5% CO2 and 95% air at 37°C. Subcultured cells were randomly divided into different groups treated with different concentrations of DES and/or 1 μM GPER antagonist G15, or 1 nM ER antagonist ICI 182780.
Immunohistochemistry
Gubernaculum cells were fixed in 4% paraformaldehyde in PBS for 15 min, washed in 0.1 M PBS for 15 min three times, and permeabilized in 0.5% Triton X-100 in PBS for 15 min. Endogenous peroxide activity was blocked by treating with 3% hydrogen peroxide for 30 min. The cells were supported on coverslips by grease-pencil markings and immersed in 5% BSA for 30 min to block nonspecific binding sites before incubation with rabbit polyclonal antibody against GPER (1:100, Santa Cruz Biotech, Santa Cruz, CA, USA) in PBS at 4°C overnight. Slides were rinsed in PBS for 15 min followed by incubation with the appropriate biotinylated goat anti-rabbit IgG for 30 min at room temperature. Then the slides were incubated with streptavidin-biotin-(peroxidase) complex at room temperature for 20 min followed by staining with a diaminobenzidine chromogenic kit. The stained cells were visualized under a microscope.
Immunoelectron microscopy
Gubernaculum testis was removed from PD3 mouse and fixed in 0.5 ml of fixation solution (pH 7.4): 4% paraformaldehyde, 0.1% glutaraldehyde 0.3% trinitrophenol, 0.1 M sodium dimethylarsenate. For proper fixation, the minimum incubation time was 4 h at 4°C. The tissues were washed with PBS, dehydrated with series of ethanol dilution, embed with LR White resin at 60°C until polymerization was completed (usually 2-3 days). The samples were cut into sections and place onto Formvar and carbon-coated nickel grids. After blocking with 20% goat serum for 1 h, the grids were incubated with rabbit polyclonal antibodies for GPER diluted at 1:50 in blocking buffer for 2 h at room temperature, followed by the incubation with anti-Rabbit IgG conjugated with 10 nm colloidal gold particles (Sigma, USA) for 1 h at room temperature. The grids were washed and dried on filter paper and observed under electron transmission microscope.
Real-time PCR
Total RNA was extracted from mouse gubernaculums testis using trizol and was used to synthesize cDNA. Specifically, 1 μL of 1 g/L RNA was mixed with 4 μL of 5 × Prime Script buffer, 1 μL RT enzyme mix I, 1 μL of oligo dT primer, 1 μL of random 6 mers, and 13 μL of DEPC water, and then incubated at 37°C for 15 min followed by 85°C for 5 s to inactivate reverse transcriptase. The cDNA was then used as the template to amplify the target genes using real-time PCR in a 20 μL system containing 2 μL of cDNA, 10 μL of 2 × SYBR Premix, 0.4 μL of Ex Taq, 0.4 μL of forward primer, 0.4 μL of reverse primer, 0.4 μL of 50 × ROX reference dye II, and 6.8 μL of dH2O under the following conditions: 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 55°C for 30 s, and 72°C for 30 s. The primers were as follows: GPER forward primer, 5’-CCCAGATGCCACTCCTAACT-3’ and reverse primer, 5’-ACCCAGTCTCCTTCCACCTT-3’; GAPDH forward primer, 5’-GGAAGGGCTCATGACCACAGT-3’, and reverse primer, 5’-GGAAGGCCATGCCAGTGA-3’. Relative expression levels of GPER and GAPDH were analyzed using image analysis software (BandScan 5.0).
Western blot analysis
Gubernaculums testis tissues and cells cultured under indicated conditions were harvested and lysed as described previously [13]. The lysates were separated by sodium dodecysulfate-polyacrylamide gel electrophoresis and transferred into the membranes, which were incubated with rabbit polyclonal antibody against GPER (1:100) or GAPDH (1:1,000) at 4°C overnight. The membranes were washed three times with TBST and incubated with peroxidase conjugated goat anti-rabbit secondary antibody (1:1,000; Santa Cruz Biotech, Santa Cruz, CA, USA) for 1 h at room temperature. Finally, the membranes were washed three times with TBST and visualized using Western Blotting Luminol Reagent (Santa Cruz Biotech, Santa Cruz, CA, USA) according to the manufacturer’s instruction.
Statistical analysis
Data were presented as mean ± SEM and analyzed by Student t and X2 tests using SPSS 13.0 software (SPSS, Chicago, IL). Differences were considered significant at P<0.05.
Results
Localization of GPER in gubernaculum testis
First we examined the localization of GPER in mouse gubernaculums testis. Immunohistochemistry analysis showed that GPER staining was strong both on the membrane and in the cytoplasm and the positive cells were located at the loosened mesenchymal part of gubernaculum testis bulb (Figure 1A). Moreover, ultrastructural analysis of subcellular localization of GPER protein by immunoelectron microscopy showed that GPER was mainly localized in the mesh-like network of the cytoplasm, which may refer to rough endoplasmic reticulum (Figure 1B). The expression of GPER mRNA was detected in the gubernaculums testis by RT-PCR (Figure 1C).
Figure 1.
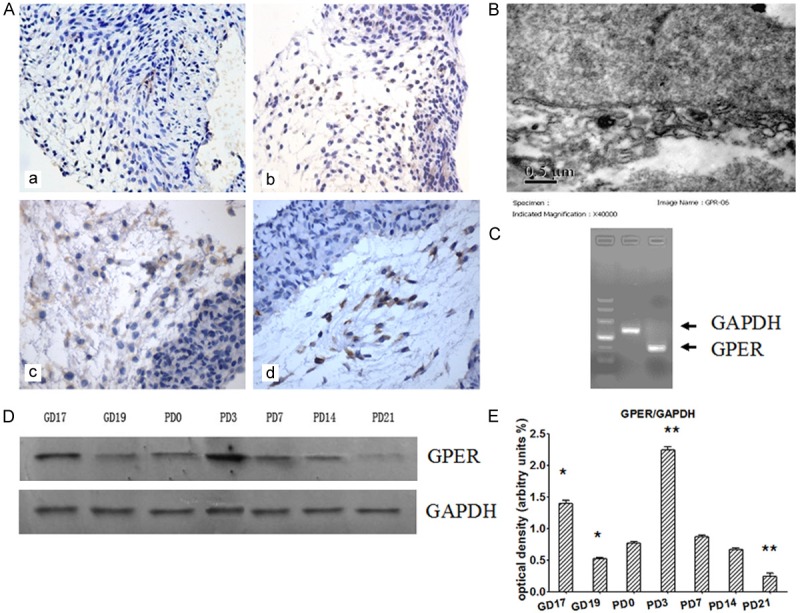
Expression of GPER in mouse gubernaculums testis. A. Immunohistochemical staining of GPER in gubernaculums testis. a. GD19, b. PD0, c. PD3, d. PD7 (× 400). B. Immunoelectron microscopy showing colloidal gold particles positively stained by GPER antibody. C. RT-PCR analysis of GPER and GAPDH expression in gubernaculums testis. D. Western blot analysis of GPER protein expression in mouse gubernaculums testis at different stages of testis descent. E. Densitometry analysis of the blots shown in D (n=3). GAPDH served as loading control. *P<0.05, **P<0.01 versus PD0 control.
Western blot analysis showed that GPER was expressed in gubernaculum testis during different stages of testicular descent, including GD17, GD19, PD0, PD3, PD7, PD14 and PD21 (Figure 1D). GPER protein level in PD3 was increased evidently and decreased in PD21. Compared with PD0, Western blot analysis showed that GPER protein level was increased in GD17 and PD3 (P<0.01), and decreased in GD19 and PD21 (P<0.5) (Figure 1E).
DES affects GEPR expression in gubernaculum testis cells
To confirm that the rapid effects of DES are mediated by GPER in gubernaculum testis cells, we detected the expression of GPER in gubernaculum testis cells treated with DES. Western blot analysis showed that GPER protein level in gubernaculum testis was inhibited by DES. Next we pretreated cells with ER inhibitor ICI 182780 or GPER inhibitor G15 before the cells were treated with DES. Compared with the DES group, pretreatment with ICI 182780 increased the level of GPER, especially at the time point of 10 min (P<0.01), but after the time point of 1 h the level of GPER was decreased obviously (P<0.05). In contrast, compared with the DES group, pretreatment with G15 decreased the level of GPER at different times (P<0.05) (Figure 2). These results suggest that DES affects GEPR expression in gubernaculum testis.
Figure 2.
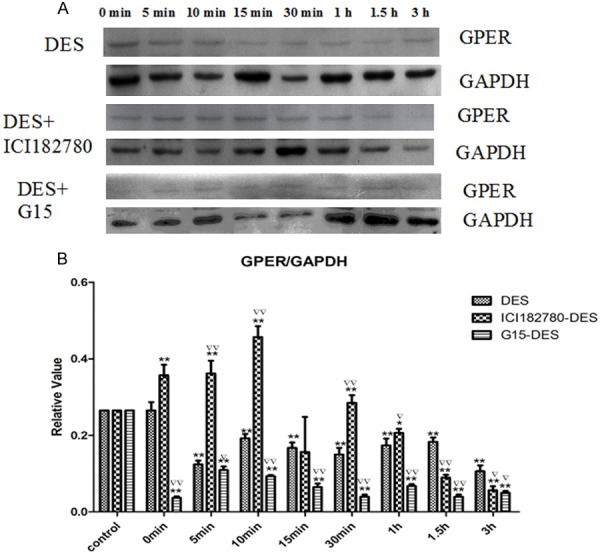
Western blot analysis of the expression of GPER in mouse gubernaculum testis cells. A. Cells were treated with DES or additionally pretreated with ICI 182780 or G15 as indicated, and GPER expression was detected by Western blot analysis. B. Densitometry analysis of the blots shown in A (n=3). *P<0.05, **P<0.01 versus the corresponding control in the left, ▽▽P<0.01, ▽P<0.05 versus DES in the same time point group.
Discussion
Environment estrogens (EEs) is one major type of environmental endocrine disruptors (EDs). Epidemiological evidence suggests that exposure to EEs is associated with deteriorating male reproductive function, such as male infertility and/or lower semen quality which are termed TDS [15]. However, the mechanism by which EEs affect the male reproduction is unclear.
EEs have been known to perturb the male reproductive system, and were thought to directly affect the testes. However, regions other than testis, such as gubernaculums have began to attract attention, since they influence testis development and testicular descent, and are involved in abnormalities of the male reproductive system [16]. We focused on the effects of EEs on gubernaculums testis for years, and had established experimental models in vivo (embryonic exposure to EEs) and in vitro (gubernaculums testis cell culture) to investigate the effects and mechanisms of EEs on the development of gubernaculums testis [13,14].
As the main receptors of estrogen, ER plays important role to mediate the biological effects of estrogens in gubernaculum testis because testis gubernaculum grew slowly, were atrophied, and even developed cryptorchid symptoms in Era knockout mice [9]. In addition, Era and Erg mRNA and proteins have been detected in rat gubernaculum testis [17]. Furthermore, recent data showed that GPER was expressed in both nonneoplastic and neoplastic human testes [18]. In agreement with these previous studies, using immunohistochemistry analysis we showed that GPER was located at the loosened mesenchymal part of gubernaculum testis bulb, and was stained both on the membrane and in the cytoplasm. RT-PCR proved that GPER mRNA was expressed. Western blot analysis showed that GPER was expressed in gubernaculum testis during different stages of testicular descent. According to the process of the normal testicular descent, the expression of GPER increased gradually in intansbdominal phase and decreased in inguinoscrotal migration phases. These data suggest that GPER is potentially involved in mediating the rapid response of gubernaculum testis cells to EEs.
DES is a non-steroidal synthetic estrogen, which has been used as estrogen therapy to prevent miscarriages and other complications of pregnancy. Recent data demonstrated the occurrence of long-term effects after developmental exposure to DES, and the possibility for adverse effects to be transmitted to subsequent generations [19]. Consequently, DES became the first and classic example of an in utero estrogenic toxicant in humans and animals. To provide evidence that GPER mediates the rapid effects of EEs, we treated gubernaculums testis cells with DES. We found that DES inhibited the expression of GPER in gubernaculum testis cells. In addition, GPER inhibitor G15 relieved DES-induced inhibition of GPER expression in gubernaculum testis cell, but ER inhibitor ICI 182780 had the converse effects on DES-induced inhibition of GPER expression in these cells. Taken together, our data suggest that the rapid effects of DES on gubernaculums testis cells are unlikely to be mediated by ER but instead mediated by GPER.
In summary, in this study we demonstrated that GPER is expressed in gubernaculum testis at tissue, cellular, and molecular levels, and DES affects the expression of GPER in the cultured gubernaculums testis cells. The nongenetic effects of DES on gubernaculums testis are unlikely to be mediated by ER but instead mediated by GPER partly. Therefore, our data provide new insight into the role of EEs in the etiology of male reproductive system and will help develop better approaches for the prevention and therapy of male reproductive malformation.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (81170555, 81341099).
Disclosure of conflict of interest
None.
References
- 1.Schultz MM, Minarik TA, Martinovic-Weigelt D, Curran EM, Bartell SE, Schoenfuss HL. Environmental estrogens in an urban aquatic ecosystem: II. Biological effects. Environ Int. 2013;61:138–149. doi: 10.1016/j.envint.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 3.Jiang XW, Li JH, Huang TH, Deng WD. Effect of prenatal exposure to diethylstilbestrol on gubernacular development in fetal male mice. Asian J Androl. 2004;6:325–329. [PubMed] [Google Scholar]
- 4.Nordkap L, Joensen UN, Blomberg Jensen M, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 5.Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol. 2010;88:910–919. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- 6.Barthold JS, Robbins A, Wang Y, Pugarelli J, Mateson A, Anand-Ivell R, Ivell R, McCahan SM, Akins RE Jr. Cryptorchidism in the orl rat is associated with muscle patterning defects in the fetal gubernaculum and altered hormonal signaling. Biol Reprod. 2014;91:41. doi: 10.1095/biolreprod.114.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation TR, Balic A, Southwell BR, Newgreen DF, Hutson JM. The hormonal control of testicular descent. Pediatr Endocrinol Rev. 2009;7:22–31. [PubMed] [Google Scholar]
- 8.Toppari J, Virtanen H, Skakkebaek NE, Main KM. Environmental effects on hormonal regulation of testicular descent. J Steroid Biochem Mol Biol. 2006;102:184–186. doi: 10.1016/j.jsbmb.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JE, Washburn T, Eddy EM, Korach KS, Temelcos C, Hutson JM. Early development of the gubernaculum and cremaster sac in estrogen receptor knockout mice. Urol Res. 2001;29:163–167. doi: 10.1007/s002400100180. [DOI] [PubMed] [Google Scholar]
- 10.Meyer MR, Haas E, Prossnitz ER, Barton M. Nongenomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallacides A, Chesnel A, Ajj H, Chillet M, Flament S, Dumond H. Estrogens promote proliferation of the seminoma-like TCam-2 cell line through a GPER-dependent ERa36 induction. Mol Cell Endocrinol. 2012;350:61–71. doi: 10.1016/j.mce.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Newbold RR. Developmental exposure to endocrine-disrupting chemicals programs for reproductive tract alterations and obesity later in life. Am J Clin Nutr. 2011;94(Suppl 6):1939s–1942s. doi: 10.3945/ajcn.110.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Li JH, Ma L, Huang TH, Jiang XW. Diethylstilbestrol impairs the morphology and function of mouse gubernaculum testis in culture. Cell Biol Toxicol. 2012;28:397–407. doi: 10.1007/s10565-012-9231-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Li JH, Duan SX, Lin QJ, Ke S, Ma L, Huang TH, Jiang XW. G protein-coupled estrogen receptor-protein kinase A-ERK-CREB signaling pathway is involved in the regulation of mouse gubernaculum testis cells by diethylstilbestrol. Arch Environ Contam Toxicol. 2014;67:97–103. doi: 10.1007/s00244-013-9976-3. [DOI] [PubMed] [Google Scholar]
- 15.Giwercman A. Estrogens and phytoestrogens in male infertility. Curr Opin Urol. 2011;21:519–526. doi: 10.1097/MOU.0b013e32834b7e7c. [DOI] [PubMed] [Google Scholar]
- 16.Hutson JM, Nation T, Balic A, Southwell BR. The role of the gubernaculum in the descent and undescent of the testis. Ther Adv Urol. 2009;1:115–121. doi: 10.1177/1756287209105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staub C, Rauch M, Ferrie`re F, Tre´pos M, Dorval-Coiffec I, Saunders PT, Cobellis G, Flouriot G, Saligaut C, Jégou B. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biol Reprod. 2005;73:703–712. doi: 10.1095/biolreprod.105.042796. [DOI] [PubMed] [Google Scholar]
- 18.Rago V, Romeo F, Giordano F, Maggiolini M, Carpino A. Identification of the estrogen receptor GPER in neoplastic and nonneoplastic human testes. Reprod Biol Endocrinol. 2011;9:135. doi: 10.1186/1477-7827-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES) Int J Androl. 2010;33:377–384. doi: 10.1111/j.1365-2605.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


