Abstract
Objective: Our objective is to explore the effect of curcumin on permeability of coronary artery and expression of related proteins in rat coronary atherosclerosis heart disease model. Methods: 45 healthy male Wistar rats of clean grade were selected and divided into treatment group, model control group and blank control group. The rats in the treatment group and model control group received high-fat diet for 12 weeks and intraperitoneal injection of VD3 to establish rat coronary atherosclerosis heart disease model. After modeling, the rats in the treatment group received gavage of 100 mg/(kg·d) curcimin, and the rats in the model control group and blank control group received gavage of 5 ml/(kg·d) distilled water, the intervention time was 4 weeks. After intervention, the rats were killed, and the hearts were dissected to obtain the samples of coronary artery. After embedding and frozen section, immunofluorescence method was used to detect the change of endarterium permeability in 3 groups, Western blot was used to detect matrix metalloproteinase-9 (MMP-9) and CD40L in coronary artery tissue, and enzyme linked immunosorbent assay (ELISA) was used to detect serum tumor necrosis factor-α (TNF-α) and C reaction protein (CRP). Results: After modeling, compared with the blank control group, total cholesterol (TC), triglyceride (TG) and low density lipoprotein cholesterin (LDL-c) in the treatment group and model control group were significantly higher (P<0.05), however, high density lipoprotein cholesterin (HDL-c) was significantly lower. The pathological sections showed that there was lipidosis in rat coronary artery in treatment group and model control group, indicating that the modeling was successful. Immunofluorescence showed that there was only a little fluorochrome permeability in artery in blank control group, there was some fluorochrome permeability in artery in the treatment group and there was a lot of fluorochrome permeability in artery in the model control group. MMP-9 and CD40L in coronary artery tissue in the model control group were significantly higher than the treatment group (P<0.05), MMP-9 and CD40L in coronary artery tissue in the treatment group were significantly higher than the blank control group (P<0.05); serum TNF-α and CRP in the model control group were significantly higher than the treatment group (P<0.05), which were significantly higher in the treatment group than the blank control group (P<0.05). Conclusion: Rat coronary atherosclerosis heart disease model can be successfully established by feeding with high-fat diet and intraperitoneal injection of VD3, the permeability of coronary artery in coronary heart disease rat model is significantly increased, which may be related to up-regulation of MMP-9, CD40L, TNF-α and CRP expression. Application of curcumin can inhibit expression of MMP-9, CD40L, TNF-α and CRP to improve the permeability of coronary artery.
Keywords: Curcumin, coronary atherosclerosis heart disease, coronary artery, permeability, MMP-9, CD40L, TNF-α, CRP
Introduction
Coronary heart disease (CHD) is a common cardiovascular disease, which is also an important reason to cause human death [1-3]. Curcumin is a polyphenols compound separated from turmeric [4,5]. It is found that curcumin has many pharmacological effects including antioxidant, anti-inflammation, eliminating free radicals, anti-tumor, lipid regulation and anti-coagulation [6,7]. And clinical trials showed no severe toxic or side effect [8,9]. It is also reported that after application of curcumin in coronary heart disease, the lipid level is effectively controlled and the incidence of cardiovascular event is reduced [10-12]. In this study, we explored the mechanism of curcumin in treating coronary heart disease by studying the effect of curcumin in rat coronary atherosclerosis heart disease model.
Materials and methods
Experimental animals
45 healthy male Wistar rats of clean grade were provided by the Animal Center of The Third Military Medical University, the body weight was 220-250 grams and the age was 9 weeks. The rearing conditions: room temperature (24±1°C), relative humidity (55±5)%, light and darkness for 12 hours alternatively, the rats were fed for 2 weeks to adapt the environment.
Instruments and reagents
The microplate reader was Multiskan MK2 purchased from Lab system Ltd, the centrifugal machine was purchased from Shanghai Anting Scientific Instrument Factory, the electrophoresis apparatus was purchased from Beijing Liuyi Instrument Factory, Modulus multi-functional photometer was purchased from Turner BioSystems, fluorescence quantization reagent was purchased from Takara Bio Company. Goat anti-mouse MMP-9 antibody and goat anti-mouse CD40L antibody were purchased from Shanghai Yuanye Biological Techonology Co, Ltd. Enzyme linked immunosorbent assay (ELISA) kit was purchased from Beijing Kangwei Century Biological Technology Co, Ltd.
Modeling, grouping and treatment
45 rats were randomly divided into treatment group, model control group and blank control group. The establishment of model in the treatment group and the model group were as follows: The rats were fed with high-fat diet for 12 weeks (4% cholesterol, 10% lard, 5% white sugar, 0.5% sodium cholate, 0.2% propylthiouracil and 80.3% basal feed), and intraperitoneal injection of 2 mL/kg VD3 (600000 IU/kg) was applied every day for 3 days since the feeding started. The rats in the blank control group were fed with basal feed, and intraperitoneal injection of 2 mL/kg normal saline was applied every day for 3 days since the feeding started. After feeding for 12 weeks, the venous blood was collected for blood lipid detection, and 2 rats were taken from the treatment group and the model control group for detection of heart pathology to confirm whether the modeling was successful. After the model was successfully built, the rats in the model group received gavage of 100 mg/(kg·d) curcumin, and the rats in model control group and blank control group received gavage of 5 ml/(kg·d) distilled water, the intervention time of drug was 4 weeks [13].
Sample collection
After intervention, intraperitoneal injection of 25% urethane (4 mL/kg) was used for anesthesia. The rats were fixed on operating table on supine position, the abdominal skin and muscle were scissored by scissor, and the arterial blood was collected by injection syringe. Then the chest was opened to expose the heart, and the coronary artery tissue was immediately collected. The coronary artery tissue was incised longitudinally, the coronary artery tissues of the same location were collected and preserved in -70°C refrigerator. After being kept still for 30 minutes, the samples were centrifuged for 20 minutes (3000 r/min), the supernatant was collected and preserved in -20°C refrigerator.
The permeability of coronary artery detected by immunofluorescence method
The samples were taken from -70°C refrigerator and put in ice box, freezing microtome was used to slice the samples, which were then put on processed glass slides. PBS was used to wash the samples for 3 times with 10 minutes/time, after being dried, a circle was drew around the sample, and then 200 μl 5% skimmed milk was put into the circle and placed at 4°C for 24 hours. 5% skim milk was discarded, 200 μl Rhodamin diluted by 1:200 was added and placed at 4°C for 2 hours. Rhodamin was discarded, and the samples were washed by PBS for 10 minutes, 0.05% Tween for 10 minutes, and again PBS for 2 times with 10 minutes/time. The samples were dried at room temperature, and covered by single-layer cover glass. And then the samples were observed and photographed under the fluorescence microscope. Fluorescent quantitative analysis system was used to detect the thickness of permeated dye [14].
Matrix metalloprotein-9 (MMP-9) and CD40L detected by Western blot
A part of coronary artery tissue was sliced and weighted, the weight of every sample was around 100 mg. 1000 μL RIPA cell lysis buffer was added and then centrifuged for 5 minutes (3000 r/min) to collect the supernatant. SDS-PAGE kit preparation kit was used. The loading buffer was added in protein samples which were then loaded in SDS-PAGE wells. The spacer gel was electrophoresed by 80V for 30 minutes and the separation gel was electrophoresed by 120V for 1 hour. After transmembrane and sealing, the anti MMP-9 and anti CD40L anbitobodies were diluted, and the TBST was used to wash the membranes for 3 times with 5 minutes/time. According to the related reference, ECL kit was used to detect the protein, SIM image analyzer was used for scanning and gray value analysis software Quantity one was used to analyze the gray value of the bends.
Tumor necrosis factor-α (TNF-α) and C reaction protein (CRP) detected by ELISA
TNF-α and CRP were detected by ELISA. The procedures were according to the protocol in the kit. Firstly, the serum sample was diluted by 2 times, which was then added in 96-well plate. The reaction wells were sealed, and incubated in 37°C incubator for 90 minutes. 350 μL scrubbing solution was used to wash the plates for 5 times, biotinylated antibody solution was added in every well and incubated in 37°C incubator for 60 minutes. The plates were again washed for 5 times, and enzyme conjugate was added in every well and incubated in 37°C incubator for 30 minutes. The plates were washed for 5 times, and chromogenic substrate solution was added and incubated for 15 minutes. After adding stop buffer, OD450 value was detected and the standard curve was drawn to calculate TNF-α and CRP concentrations [15].
Statistical analysis
SPSS14.0 was used for statistical analysis, the comparisons among measurement data in three groups were analyzed by one-way ANOVA, multiple comparisons were analyzed by LSD-t test, the data which were not conformed to normal distribution and homogeneity of variance were analyzed by Kruskal-Wallis test, and multiple comparisons were analyzed by Nemenyi test. Measurement data were presented as (x±s), the inspection level was set as α=0.05, P<0.05 was considered as statistically different.
Results
Comparison of blood lipid levels after modeling and intervention among 3 groups
After modeling, compared with the blank control group, total cholesterol (TC), triglyceride (TG) and low density lipoprotein cholesterin (LDL-c) in the treatment group and model control group were significantly higher (P<0.05), however, high density lipoprotein cholesterin (HDL-c) was significantly lower. TC, TG and LDL-c in the treatment group were significantly lower than model control group, and HDL-c was significantly higher than the model control group (P<0.05). As shown in Table 1.
Table 1.
Comparison of blood lipid levels after model establishment and intervention among 3 groups (x±s, mmol/L)
| Group | n | TG | TC | LDL-c | HDL-c |
|---|---|---|---|---|---|
| Treatment group | 15 | 1.22±0.22 | 4.23±1.05 | 4.58±0.56 | 1.08±0.14 |
| Model control group | 15 | 2.42±0.33Δ | 11.82±2.12Δ | 12.02±2.59Δ | 0.32±0.15Δ |
| Blank control group | 15 | 0.84±0.11Δ,* | 2.26±0.32Δ,* | 1.93±0.26Δ,* | 1.54±0.26Δ,* |
| F | 222.86 | 209.89 | 189.24 | 272.50 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
means P<0.05 when compared with the treatment group;
means P<0.05 when compared with model control group.
Comparison of coronary permeability after modeling and intervention among 3 groups
Immunofluorescence showed that there was only a little fluorochrome permeability in artery in blank control group, there was some fluorochrome permeability in artery in the treatment group and there was a lot of fluorochrome permeability in artery in the model control group, the thickness of permeated dye in the treatment was significantly lower than the model control group (P<0.05), which was significantly lower in the blank control group than the treatment group (P<0.05). As shown in Table 2 and Figure 1.
Table 2.
Comparisons of coronary artery permeability related proteins in 3 groups
| Group | n | MMP-9 | CD40L | TNF-α (U/ml) | CRP (mg/L) |
|---|---|---|---|---|---|
| Treatment group | 15 | 4.31±1.02 | 0.42±0.04 | 3.54±0.52 | 6.54±1.21 |
| Model control group | 15 | 8.45±2.13 | 0.68±0.05 | 11.43±2.56 | 10.42±4.25 |
| Blank control group | 15 | 1.05±0.67 | 0.35±0.03 | 2.02±0.28 | 2.87±1.24 |
| F | 102.71 | 272.10 | 166.37 | 30.45 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
Figure 1.
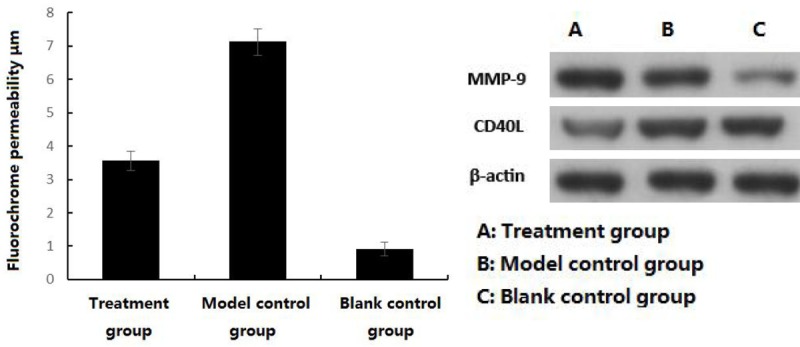
Comparison of coronary permeability after modeling and intervention among 3 groups.
Comparisons of coronary artery permeability related proteins among 3 groups
MMP-9 and CD40L in coronary artery tissue in the model control group were significantly higher than the treatment group (P<0.05), MMP-9 and CD40L in coronary artery tissue in the treatment group were significantly higher than the blank control group (P<0.05); serum TNF-α and CRP in the model control group were significantly higher than the treatment group (P<0.05), which were significantly higher in the treatment group than the blank control group (P<0.05). As shown in Figure 1.
Discussion
At present, the mechanism of coronary heart disease is not completely clear yet. A lot of epidemiological evidences have proven that the incidence of coronary heart disease is closely related to metabolic disorder of blood lipid in body, and feeding high-fat diet can establish atherosclerotic rat model also indicates the high correlation between them [16-19]. In this study, we successfully established coronary atherosclerotic heart disease rat model by feeding high-fat diet combined with intraperitoneal injection of VD3, the blood lipid level was significantly higher than the blank control group and the pathological results showed that there was atheromatous plaque in coronary artery [20,21]. Curcumin is the effective component of traditional Chinese medicine curcuma, the molecular weight is low and it is a natural organic-compound of polyphenols [22]. In the recent years, the researches about curcumin are further and further; several physiological and pharmacological effects have been found. One of the effects is the therapeutic effect of curcumin on coronary heart disease [23-25]. Scholars have found that curcumin has significant lipid regulation effect, which can affect the activity of lipoprotein related metabolic enzymes, increase the content of lipoprotein, mobilize the reverse transportation of cholesterol and accelerate cholesterol metabolism pathway to decrease TC, TG and LDL-C levels, and increase HDL-C level [26]. In this study, serum TC, TG and LDL-C in the treatment group were significantly lower than model control group, and HDL-C level was significantly higher than the model control group, which was in accordance with the above researches.
At present, it is considered that the main theory of atherosclerosis is lipid deposition theory. In this theory, it is considered that lipid is deposited after endothelium injury to induce inflammatory cell infiltration and phagocytosis, plaque calcification and bleeding [27]. Thus, the permeability is closely related to atheromatous plaque formation. The objective of this study is to explore the effect of curcumin on permeability of coronary artery and the possible mechanism. According to the related references, fluorescence permeation method was used to judge the permeability of artery endothelium [28]. The results showed that there was only a little fluorochrome permeability in artery in blank control group, there was some fluorochrome permeability in artery in the treatment group and there was a lot of fluorochrome permeability in artery in the model control group, indicating that curcumin can improve the permeability of atherosclerotic coronary artery to inhibit lipid deposition and inflammatory cell infiltration, and delay and stabilize atheromatous plaque formation. In this study, we further explored the possible mechanism of curcumin in improving coronary artery permeability, especially the expressions of coronary and inflammation related proteins. MMP-9 is synthesized by various matrix cells and macrophages, which mainly participates in degradation of active substance in extracellular matrix (ECM) in different tissues [29]. It is proven that curcumin has anti-endothelial injury effect and this effect is related to inhibition of MMP, the possible pathway is CD40/CD40L ligand pathway [30]. The results in our study have verified this finding. MMP-9 and CD40L in the treatment group were significantly lower than the model control group. Except the above conclusion, we also explored the effect of curcumin on inflammation level in rat coronary heart disease model. The results showed that compared with the model control group, TNF-α and CRP levels in the treatment group were significantly lower. TNF-α and CRP both participate in the effect of ox-LDL on inflammatory reaction in coronary endothelium injury, and curcumin has strong anti-inflammatory effect which can inhibit many inflammatory reaction mediators including epoxidase, lipoxidase, TNF-α, IFN-γ and CRP et al to effectively protect endothelium of vessels [31,32]. Thus, the improvement of coronary artery permeability by curcumin may be related to its anti-inflammatory effect.
In conclusion, coronary atherosclerotic heart disease rat model can be established by high-fat diet and intraperitoneal injection of VD3. The coronary artery permeability in coronary heart disease is significantly increased, which may be related to the up-regulation of MMP-9, CD40L, TNF-α and CRP, application of curcumin can inhibit the expressions of MMP-9, CD40L, TNF-α and CRP to improve the permeability of coronary artery.
Disclosure of conflict of interest
None.
References
- 1.Kumbhani DJ, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Deedwania P, Grau-Sepulveda M, Schwamm LH, Bhatt DL Get With the Guidelines Steering Committee and Investigators. Temporal trends for secondary prevention measures among patients hospitalized with coronary artery disease. Am J Med. 2015;128:426.e1–9. doi: 10.1016/j.amjmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Pello AM, Cristóbal C, Tarín N, Huelmos A, Aceña Á, Carda R, González-Casaus ML, Alonso J, Lorenzo Ó, Blanco-Colio L, Martín-Ventura JL, Franco Peláez JA, Mahíllo-Fernández I, Farré J, López-Bescós L, Egido J, Tuñón J. Differential profile in inflammatory and mineral metabolism biomarkers in patients with ischemic heart disease without classical coronary risk factors. J Cardiol. 2014;19:22–7. doi: 10.1016/j.jjcc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Karino S, Willcox BJ, Fong K, Lo S, Abbott R, Masaki KH. Total and differential white blood cell counts predict eight-year incident coronary heart disease in elderly Japanese-American men: The Honolulu Heart Program. Atherosclerosis. 2015;238:153–8. doi: 10.1016/j.atherosclerosis.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Teiten MH, Dicato M, Diederich M. Hybrid Curcumin Compounds: A New Strategy for Cancer Treatment. Molecules. 2014;19:20839–63. doi: 10.3390/molecules191220839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran JM, Rodriguez-Velasco FJ, Roncero-Martin R, Vera V, Pedrera-Zamorano JD. Cytotoxic effects of curcumin in osteosarcoma cells. Int J Nanomedicine. 2014;9:5273–5. doi: 10.2147/IJN.S75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal R, Sandhu SK, Sharma I, Kaur IP. Development and evaluation of curcumin-loaded elastic vesicles as an effective topical anti-inflammatory formulation. AAPS PharmSciTech. 2015;16:364–74. doi: 10.1208/s12249-014-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuelli L, Di Stefano E, Fantini M, Mattera R, Benvenuto M, Marzocchella L, Sacchetti P, Focaccetti C, Bernardini R, Tresoldi I, Izzi V, Mattei M, Frajese GV, Lista F, Modesti A, Bei R. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5:10745–62. doi: 10.18632/oncotarget.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman MM, Nassan MA, Ismail TA. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement Altern Med. 2014;14:457. doi: 10.1186/1472-6882-14-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohankumar K, Pajaniradje S, Sridharan S, Singh VK, Ronsard L, Banerjea AC, Selvanesan BC, Coumar MS, Periyasamy L, Rajagopalan R. Apoptosis induction by an analog of curcumin (BDMC-A) in human laryngeal carcinoma cells through intrinsic and extrinsic pathways. Cell Oncol (Dordr) 2014;37:439–54. doi: 10.1007/s13402-014-0207-3. [DOI] [PubMed] [Google Scholar]
- 10.Abo-Salem OM, Harisa GI, Ali TM, El-Sayed el-SM, Abou-Elnour FM. Curcumin ameliorates streptozotocin-induced heart injury in rats. J Biochem Mol Toxicol. 2014;28:263–70. doi: 10.1002/jbt.21562. [DOI] [PubMed] [Google Scholar]
- 11.Arora R, Kuhad A, Kaur IP, Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur J Pain. 2015;19:940–952. doi: 10.1002/ejp.620. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MH, Ramanathan M, Sankar V. Preparation, characterization and in vitro cytotoxicity assay of curcumin loaded solid lipid nanoparticle in IMR32 neuroblastoma cell line. Pak J Pharm Sci. 2014;27:1281–5. [PubMed] [Google Scholar]
- 13.Nakazawa G, Shinke T, Ijichi T, Matsumoto D, Otake H, Torii S, Hiranuma N, Ohsue T, Otsuka F, Shite J, Hirata K, Ikari Y. Comparison of vascular response between durable and biodegradable polymer-based drug-eluting stents in a porcine coronary artery model. EuroIntervention. 2014;10:717–23. doi: 10.4244/EIJV10I6A124. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS One. 2014;9:e108577. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basturk T, Unsal A, Ulas T, Koc Y, Sakaci T, Ahbap E, Borlu F. Effects of rosiglitazone treatment on insulin resistance and TNF-alpha levels in patients with chronic kidney disease: a prospective study. Eur Rev Med Pharmacol Sci. 2012;16:1519–24. [PubMed] [Google Scholar]
- 16.Sbarouni E, Voudris V, Georgiadou P, Hamilos M, Steg PG, Fox KM, Greenlaw N, Ferrari R, Vardas PE. Clinical presentation and management of stable coronary artery disease: insights from the international prospective CLARIFY registry - results from the Greek national cohort. Hellenic J Cardiol. 2014;55:442–7. [PubMed] [Google Scholar]
- 17.Alnouri F, Wood D, Kotseva K, Ibrahim ME Which statin worked best to achieve lipid level targets in a European registry. A post-hoc analysis of the EUROASPIRE III for coronary heart disease patients. J Saudi Heart Assoc. 2014;26:183–91. doi: 10.1016/j.jsha.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musa HH, Tyrab EM, Hamid MM, Elbashir EA, Yahia LM, Salih NM. Characterization of lipid profile in coronary heart disease patients in Sudan. Indian Heart J. 2013;65:232–3. doi: 10.1016/j.ihj.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadaegh F, Mohebi R, Cheraghi L, Tohidi M, Moghaddam NB, Bozorogmanesh M, Sheikholeslami F, Azizi F. Do different metabolic syndrome definitions predict cerebrovascular events and coronary heart disease independent of their components?: 9 years follow-up of the tehran lipid and glucose study. Stroke. 2012;43:1669–71. doi: 10.1161/STROKEAHA.112.650812. [DOI] [PubMed] [Google Scholar]
- 20.Pinies JA, González-Carril F, Arteagoitia JM, Irigoien I, Altzibar JM, Rodriguez-Murua JL, Echevarriarteun L Sentinel Practice Network of the Basque Country. Development of a prediction model for fatal and non-fatal coronary heart disease and cardiovascular disease in patients with newly diagnosed type 2 diabetes mellitus: the Basque Country Prospective Complications and Mortality Study risk engine (BASCORE) Diabetologia. 2014;57:2324–33. doi: 10.1007/s00125-014-3370-1. [DOI] [PubMed] [Google Scholar]
- 21.Munshi RP, Joshi SG, Rane BN. Development of an experimental diet model in rats to study hyperlipidemia and insulin resistance, markers for coronary heart disease. Indian J Pharmacol. 2014;46:270–6. doi: 10.4103/0253-7613.132156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain SK, Gill MS, Pawar HS, Suresh S. Novel Curcumin Diclofenac Conjugate Enhanced Curcumin Bioavailability and Efficacy in Streptococcal Cell Wall-induced Arthritis. Indian J Pharm Sci. 2014;76:415–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Shetty D, Kim YJ, Shim H, Snyder JP. Eliminating the heart from the curcumin molecule: monocarbonyl curcumin mimics (MACs) Molecules. 2014;20:249–292. doi: 10.3390/molecules20010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydin MS, Caliskan A, Kocarslan A, Kocarslan S, Yildiz A, Günay S, Savik E, Hazar A, Yalcin F. Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int J Surg. 2014;12:601–5. doi: 10.1016/j.ijsu.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Katanasaka Y, Sunagawa Y, Hasegawa K, Morimoto T. Application of curcumin to heart failure therapy by targeting transcriptional pathway in cardiomyocytes. Biol Pharm Bull. 2013;36:13–7. doi: 10.1248/bpb.b212022. [DOI] [PubMed] [Google Scholar]
- 26.Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, Ernie S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. 2008;40:201–10. [PubMed] [Google Scholar]
- 27.Tuso P, Stoll SR, Li WW. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm J. 2015;19:62–7. doi: 10.7812/TPP/14-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Nino WR, Tapia E, Zazueta C, Zatarain-Barrón ZL, Hernández-Pando R, Vega-García CC, Pedraza-Chaverrí J. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid Based Complement Alternat Med. 2013;2013:424692. doi: 10.1155/2013/424692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jana S, Rudra DS, Paul S, Snehasikta S. Curcumin delays endometriosis development by inhibiting MMP-2 activity. Indian J Biochem Biophys. 2012;49:342–8. [PubMed] [Google Scholar]
- 30.Jana S, Paul S, Warnakar SS. Curcumin as anti-endometriotic agent: implication of MMP-3 and intrinsic apoptotic pathway. Biochem Pharmacol. 2012;83:797–804. doi: 10.1016/j.bcp.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 31.Heeba GH, Mahmoud ME, Hanafy AA. Anti-inflammatory potential of curcumin and quercetin in rats: Role of oxidative stress, heme oxygenase-1 and TNF-alpha. Toxicol Ind Health. 2012;30:551–560. doi: 10.1177/0748233712462444. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–92. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]


