Abstract
Introduction: Lupus erythematosus is a multisystemic disease that is characterized by autoantibody production and immune complex deposition in such tissues as the mucosa, joints, the central nervous system, and skin. Cutaneous lupus erythematosus is categorized as acute, subacute, and chronic. Chronic cutaneous lupus erythematosus comprises discoid lupus erythematosus (DLE) and lupus profundus (LP). Aim: To analyze the expression of proapoptotic molecules in patients with lupus erythematosus discoid and lupus profundus. Material and methods: Descriptive study, the study groups comprised 10 cases of LP and 10 cases of DLE, and a control. Skin samples of cases and controls were processed for immunohistochemistry and by TUNEL technique. The database and statistical analysis was performed (statistical test X2) SPSS (Chicago, IL, USA). Results: Apoptotic features were broadly distributed along the skin biopsies in epidermal keratinocytes as well as at dermis. By immunohistochemistry the expression of Fas receptor and Fas-L was higher in the skin of lupus patients compared with controls. We also noted differences in Fas-L, -Fas, and -Bax proteins expression intensity in discoid lupus erythematosus patients in the epidermis, and hair follicles. Conclusions: Fas and Fas-L are expressed similarly in LP and DLE.
Keywords: Apoptosis, discoid lupus, lupus profundus, chronic cutaneous lupus erythematosus, proapoptotic molecules
Introduction
Lupus erythematosus (LE) is a multisystemic disease that is characterized by autoantibody production and immune complex deposition in such tissues as the mucosa, joints, the central nervous system, and skin. Its diagnosis and classification is based on clinical and serological correlation [1].
Cutaneous lupus erythematosus (CLE) is categorized as acute, subacute, and chronic [2-4]. Chronic cutaneous lupus erythematosus (CCLE) comprises discoid lupus erythematosus (DLE) and lupus profundus (LP) [5].
DLE is the most common type of CLE, affecting the face, scalp, ears, neck, and the extensor surfaces of thoracic limbs [6]. DLE occurs in women of reproductive age (20-40 years), ranging in prevalence from 14.6 to 68 per 100,000 cases of lupus, and the number of diagnosed cases is expected to increase due to the development of new biomarkers that can be used to diagnose the disease.
Systemic lupus erythematosus (SLE) is more common in women and has no predilection for any race [7]; the diagnostic criteria of the American College of Rheumatology for SLE include malar rash; discoid rash; photosensitivity; oral ulcers; arthritis; serositis; renal, neurological, hematological, and immunological disorders; and the presence of antinuclear antibodies [8].
In most frequent dermatological consultations of CLE cases correspond to DLE and LP, both dermatologic features display lower morbidity and mortality that the SLE, in this the quality of life is practically limited to physical appearance.
Five percent of patients with DLE progress to SLE that tends to be less aggressive than in those in whom systemic disease begins abruptly [9,10].
This condition develops in genetically susceptible subjects who are exposed to environmental factors, such as UV radiation, infection, and drugs, which accelerate progression of the disease [11].
The clinical presentation consists of erythema, hyper- or hypopigmentation, plaques, and atrophy [4,5]. LP is characterized by an inflammatory infiltrate in the subcutaneous tissue and deep dermis, manifesting clinically as erythema with subcutaneous nodules and an erythematous surface or fovea when the evolution is chronic.
Local skin damage due to physical, chemical, and biological factors (eg, environmental, infectious, hormonal, and stress-related) induces apoptosis, in which antigens and intracellular molecules translocate in the cell surface [6]. Apoptosis is an active form of programmed cell death in response to external or internal molecular signals and is detected directly and indirectly. TUNEL (transferase-mediated dUTP nick-end labeling) is a direct method, whereas immunohistochemistry is an indirect approach, in which the expression of molecules that regulate apoptosis is analyzed, such as Fas, Fas-L, Bax, Bcl-2, and p53 [12].
The aim of this study was to analyze the expression of proapoptotic molecules in patients with lupus erythematosus discoid and lupus profundus, this is of importance because its clinical implication in skin regeneration and reparative process in lupus profundus.
Material and methods
This descriptive study was conducted at the Department of Dermatology of General Hospital “Dr. Manuel Gea González” and in the Department of Immunology of the Universidad Autónoma de Zacatecas. (Approved by the ethics committee and research of the General Hospital “Dr. Manuel Gea González” #06-40-2011). Patients signed informed consent for diagnosis and treatment.
The study groups comprised 10 cases of LP and 10 cases of DLE, and a control (healthy tissue without histological alteration).
Skin samples of cases and controls were processed for immunohistochemistry (IHC). Two-micrometer-thick sections were deparaffinized for 10 minutes at 60°C and hydrated in xylene and an alcohol series (100%, 95%, and 70%). After being washed with 1X phosphate-buffered saline (PBS) for 5 minutes, they were heated for 1 minute in citrate buffer, pH 6 for antigen retrieval, and endogenous peroxidase was blocked for 10 minutes with 3% H2O2 dissolved in metanol.
The tissues were incubated for 18 hours at 4°C in a moist chamber with monoclonal anti-Fas-L. (®RDI-CD95Lamb. RDI Flanders, NJ, USA), antibody -Fas (DAKO) and anti-Bax (®RDI-BAX-amb-A7 RDI Flanders, NJ, USA), diluted 1:100. Then, the sections were incubated with HRP-conjugated anti-mouse IgG (Cat. 50695148 Zymed San Francisco, California) for 4 h and washed twice with PBS for 5 minutes. The color reaction was induced by 3,3’-diaminobenzidine-0.06% H2O2 (Sigma, St Louis, MO), and the reaction was stopped with 0.5 M sulfuric acid, and counterstained with hematoxylin for 1 minute. After a dehydration step in alcohol, the specimens were cleared with xylene and mounted in synthetic resin for microscopy (Olympus B-Max BX-40) at 20X and 40X.
The analysis was performed by 2 investigators whom examined the tissues in a blinded manner, ten random fields were tested to determine the bit rates of immunoreactivity. The images were analyzed with Image-Pro Plus, Version 7.0 for WindowsTM.
Apoptosis was measured by DNA fragmentation with an apoptosis detection kit (Roche TUNEL-Apo-AP; Cat 11684795910 Penzberg, Germany). Tissues were deparaffinized and hydrated as in the IHC experiments. After being washed in PBS for 5 minutes, the sections were incubated with TUNEL mixture for 1 hour at 37°C in the dark.
The samples were washed in PBS and mounted in fluorescence mounting medium (Dako, Cat. S3023, Dakocytomation), for analysis at 20X and 40X under a fluorescence microscope (Olympus B-Max BX-40).
To distinguish apoptotic and nonapoptotic samples, cells were counterstained with propidium iodide. As a positive control for the TUNEL staining, a sample was treated with DNase-1 for 30 minutes, and the negative control was incubated with labeling solution without enzyme.
The database and statistical analysis was performed (statistical test X2) SPSS (Chicago, IL, USA).
Results
Apoptotic features were broadly distributed along the skin biopsies in epidermal keratinocytes as well as at dermis. By immunohistochemistry the expression of Fas receptor and Fas-L was higher in the skin of lupus patients compared with controls. We also noted differences in Fas-L, -Fas, and -Bax proteins expression intensity in DLE patients in the epidermis, and hair follicles, as well as long inflammatory infiltrates of DLE and other groups of lupus (X2 = 11.000 P = 0.027) (Table 1).
Table 1.
Expression of proapoptotic molecules
| DLE | LP | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Antibody | Location | Intensity | % | Location | Intensity | % |
| Fas-L | Epidermis | Mild | 70 | Epidermis | Mild | 40 |
| Moderate | 20 | Moderate | 50 | |||
| Severe | 10 | Severe | 10 | |||
| Hair follicle | Mild | 40 | Hair follicle | Mild | 30 | |
| Moderate | 50 | Moderate | 60 | |||
| Severe | 10 | Severe | 10 | |||
| Inflammatory infiltrate | Mild | 70 | Inflammatory infiltrate | Mild | 90 | |
| Moderate | 30 | Severe | 10 | |||
| Fas | Epidermis | Mild | 70 | Epidermis | Mild | 70 |
| Moderate | 30 | Moderate | 30 | |||
| Hair follicle | Negative | 10 | Hair follicle | Negative | 10 | |
| Mild | 60 | Mild | 60 | |||
| Moderate | 30 | Moderate | 30 | |||
| Inflammatory infiltrate | Mild | 70 | Inflammatory infiltrate | Negative | 10 | |
| Moderate | 30 | Mild | 70 | |||
| Moderate | 20 | |||||
| Bax | Epidermis | Mild | 50 | Epidermis | Mild | 70 |
| Moderate | 50 | Moderate | 20 | |||
| Severe | 10 | |||||
| Hair follicle | Mild | 40 | Negative | 10 | ||
| Moderate | 40 | Hair follicle | Mild | 70 | ||
| Severe | 10 | Moderate | 10 | |||
| Severe | 10 | |||||
| Inflammatory infiltrate | Mild | 40 | Inflammatory infiltrate | Negative | 10 | |
| Moderate | 40 | Mild | 70 | |||
| Severe | 10 | Moderate | 10 | |||
| Severe | 10 | |||||
Discussion
By present studies we demonstrated the presence of increased apoptosis in lupus determined by TUNEL and immunohistochemistry technique. The pathophysiology of lupus erythematosus is complex-in addition to apoptotic factors, the lack of elimination of apoptotic debris likely effects the production of autoantibodies against intracellular molecules, such as anti-DNA [13].
Apoptosis is physiologically programmed cell death that can be initiated by a permanently active mechanism (extrinsic pathway) or by irreparable DNA damage due to external factors (intrinsic pathway).
Apoptosis begins in the membrane with a signal that is induced by cellular damage and by the binding of ligands to their receptors and is characterized by the loss of membrane integrity [14,15].
The extrinsic pathway of apoptosis is effected by cytokine receptors and ligands from the tumor necrosis factor family. Fas-L and Fas receptor mediate immune regulation and the development of autoimmunity, and mutations in Fas and its ligand cause severe lymphadenopathy and autoimmunity in humans and mice. The function of Fas-L is to stimulate Fas receptor on the cell membrane, initiating signal transduction and activating caspases [16].
Fas receptor triggers apoptosis [17] and has other nonapoptotic signaling functions that are poorly understood, such as in extracellular signaling, invasion, and cell proliferation [18].
Based in our findings, the Fas-L and its Fas receptor are expressed at similar levels in LP and DLE (mild to moderate), our findings are consistent with other authors that demonstrated increased activity of the extrinsic apoptotic pathway.
Bax is a proapoptotic member of the Bcl-2 family that regulates intrinsic apoptotic signaling [19]. Bax is expressed in the cytoplasm in an inactive form and is activated in the early stages of apoptosis. It is associated with mitochondria through poorly understood mechanisms and induces conformational changes [20].
Ohsako et al. studied SLE activity, reporting significant expression of Bcl2 and Bax in cases of active versus inactive disease. This group also discussed the hypothesis of Oltvai, in which overexpression of Bax accelerates cell death and proposed that the anti-Bcl2 and -Bax can be used as indicators of the rate of programmed cell death [21].
We found that the protein Bax is expressed in the DLE in the epidermis, hair follicle, and inflammatory infiltrate at mild to moderate levels, and in LP, the intensity rose slightly in 70% of cases, regardless of histological localization. The increased intensity of Bax in DLE with respect to LP is likely to be associated with greater apoptosis.
Faurschou et al., measured the level of renal cell apoptosis in biopsies from 35 patients with lupus nephritis by TUNEL staining and observed positive tubular cells and high levels of mononuclear tubule interstitial infiltration in a cohort of patients with SLE, indicating that the level of apoptosis in tubular cells is linked to the severity of inflammation in this type of lupus [22].
In our study, we noted 100% TUNEL positivity in DLE and LP; in DLE, the intensity was severe in 60% and mild in 40% of cases, versus 80% and 20% in LP, respectively. Thus, we infer that there is greater apoptotic activity in LP, similar to the results of Faurschou, who also use TUNEL, although we analyzed skin, whereas they studied kidney.
Our results are consistent with Chong et al., who also demonstrated the presence of apoptosis in the epidermis of cutaneous lupus by TUNEL. Nevertheless, they failed to note any positivity in LP, whereas our results were similar in DLE and DL.
In LP patients, Fas-L and Bax expression differed in the epidermis and inflammatory infiltrate (X2 = 13.571 P = 0.009). With regards to apoptotic features, all DLE and LP cases display positive TUNEL meanwhile in the healthy controls the TUNEL tag was irrelevant (Figure 1). The distribution of apoptotic cells in DLE samples occurred in the epidermis, hair follicle, and inflammatory infiltrates. In conclusion, Fas and Fas-L are expressed similarly in LP and DLE.
Figure 1.
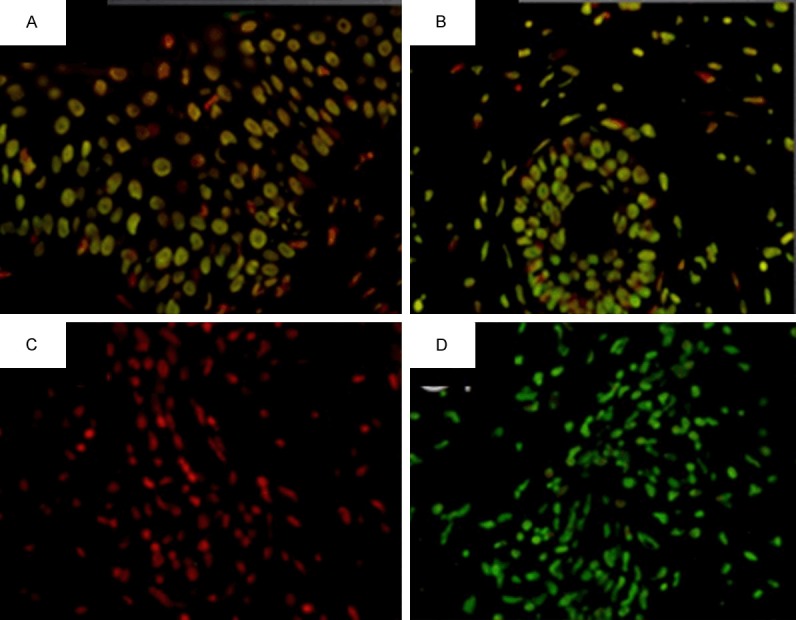
TUNEL staining of lupus profundus (400 ×). A. Positivity in the epidermis. B. Reaction in the hair follicle and adjacent lymphocytic infiltrate. C. Control. D. Control +.
Disclosure of conflict of interest
None.
References
- 1.Crowson AN. Superficial and deep perivascular dermatitis. In: Barnhill RL, Crowson AN, editors. Textbook of Dermatopathology. 2nd edition. USA: McGraw-Hill; 2004. pp. 89–92. [Google Scholar]
- 2.Beutner EH, Blaszczyk M, Jablonska S, Chorzelski TP, Kumar V, Wolska H. Studies on criteria of the European Academy of Dermatology and Venerology for the classification of cutaneous lupus erythematosus. I. Selection of clinical groups and study factors. Int J Dermatol. 1991;30:411–7. doi: 10.1111/j.1365-4362.1991.tb03896.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilliam JN. The cutaneous signs of lupus erythematosus. Cont Educ Fam. Phys. 1977;6:34–70. [Google Scholar]
- 4.Sontheimer RD. Lupus-Specific Skin Disease (Cutaneous LE) In: Wallace D, Hannahs B, editors. Dubois’ Lupus Erythematosus. 7th edition. USA: Lippincott Williams and Wilkins; 2007. pp. 576–613. [Google Scholar]
- 5.Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19:1050–70. doi: 10.1177/0961203310370048. [DOI] [PubMed] [Google Scholar]
- 6.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 7.Callen JP. Update on the management of cutaneous lupus erythematosus. Br J Dermatol. 2004;151:731–6. doi: 10.1111/j.1365-2133.2004.06196.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 9.Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121–4. doi: 10.1016/j.clindermatol.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Tebbe B, Mansmann U, Wollina U, Auer-Grumbach P, Licht-Mbalyohere A, Arensmeier M, Orfanos CE. Markers in cutaneous lupus erythematosus indicating systemic involvement. A multicenter study on 296 patients. Acta Derm Venereol. 1997;77:305–8. doi: 10.2340/0001555577305308. [DOI] [PubMed] [Google Scholar]
- 11.Hahn BH. An Overview of Pathogenesis of Systemic Lupus Erythematosus. In: Wallace D, Hanh B, editors. Dubois’ Lupus Erythematosus. USA: Lippincott Williams and Wilkins; 1997. Chapter 5. [Google Scholar]
- 12.Panizo A, Vega F. Estudio de la apoptosis mediante la técnica de TUNEL. Rev Esp Patol. 1997;30:243–245. [Google Scholar]
- 13.Navarrete CL, Ibáñez C. Rol de la apoptosis en la fisiopatología del lupus eritematoso sistemico. Rev Chil Reumatol. 2008;24:30–38. [Google Scholar]
- 14.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–22. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 15.Alfaro ME, García CC, Dueñas GA. Métodos de detección de la apoptosis, aplicaciones y limitaciones. Rev Inst Nal Cancerol Méx. 2000;46:275–80. [Google Scholar]
- 16.O’ Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, Smyth MJ, Bouillet P, Robb L, Strasser A. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–63. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phil Dash. Apoptosis; Basic Medical Sciences, St.George‘s, University of London. 1994. http://www.ppgorgsistem.ics.ufba.br/arquivos/fatima/Apoptosis.pdf.
- 18.Brint E, O’Callaghan G, Houston A. Life in the Fas lane: differential outcomes of Fas signaling. Cell Mol Life Sci. 2013;70:4085–99. doi: 10.1007/s00018-013-1327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarnescu O, Brehar FM, Chivu M, Ciurea AV. Immunohistochemical localization of caspase-3, caspase-9 and Bax in U87 glioblastoma xenografts. J Mol Histol. 2008;39:561–9. doi: 10.1007/s10735-008-9196-8. [DOI] [PubMed] [Google Scholar]
- 20.Lalier L, Cartron PF, Olivier C, Logé C, Bougras G, Robert JM, Oliver L, Vallette FM. Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ. 2011;18:528–37. doi: 10.1038/cdd.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohsako S, Hara M, Harigai M, Fukasawa C, Krajewski S, Reed J, Kashiwazaki S. The Bcl-2/Bax ratio of lymphocytes from human systemic lupus erythematosus patients. Japanese J Rheumatol. 1997;7:305–313. [Google Scholar]
- 22.Faurschou M, Penkowa M, Andersen CB, Starklint H, Jacobsen S. Renal cell apoptosis in human lupus nephritis: a histological study. Lupus. 2009;18:994–9. doi: 10.1177/0961203309106175. [DOI] [PubMed] [Google Scholar]


