Abstract
Background: MicroRNAs (miRNAs) play critical roles in hepatocellular carcinoma (HCC) development and progression. Aberrant miR-21 expression has been reported in several cancers. However, the clinical significance of miR-21 in human HCC is still unclear. Methods: A total of 112 patients with primary HCC who underwent a curative liver resection were included in this retrospective study. The differentially expressed amount of the miR-21 was validated by quantitative real-time PCR (qRT-PCR). Survival rate was analyzed by log-rank test, and survival curves were plotted according to Kaplan-Meier. Multivariate analysis of the prognostic factors was performed with Cox regression model. Results: As revealed by qRT-PCR analysis, miR-21 expression was significantly upregulated in HCC tissues when compared with adjacent non-tumor tissues (P<0.05). High miR-21 expression level was observed to be closely correlated with tumor differentiation, TNM stage and vein invasion (P<0.05). Patients who had high miR-21 expression had a shorter overall survival than patients who had low miR-21 expression (P<0.05). Moreover, multivariate analysis of the prognosis factors with a Cox proportional hazards model showed that high miR-21 expression was a significant independent predictor of poor survival in HCC (P<0.05). Conclusion: Our results suggested that increased expression of miR-21 was significantly correlated with tumor progression and could be a novel potential biomarker for HCC prognosis.
Keywords: miRNA-21, hepatocellular carcinoma, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most malignant solid tumors and is the second leading cause of cancer related mortality [1]. HCC is a complex malignant tumor, and its development and progression is influenced by various factors. Two important risk factors for developing HCC are cirrhosis and hepatitis B virus infection [2]. Currently, surgery is the most common treatment for HCC. However, the recurrence rate is high and surgery affects long-term recovery and survival in HCC patients after curative resection [3]. For advanced HCC patients, several novel molecular targeted therapies can be applied but with limited therapeutic effects [4]. Therefore, it is urgent and necessary to identify prognostic factors which could predict recurrence, metastasis and prognosis for patients with HCC to improve the individual treatment.
MicroRNAs (miRNAs) are emerging as a new class of regulatory molecules involved in numerous biologic processes [5]. miRNAs are single-stranded and highly conserved small non-coding RNAs that recognize complementary sequences in the 3’untranslated region of target mRNAs, leading to the reduction of protein expression either by mRNA degradation and/or by translational repression [6]. miRNA deregulation is a common feature of human malignancies as they control the expression of oncogenes or tumor suppressors acting as onco-miRNAs or tumor suppressor miRNAs themselves [7].
Recent studies have shown that increased expression of miR-21 could increase cell proliferation, migration and invasion in a variety of cancer cells [8,9]. Some studies have found that miR-21 could serve as a diagnostic and prognostic marker for human malignancies, such as breast cancer, colorectal cancer, lung cancer and pancreatic cancer [10-13]. However, whether the expression level of miR-21 is associated with the prognosis of patients with HCC is still unclear. The aim of our study was to investigate the clinical significance and prognostic value of miR-21 in HCC.
Materials and methods
Patients and tissue samples
A total of 112 patients with primary HCC who underwent a curative liver resection at the Affiliated Tumour Hospital of Zhengzhou University, were included in this retrospective study. These patients were diagnosed as HCC between 2007 and 2013. The tissues were immediately frozen in liquid nitrogen after surgical removal and stored at -80°C until use. None of the patients recruited in this study had undergone preoperative chemotherapy or radiotherapy. HCC diagnosis was based on WHO criteria. Tumor staging was determined according to the sixth edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer. The characteristics of patients were shown in Table 1. The study was approved by the Research Ethics Committee of Affiliated Tumour Hospital of Zhengzhou University. Informed consent was obtained from all Patients.
Table 1.
Correlations between miR-21 expression and clinicopathological features in HCC patients
| miR-21 expression | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | N=112 | High (n=59) | Low (n=53) | P value |
| Gender | 0.929 | |||
| Male | 66 | 35 | 31 | |
| Female | 46 | 24 | 22 | |
| Age (years) | 0.224 | |||
| <50 | 49 | 29 | 20 | |
| ≥50 | 63 | 30 | 33 | |
| Tumor size (cm) | 0.302 | |||
| ≤5 | 43 | 20 | 23 | |
| >5 | 69 | 39 | 30 | |
| Tumor number | 0.204 | |||
| Solitary | 62 | 36 | 26 | |
| Multiple | 50 | 23 | 27 | |
| Serum AFP (ug/l) | 0.316 | |||
| <400 | 75 | 42 | 33 | |
| ≥400 | 37 | 17 | 20 | |
| Liver cirrhosis | 0.092 | |||
| Absence | 13 | 4 | 9 | |
| Presence | 99 | 55 | 44 | |
| Tumor differentiation | 0.000 | |||
| Well+Moderate | 57 | 15 | 42 | |
| Poor | 55 | 44 | 11 | |
| TNM stage | 0.002 | |||
| I-II | 48 | 17 | 31 | |
| III-IV | 64 | 42 | 22 | |
| Vein invasion | 0.003 | |||
| Presence | 19 | 16 | 3 | |
| Absence | 93 | 43 | 50 | |
AFP, Alpha Fetal Protein; TNM, Tumor/Nodes/Metastases.
RNA Isolation and qRT-PCR
Total RNA isolation from tissues was performed using mirVana miRNA Isolation Kit (Applied Biosystems). RNA concentrations were measured using the SPECTRAmax microplate spectrophotometer (Molecular Devices Corp). Primers for miR-21 and endogenous control U6 snRNA were obtained from Applied Biosystems. cDNA was generated using the PrimeScript RT reagent kit (Takara) in a 20 µl final reaction volume containing 0.5 µg of RNA, 0.5 µl Prime-Script RT enzyme mix, and 4 µl 5×PrimeScript buffer, and 1 µl RT primer, and incubated at 42°C for 60 min and at 85°C for 5 min. Quantitative real-time PCR assay was performed to evaluate miR-21 expression using SYBR Premix Ex Taq (Takara) and measured in a LightCycler 480 System (Roche). The amplification profile was denatured at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. The relative expression of miR-21 was calculated and normalized using the 2-ΔΔCt method relative to U6 small nuclear RNA.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software (IBM). Relationships between miR-21 expression level and other parameters were studied using the chi-square test and Fisher’s exact test or independent t test. The Kaplan-Meier method was used to estimate survival, log-rank test was used to test differences between the survival curves. A multivariate survival analysis was performed for all parameters that were significant in the univariate analyses using the Cox regression model. Differences were considered statistically significant when P was less than 0.05.
Results
The expression of miR-21 is significantly upregulated in human HCC tissues
The qRT-PCR assay was performed to determine the expression level of miR-21 in 112 cases of HCC and their matched non-tumor tissues. Our data showed that miR-21 expression was significantly higher in HCC tissues compared with adjacent non-tumor tissues (P<0.05, Figure 1). The 112 HCC patients were classified into two groups according to the mean of miR-21 expression level as determined by qRT-PCR. 59 cases were placed in the high expression group and 53 in the low expression group.
Figure 1.
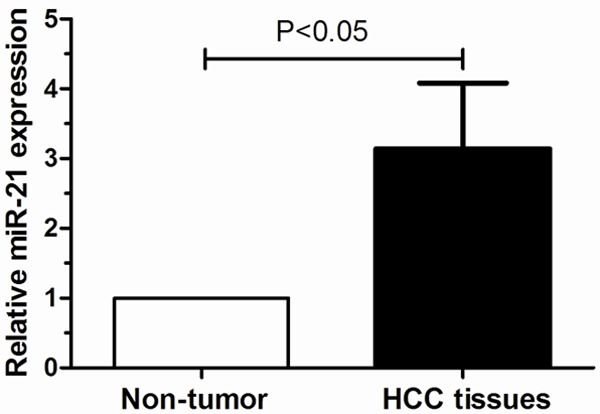
Relative expression levels of miR-21 in 112 paired HCC and adjacent non- tumor tissues.
Association of miR-21 expression with clinicopathological features of HCC patients
Next, the clinicopathological significance of miR-21 expression in human HCC was analyzed. As shown in Table 1, high miR-21 expression level was observed to be closely correlated with tumor differentiation, TNM stage and vein invasion (P<0.05). However, there were no significant correlations between miR-21 expression level and other clinicopathologic factors, including age, gender, tumor number, tumor size, serum AFP level and liver cirrhosis (P>0.05).
Association of miR-21 expression with prognosis of HCC patients
Considering that the level of miR-21 expression was significantly correlated with tumor differentiation, TNM stage and vein invasion, we hypothesized that miR-21 might affect the prognosis of HCC patients. Kaplan-Meier overall survival curve of the HCC patients according to the status of miR-21 level was examined. The results indicated overall survival of patients in the high miR-21 group showed a poor survival rates than those who were in the low miR-21 group (P<0.05, Figure 2), suggesting that high expression of miR-21 is associated with poor prognosis in HCC patients. A multivariate analysis of the prognosis factors with a Cox proportional hazards model showed that high miR-21 expression, poor tumor differentiation, high TNM stage and presence of vein invasion were significant independent predictors of poor survival in HCC (P<0.05, Table 2).
Figure 2.
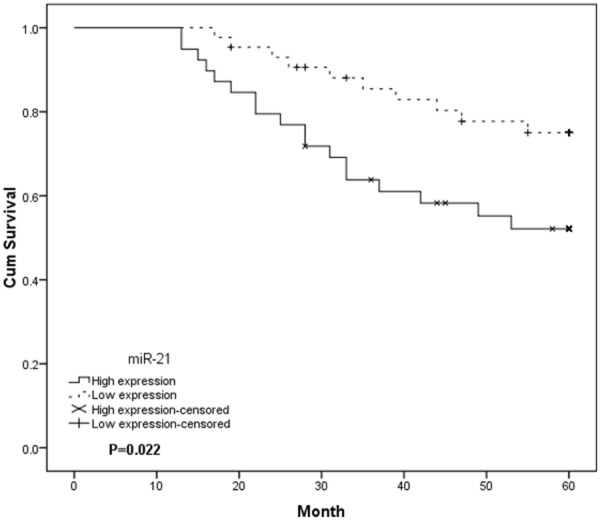
Kaplan-Meier analysis of the overall survival of HCC patients depending on the miR-21 expression level. The group with high miR-21 expression exhibited a significantly shorter survival compared with the group with low miR-21 expression.
Table 2.
Univariate and multivariate analysis of parameters associated with overall survival of HCC patients
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Gender | 0.583 | 0.312-2.657 | 0.383 | |||
| Age | 1.192 | 0.691-3.146 | 0.217 | |||
| Tumor size | 2.813 | 0.726-5.665 | 0.117 | |||
| Tumor number | 2.217 | 0.819-7.621 | 0.094 | |||
| Serum AFP level | 1.984 | 0.592-4.703 | 0.162 | |||
| Liver cirrhosis | 3.256 | 0.738-5.239 | 0.114 | |||
| Tumor differentiation | 2.961 | 0.395-5.284 | 0.008 | 2.731 | 0.353-4.193 | 0.007 |
| TNM stage | 3.941 | 1.987-9.758 | 0.001 | 3.305 | 1.721-7.841 | <0.001 |
| Vein invasion | 3.642 | 2.283-7.981 | 0.004 | 3.185 | 1.893-7.059 | 0.002 |
| miR-21 expression | 2.417 | 1.522-8.467 | 0.011 | 2.275 | 1.394-7.924 | 0.005 |
Discussion
The dysregulation of miRNAs is common in various carcinomas and plays an important role in cancer progression by altering normal gene expression [14]. It has been noted that alterations in single miRNA expression correlate highly with the progression and prognosis of human tumors. Thus, identification of miRNA molecular profiles associated with the prognosis of patients with HCC may not only elucidate the underlying biological mechanisms involved in the development or progression of the disease but also provide the opportunity to identify novel targets for HCC therapy [15,16].
Previous studies showed miR-21 was increased in several types of cancers, such as breast cancer, colon cancer, lung cancer and pancreatic cancer [10-13]. In agreement with these studies, we confirmed that the expression levels of miR-21 in HCC tissues. Our data showed that miR-21 was upregulated in human HCC tissues when compared to adjacent non-tumor tissues. Furthermore, the high expression of miR-21 was observed to be closely correlated with tumor differentiation, TNM stage and vein invasion, indicating miR-21 may be involved in HCC progression. Previously, Numbers studies showed that upregulated expression of miR-21 was closely associated with poor survival outcome in cancers. For example, Yan et al showed miR-21 was overexpression in human breast cancer and associated with advanced clinical stage and lymph node metastasis. Furthermore, the overall survival rates of patients with high miR-21 expression were significantly worse than those of patients with low miR-21 expression [17]. Faltejskova et al found that miR-21 were significantly increased in colorectal cancer (CRC) tumor tissue and associated with advanced clinical stage. Kaplan-Meier analysis proved that the miR-21 expression levels are correlated to shorter overall survival of CRC patients [18]. Hu et al found that miR-21 were expressed at higher levels in the laryngeal squamous cell carcinoma samples compared to the normal samples; furthermore, they indicated that patients with high miR-21 expression in tumor tissues had poorer prognoses compared to patients with lower miR-21 expression [19].
However, whether the expression level of miR-21 is associated with the prognosis of patients with HCC is still unknown. In the present study, our data indicated that miR-21 was significantly higher in HCC tissues compared with adjacent non-tumor tissues. High miR-21 expression level was observed to be closely correlated with tumor differentiation, TNM stage and vein invasion. The overall survival of patients in the high miR-21 group showed significantly poor survival rates than those who were in the low miR-21 group, suggesting that high expression of miR-21 is associated with poor prognosis in HCC patients. Furthermore, multivariate analysis of the prognosis factors with a Cox proportional hazards model suggested that high miR-21 expression was a significant independent predictor of poor survival in HCC.
Taken together, the current study indicated that upregulation of miR-21 was significantly correlated with tumor progression and may be a potent prognostic marker of HCC. However, this study has several limits. First, as the number of patients in this study is smaller, a larger case population is needed to confirm the prognostic value of miR-21 expression in HCC. Second, further investigation of the cell biology of miR-21 and its potential as a therapeutic target in HCC are clearly warranted.
Disclosure of conflict interest
None.
References
- 1.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Selcuklu SD, Donoghue MA, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 10.Qian B, Katsaros D, Lu L, Preti M, Durando A, Arisio R, Mu L, Yu H. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res Treat. 2009;117:131–140. doi: 10.1007/s10549-008-0219-7. [DOI] [PubMed] [Google Scholar]
- 11.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 12.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137:557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 13.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, He X. The role of microRNAs in liver cancer progression. Br J Cancer. 2011;104:235–240. doi: 10.1038/sj.bjc.6606010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, Pan Z, Hu X, Zhao Y, Xie H, Jiang G, Chen T, Wang J, Zheng S, Cheng J, Wan D, Yang S, Li Y, Gu J. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 16.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 17.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faltejskova P, Besse A, Sevcikova S, Kubiczkova L, Svoboda M, Smarda J, Kiss I, Vyzula R, Slaby O. Clinical correlations of miR-21 expression in colorectal cancer patients and effects of its inhibition on DLD1 colon cancer cells. Int J Colorectal Dis. 2012;27:1401–1408. doi: 10.1007/s00384-012-1461-3. [DOI] [PubMed] [Google Scholar]
- 19.Hu A, Huang JJ, Xu WH, Jin XJ, Li JP, Tang YJ, Huang XF, Cui HJ, Sun GB. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: association with patient survival. Am J Transl Res. 2014;6:604. [PMC free article] [PubMed] [Google Scholar]


