Abstract
Objective: To explore the changes of peripheral blood monocytes subsets in acute coronary syndrome (ACS) and its clinical significance. Methods: A total of 68 ACS patients and 27 healthy subjects (HS) were enrolled. Monocyte subset analysis was performed using flow cytometry: CD14++CD16-(Mon1), CD14++CD16+ (Mon2), and CD14+CD16++ (Mon3). Results: 1. The number of Mon1 and Mon3 were significantly increased in ACS patients compared with HS (P<0.05) and Mon2 decreased in ACS patients (P<0.05). 2. The number of Mon1, Mon2, Mon3 was positively correlated with WBC count (P<0.05). The Mon2% was negatively correlated with the serum levels of LDH, CK, CK-MB (P<0.05). The number of Mon1, Mon3 was positively correlated with the serum levels of LDH, CK, CK-MB (P<0.05). Conclusion: The changes in different subsets of monocytes may be associated with pathogenesis of ACS and myocardial injury. The findings might be useful in the assessment of myocardial injury.
Keywords: Acute coronary syndrome, monocytes, CD14, CD16
Introduction
Coronary atherosclerotic heart disease (CHD) is mainly due to the insufficient myocardial blood supply, which can lead to angina, myocardial infarction, and sudden death. The incidence of CHD peaked in the 1960s and 1970s. In spite of several effective preventive measures taken in North America, Western Europe and Australia, CHD is still one of the leading causes of death in adults in most developed countries. In the developing country, it also showed a rising trend year by year. The involvement of inflammation in the pathophysiology of CHD has been identified in recent years, and monocytes were found to be the key participants in this process [1]. It is shown that monocyte count is an independent risk factor for coronary heart disease [2]. Recently research showed that circulating monocytes can be divided into three subtypes according to their surface expression of CD14 and CD16, which were a classical (cD14++/cD16-, Mon1), an intermediate (cD14++/cD16+, Mon2), and a non-classical (cD14+/cD16++, Mon3), with percentage of subsets around 83-85%, 4-5%, and 7-11% respectively [3,4]. In humans, the disturbed balance of monocyte subsets has been linked to various diseases [5], indicating the importance of the complex monocyte subset ratio. Similarly, the role of each monocyte subset in atherosclerosis has also been investigated in humans. Here we report the changes of peripheral blood monocytes subsets in 68 patients with acute coronary syndrome (ACS), and explore its relationship with other laboratory index. Our work might provide useful clues to the diagnosis, treatment and pathogenesis of ACS.
Materials and methods
Materials
Instruments
FACS Calibur flow cytometry with CELL Quest software (BD Company, United States).
Reagent
CD45-PerCP (#347464), the CD14-FITC (#347493) and isotype control Mouse IgG2a-FITC (#349051) were obtained from the BD Bioscience Companies. PE Mouse Anti-Human CD16 (Clone: 3G8) and the isotype control Mouse IgG1-PE (clone: 349043) were obtained from the BD Phamingen Company. Lactate dehydrogenase (LDH), creatine kinase (CK), myoglobin (CK-MB), total cholesterol (CHOL), triglyceride (TG), glucose (GLU), low density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c), apolipoprotein A (Apo-A), apolipoprotein B (Apo B) kit were obtained from Roche Company.
Study subjects
Sixty eight cases of ACS confirmed by coronary angiography from Taizhou Peoples’ Hospital were enrolled, which includes 54 male and 14 female cases, 27 healthy subjects were recruited as control group, including 22 male and 5 female cases. In all cases, an informed patient consent was obtained. The mean age of patients was 65.71±11.51 years old, and the mean age of the healthy subjects was 63.11±7.26 years old. Patients with ACS and following concurrent diseases were excluded from the studies: severe dysfunction of liver and kidney, infectious diseases, blood system diseases, rheumatic autoimmune diseases and malignant tumors.
Methods
Measurement of monocyte subsets
Flow cytometry sample processing
Peripheral blood samples were obtained from patients with the acute onset of ACS, 2 ml of blood collected into tubes with EDTA anticoagulant. Each sample was divided into two groups, with CD45-PerCP, CD14-FITC, PE Mouse Anti-Human CD16 in the testing group and CD45-PerCP, Mouse IgG2a, Mouse IgG1-PE in the isotype control group. Cell staining was performed following product manual.
Flow cytometry instrument settings and data analysis
The voltage of FSC, SSC, FL1, FL2,FL3 were adjusted by FACS Comp. Instrumental settings were adjusted according to corresponding isotype controls. CD45+ cells were selected for further analysis. Monocytes were obtained and analyzed by CELL Quest software (2000 events). The gating and analysis of different subsets of monocytes were illustrated in Figure 1.
Figure 1.
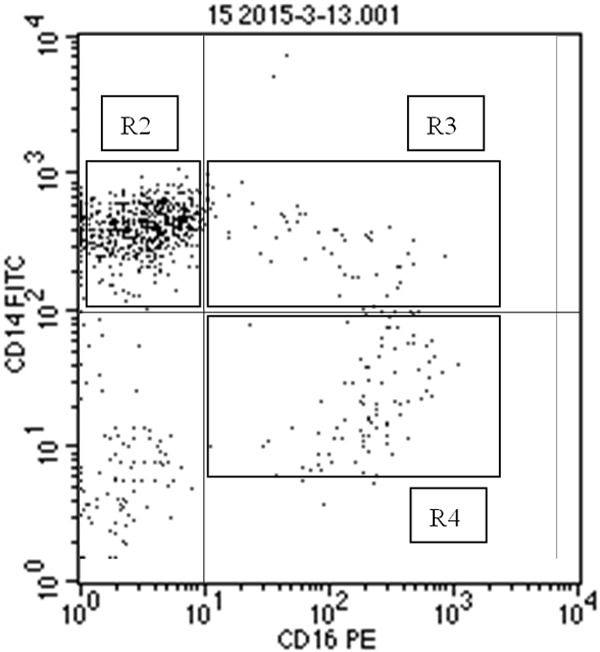
Detection of different monocytes subsets. Blood samples from ACS patients and HS were collected, stained, and analyzed as described in the Materials & Methods. A representative histogram was shown. R1: monocytes, R2: Mon1 (CD14++CD16-), R3: Mon2 (CD14++CD16+), R4: Mon3 (CD14+CD16+).
Other laboratory tests
Blood samples were drawn after overnight fasting within 24 h after hospitalization. Fasting venous blood serum lactate dehydrogenase (LDH), CK, total cholesterol (TC), CK-MB, triglyceride (TG), blood glucose (GLU), low density lipoprotein cholesterol (LDL-c) and high density lipoprotein cholesterol (HDL-c), apolipoprotein A (Apo-A), apolipoprotein B (Apo-B) were analyzed with standard methods at the laboratory of Taizhou Peoples’ Hospital. In addition, the white blood cell count (WBC), the monocytes count (M), the percentage of neutrophils (N%), the percentage of mononuclear cells (M%) and the percentage of lymphocytes (L%) were also measured.
Statistical analysis
The statistical analysis of the data was performed using the Excel and Sigma Plot 12.0 software. Continuous data are presented as the arithmetic mean and its standard deviation (M ± SD). The differences between two groups were analyzed with t-test and Pearson analysis. The differences were considered statistically significant at P<0.05.
Results
The routine tests in ACS patients and HS
Our results showed that the WBC count, N%, M% were significantly increased in ACS patients compared with HS (P<0.05), the N% was significantly lower (P<0.05). The serum level of LDH, CK, CK-MB, CHOL, LDL, Apo-A were significantly different (P<0.05, Table 1).
Table 1.
The routine tests in ACS patients and HS
| HS | ACS | Statistical analysis | ||
|---|---|---|---|---|
| N (M/F) | 27 (22/5) | 68 (54/14) | χ2=0.00323 | P=0.955 |
| Age | 63.11±7.26 | 65.71±11.51 | t=1.086 | P=0.280 |
| WBC (×109/L) | 6.864±2.396 | 7.451±2.536 | t=1.033 | P=0.304 |
| N (%) | 58.64±5.68 | 65.63±12.62 | t=2.762 | P=0.007# |
| L (%) | 31.07±5.67 | 23.57±11.35 | t=3.268 | P=0.002# |
| M (%) | 7.64±2.08 | 9.04± 2.69 | t=2.429 | P=0.017# |
| M (×109/L) | 0.514±0.177 | 0.677±0.332 | t=2.413 | P=0.018# |
| LDH (U/L) | 180.22±21.93 | 262.53±188.38 | t=2.257 | P=0.026# |
| CK (U/L) | 89.34±19.82 | 229.56±315.14 | t=2.303 | P=0.024# |
| CK-MB (U/L) | 12.00±5.35 | 24.76±29.79 | t=2.206 | P=0.030# |
| GLU (mmol/L) | 5.32±0.58 | 5.92±2.10 | t=1.458 | P=0.148 |
| CHOL (mmol/L) | 3.73±0.96 | 4.64±0.45 | t=2.070 | P=0.041 |
| TG (mmol/L) | 1.68±1.02 | 1.86±2.26 | t=0.397 | P=0.692 |
| HDL-c (mmol/L) | 1.22±0.37 | 1.23±0.47 | t=0.0989 | P=0.921 |
| LDL-c (mmol/L) | 2.49±0.36 | 1.88±0.58 | t=5.081 | P≤0.001# |
| Apo-A (g/L) | 1.27±0.19 | 1.15±0.24 | t=2.323 | P=0.022# |
| APO-B (g/L) | 0.87±0.13 | 0.87±0.53 | t=0.000 | P=1.000 |
The difference between ACS patients and HS: P values <0.05.
The distribution of monocyte subsets in ACS patients and HS
Our results showed that the Mon1%, Mon1 cell count and Mon3 cell count significantly increased in ACS patients compared with HS (P<0.05). The Mon2% was significantly lower (P<0.05, Table 2).
Table 2.
The distribution of monocyte subsets in ACS patients and HS
| HS | ACS | Statistical analysis | ||
|---|---|---|---|---|
| N | 27 | 68 | t | P |
| M (%) | 7.64±2.08 | 9.04±2.69 | 2.429 | 0.017 |
| M (×109/L) | 0.514±0.177 | 0.677±0.332 | 2.413 | 0.018 |
| monocyte subsets (%) | ||||
| Mon1 (%) | 77.89±14.41 | 85.52±6.85 | 3.496 | <0.001 |
| Mon2 (%) | 4.71±1.79 | 3.47±2.17 | 2.628 | 0.010 |
| Mon3 (%) | 9.54±3.39 | 10.73±5.94 | 0.978 | 0.331 |
| Monocytes cell count (/µL) | ||||
| Mon1 (/µL) | 388.2±123.6 | 569.4±23.8 | 3.034 | 0.003 |
| Mon2 (/µL) | 24.1±12.1 | 23.1±19.1 | 0.258 | 0.797 |
| Mon3 (/µL) | 44.89±10.44 | 71.38±49.46 | 2.750 | 0.007 |
The correlation analysis of monocyte subsets and other laboratory parameters in ACS patients
Our results showed that the Mon1, Mon2, Mon3 cell count was significantly positively correlated with the WBC count (P<0.05). Mon2% was significantly negatively correlated with the serum level of LDH and CK-MB (P<0.05). The Mon1, Mon3 cell count was significantly positively correlated with the serum level of LDH, CK and CK-MB (P<0.05, Table 3).
Table 3.
The correlation analysis of monocyte subsets and other laboratory parameters in ACS
| Mon1 (%) | Mon2 (%) | Mon3 (%) | Mon1 | Mon2 | Mon3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| WBC | -0.089 | 0.471 | -0.131 | 0.258 | 0.095 | 0.441 | 0.665 | <0.001 | 0.365 | 0.00219 | 0.617 | <0.001 |
| N% | -0.247 | 0.0425 | 0.0385 | 0.755 | 0.281 | 0.0202 | 0.118 | 0.336 | -0.0132 | 0.915 | 0.237 | 0.0513 |
| L% | 0.179 | 0.144 | -0.0605 | 0.624 | -240 | 0.0488 | -0.284 | 0.0189 | -0.126 | 0.305 | -0.307 | 0.0108 |
| LDH | 0.0783 | 0.526 | -0.232 | 0.0572 | -0.0067 | 0.957 | 0.415 | <0.001 | 0.03 | 0.808 | 0.335 | 0.00516 |
| CK | -0.0495 | 0.688 | -0.289 | 0.017 | 0.115 | 0.351 | 0.331 | 0.00576 | -0.0393 | 0.75 | 0.464 | 0.001 |
| CK-MB | -0.139 | 0.257 | -0.246 | 0.0431 | 0.207 | 0.0904 | 0.305 | 0.0113 | -0.0412 | 0.739 | 0.518 | <0.001 |
| GLU | 0.0918 | 0.457 | -0.0669 | 0.588 | -0.067 | 0.587 | -0.0145 | 0.907 | -0.0952 | 0.44 | -0.0286 | 0.817 |
| CHOL | -0.0645 | 0.601 | -0.0113 | 0.927 | -0.0287 | 0.817 | -0.0439 | 0.722 | -0.0545 | 0.659 | -0.121 | 0.328 |
| TG | 0.179 | 0.143 | 0.0199 | 0.872 | -0.183 | 0.136 | -0.158 | 0.199 | -0.104 | 0.399 | -0.231 | 0.0582 |
| HDL | -0.059 | 0.632 | -0.0101 | 0.935 | 0.00706 | 0.954 | -0.0841 | 0.495 | -0.112 | 0.362 | -0.0875 | 0.478 |
| LDL | -0.0698 | 0.572 | 0.0986 | 0.424 | -0.0707 | 0.567 | 0.133 | 0.281 | 0.15 | 0.223 | -0.0183 | 0.882 |
| Apo-A | -0.0938 | 0.447 | -0.162 | 0.188 | -0.0255 | 0.836 | -0.0715 | 0.562 | -0.195 | 0.11 | -0.0722 | 0.532 |
| APO-B | -0.0593 | 0.631 | 0.083 | 0.501 | 0.0353 | 0.775 | -0.0219 | 0.859 | 0.0399 | 0.747 | -0.047 | 0.703 |
Discussion
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and leukocytes in the arterial vessel wall [6]. It is caused by the interaction of many factors. Monocytes can not only phagocytose or remove metabolites, but also control the inflammatory response by promoting the apoptosis of other inflammatory cells [7]. The formation of atherosclerotic plaques includes the adhesion between monocytes and endothelial cell, the migration to the arterial wall and lipid deposition [8]. It is revealed that except for monocyte/macrophages, other inflammatory cells, such as the number of neutrophils, T cells and the like are quite rare in the plaque [9]. Therefore, monocyte/macrophage cells play critical roles in the initiation and progression of atherosclerosis. But the exact mechanism of continuous inflammatory responses in plaque, the formation of foam cells and the spread of inflammation remains unclear.
Recently, monocytes have been observed to be heterogeneous, could be divided into three subsets: Mon1, Mon2 and Mon3. Mon1 have the effection of phagocytosis, proteolysis and pro-inflammation [10]. They can also secret proinflammatory cytokines and adhesion molecules, and differentiate into proinflammatory M1 macrophages, which help the immune clearance [11]. Katarina et al reported that Mon1 cell count and Mon1% were significantly higher in patients with coronary heart disease than in HS, indicating Mon1 can be used as an independent risk factor for CHD [12]. Jaipersad et al showed that the increased Mon1 cell count associated with thickness of the carotid intima-media, the degree of carotid artery stenosis and systemic atherosclerosis [13]. Krychtiuk et al demonstrated that Mon1 cell count was negatively correlated with small HDL (high density lipoprotein) in patients with stable coronary artery disease [14]. Our study showed that the increased Mon1 cell count was significantly positively correlated with the WBC count, suggesting that the incidence of coronary syndrome is closely related with inflammation. In addition, The Mon1 cell count was significantly positively correlated with the serum level of LDH, CK and CK-MB, indicating that Mon1 may play a proinflammatory role in the acute onset of ACS. They might migrate to the intimal layer and transform into the M1 macrophages, leading to myocardial injury by inflammation.
Mon2 cells mainly secret IL-10 (interleukin-10), HO-1 (haem oxygenase-1) immunoregulatory factor [15,16]. They have the effects of anti-inflammation and immunomodulation. They could also differentiate into the anti-inflammatory M2 macrophages [17]. When stimulated by inflammation, CD163 fall off from Mon2, accompanied with the transformation of Mon2 to Mon1. And only Mon2 has the potential to convert to Mon1 [18]. It has been reported that the CD14++CD16+ monocytes (Mon2) and total CD16+ monocytes cell count were significantly higher in patients with coronary heart, and the total CD16+ monocytes cell count correlated with the and instability of coronary atherosclerotic plaque [17]. The Mon2 cell count was significantly increased in patients with ST-elevation myocardial infarction, and positively correlated with serum level of troponin, IL-6, IL-10 [19]. The Mon1 and Mon2 cell count was significantly elevated in patients with acute heart failure than those in chronic heart failure, stable coronary artery disease and HS. The monocyte-platelet aggregates were significantly higher in patients with acute heart failure than those in chronic heart failure and HS, but no significant difference in coronary artery disease [20]. Czepluch et al reported that Mon1 cell count was significantly higher in ACS patients, meanwhile, Mon2 significantly reduced [21]. Our study shows that the Mon2% was significantly lower in patients with acute coronary syndrome, which is consistent with Czepluch’s research. In addition, the Mon2% was significantly negatively correlated with myocardial injury markers LDH, CK-MB. The reduction of Mon2 cell count may be due to their transformation into inflammatory Mon1 cells under the stimulation of inducing factors, leading to acute exacerbation of inflammation, resulting in the acute onset of acute coronary syndrome. Meanwhile, the weakening of anti-inflammatory effects also aggravates myocardial injury.
Mon3 are attached the surface of endothelial cells in normal tissues. Tallone et al found that the Mon3 cell count increased about 90% in patients with coronary heart diseases than in HS, CD14+CD16++ monocytes may play a “parade” role in large vessels, and arrive at the endothelial injury site in time [22]. Our study showed that the Mon3 cell count was significantly increased in patients with acute coronary syndromes compared with the HS. They were positively correlated with the serum level of LDH, CK, and CK-MB. It is possible that a large number of Mon3 cells mobilized in the body when the inflammation of endothelial cells aggravate on the acute onset.
Different monocytes subsets play important roles in the formation and progression of atheromatous plaque in coronary heart disease. Therefore, more intensive study of the monocytes subsets is in urgent need. The detection of the functions of different monocyte subsets has important clinical value in the severity assessment, progression and prognosis of ACS.
Disclosure of conflict of interest
None.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Olivares R, Ducimetière P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137:49–53. doi: 10.1093/oxfordjournals.aje.a116601. [DOI] [PubMed] [Google Scholar]
- 3.Shantsila E, Wrigley B, Tapp L, Apostolakis S, Montoro-Garcia S, Drayson MT, Lip GY. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost. 2011;9:1056–1066. doi: 10.1111/j.1538-7836.2011.04244.x. [DOI] [PubMed] [Google Scholar]
- 4.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock L. The CD14+CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 8.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 9.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, Komatsu R, Ikura Y, Ogami M, Shimada Y, Ehara S, Yoshiyama M, Takeuchi K, Yoshikawa J, Becker AE. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 10.Hristov M, Weber C. Differential role of monocyte subsets in atherosclerosis. Thromb Haemost. 2011;106:757–62. doi: 10.1160/TH11-07-0500. [DOI] [PubMed] [Google Scholar]
- 11.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–45. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Björkbacka H. Elevated CD14++CD16+ Monocytes Predict Cardiovascular Events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 13.Jaipersad AS, Shantsila A, Lip GY. Expression of monocyte subsets and angiogenic markers in relation to carotid plaque neovascularization in patients with pre-existing coronary artery disease and carotid stenosis. Ann Med. 2014;46:530–8. doi: 10.3109/07853890.2014.931101. [DOI] [PubMed] [Google Scholar]
- 14.Krychtiuk KA, Kastl SP, Pfaffenberger S. Small high-density lipoprotein is associated with monocyte subsets in stable coronary artery disease. Atherosclerosis. 2014;237:589–96. doi: 10.1016/j.atherosclerosis.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skrzeczyńska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–9. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno K, Toma T, Tsukiji H, Okamoto H, Yamazaki H, Ohta K, Ohta K, Kasahara Y, Koizumi S, Yachie A. Selective expansion of CD16highCCR2- subpopulation of circulating monocytes with preferential production of haem oxygenase (HO)-1 in response to acute inflammation. Clin Exp Immunol. 2005;142:461–70. doi: 10.1111/j.1365-2249.2005.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hristov M, Weber C. Differential role of monocyte subsets in atherosclerosis. Thromb Haemost, 2011;106:757–62. doi: 10.1160/TH11-07-0500. [DOI] [PubMed] [Google Scholar]
- 18.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 19.Tapp LD, Shantsila E, Wrigley BJ. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost. 2012;10:1231–41. doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 20.Wrigley BJ, Shantsila E, Tapp LD. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail. 2013;6:127–35. doi: 10.1161/CIRCHEARTFAILURE.112.968073. [DOI] [PubMed] [Google Scholar]
- 21.Czepluch FS, Kuschicke H, Dellas C. Increased proatherogenic monocyte-platelet cross-talk in monocyte subpopulations of patients with stable coronary artery disease. J Intern Med. 2014;275:144–54. doi: 10.1111/joim.12145. [DOI] [PubMed] [Google Scholar]
- 22.Tallone T, Turconi G, Soldati G, Pedrazzini G, Moccetti T, Vassalli G. Heterogeneity of human monocytes: an optimized four-color flow cytometry protocol for analysis of monocyte subsets. J Cardiovasc Transl Res. 2011;4:211–9. doi: 10.1007/s12265-011-9256-4. [DOI] [PubMed] [Google Scholar]


