Abstract
Receptor tyrosine kinases have a single transmembrane (TM) segment that is usually assumed to play a passive role in ligand-induced dimerization and activation of the receptor. However, mutations within some of these receptors, and recent studies with the epidermal growth factor (EGF) and ErbB2 receptors have indicated that interactions between TM domains do contribute to stabilization of ligand-independent and/or ligand-induced receptor dimerization and activation. One consequence of the importance of these interactions is that short hydrophobic peptides corresponding to these domains should act as specific inhibitors. To test this hypothesis, we constructed expression vectors encoding short fusion peptides encompassing native or mutated TM domains of the EGF, ErbB2, and insulin receptors. In human cell lines overexpressing the wild-type EGF receptor or ErbB2, we observed that the peptides are expressed at the cell surface and that they inhibit specifically the autophosphorylation and signaling pathway of their cognate receptor. Identical results were obtained with peptides chemically synthesized. Mechanism of action involves inhibition of dimerization of the receptors as shown by the lack of effects of mutant nondimerizing sequences, completed by density centrifugation and covalent cross-linking experiments. Our findings stress the role of TM domain interactions in ErbB receptor function, and possibly for other single-spanning membrane proteins.
INTRODUCTION
Receptor tyrosine kinases (RTKs) are transmembrane (TM) glycoproteins that consist of a variable extracellular N-terminal domain, a single membrane spanning domain, and a large cytoplasmic portion composed of a juxtamembrane domain, the highly conserved tyrosine kinase domain, and a C-terminal regulatory region. Biochemical and structural data concur in the current idea that ligand binding stimulates monomeric receptor dimerization and trans-autophosphorylation at defined tyrosine residues through intrinsic kinase activity (Heldin, 1995; Weiss and Schlessinger, 1998; Hubbard, 1999).
Whereas ligand-induced RTK signaling is essential for normal cell proliferation, differentiation, migration, and metabolism (Ullrich and Schlessinger, 1990; Schlessinger, 2000), the aberrant activity of members of this receptor family has been shown to play a key role in the development and growth of tumor cells. Mutation and/or overexpression of many RTKs contribute to the development of a number of human cancers, by provoking ligand-independent receptor dimerization and activation (Kolibaba and Druker, 1997; Robertson et al., 2000; Blume-Jensen and Hunter, 2001). The epidermal growth factor (EGF) receptor (EGFR) family, or ErbB family, is the best studied example of such oncogenic RTKs. For example, HER2/ErbB2 is overexpressed on the surface of 25-30% of breast cancer cells, and it has been associated with a high risk of relapse and death (Slamon et al., 1989). EGFR amplification and mutations have been associated with many carcinomas (Kolibaba and Druker, 1997; Mendelsohn and Baselga, 2000).
The mechanism by which ligand-independent RTK activation occurs is not fully understood. Regions within both the extracellular and intracellular domains have been shown to be important for dimerization (Lemmon et al., 1997; Schaefer et al., 1999; Schlessinger, 2002). The TM region also has been shown to be important in receptor association. Although it was initially thought that this domain represents a passive anchor of the receptor to the membrane, a point mutation in the TM domain of one receptor, neu/ErbB-2, has been found to enhance its transforming properties by provoking dimerization and constitutive activation of this ligandless receptor (Bargmann et al., 1986; Weiner et al., 1989). Tanner and Kyte (1999) also have shown that EGF-induced dimerization is far more efficient for a fragment of EGFR that contains both the extracellular and TM domains than it is for the extracellular domain alone. Other genetic alterations in this domain have demonstrated an active role of this region in RTK dimerization and also demonstrated that dimerization is not sufficient, but that proper alignment also must occur for activation and signaling (Burke et al., 1997; Li et al., 1997; Gardin et al., 1999; Bell et al., 2000; Moriki et al., 2001).
Based on the hypothesis that the TM domains of ErbB receptors are directly involved in receptor dimerization and activation, we have studied the effect of the expression of different hydrophobic TM peptides on the activation of EGF receptors overexpressed on A431 epidermal cancer cells, and of ErbB2 receptors that are overexpressed on SK-OV3 ovarian cancer cells. Our results show that expression of peptides corresponding to the TM domain of these receptors is able to inhibit specifically the corresponding receptor activation in whole cells. Furthermore, this inhibition of activation of RTK is accompanied by an inhibition of mitogen-activated protein kinase (MAPK/extracellular signal-regulated kinase [ERK]), an enzyme that belongs to signaling pathways coupled to ErbB2 and EGF receptors and that is implicated in cell proliferation. The mechanism of action of the short peptides seems to involve inhibition of receptor dimerization.
MATERIALS AND METHODS
Cell Culture
The human epidermal adenocarcinoma cell line A431 and the human ovarian adenocarcinoma cell line SK-OV3, which overexpress epidermal growth factor receptors and ErbB2 receptors, respectively, were obtained from the American Type Culture Collection (Rockville, MD). Stock cultures were grown in DMEM and RPMI 1640, respectively, supplemented with 10% of fetal calf serum (Invitrogen, Carlsbad, CA), in a 5% CO2 humidified atmosphere.
Constructions of Expression Vectors for Short TM Domains
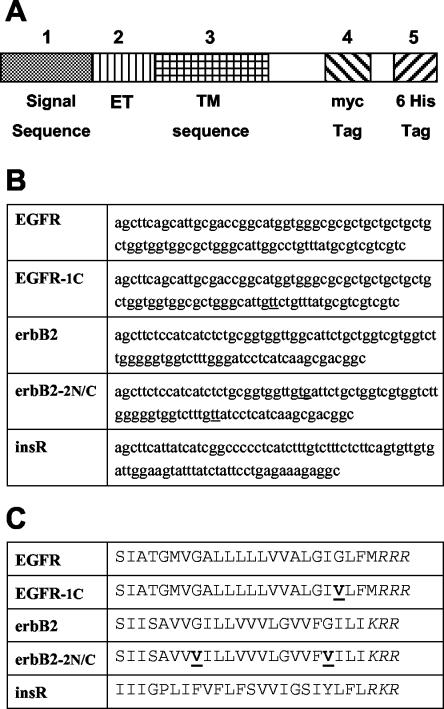
pSecTag vectors (Invitrogen) are mammalian expression vectors designed for the secretion, purification, and detection of fusion proteins. This vector contains a cytomegalovirus promoter for high level constitutive expression and a T7 priming site followed by a leader sequence, a multiple cloning site, and two tag sequences (myc and polyhistidine). It uses simian virus 40 origin for episomal replication, and it carries the zeocin and ampicillin resistance genes. The minigenes encoding the short TM sequence peptides of insulin receptor, ErbB2 receptor, epidermal growth factor receptor (GenBank accession codes M10051, M11767, and X00588, respectively), and their mutants were constructed by ligation of synthetic oligonucleotides (NAPS Göttingen, Göttingen, Germany or Proligo, Paris, France), by using the EcoRV and HindIII restriction sites. Nondimerizing mutants of the EGF receptor and ErbB2 TM domains were chosen according to Mendrola et al. (2002) and named correspondingly. The final sequence comprised the signal peptide and an adjacent extracellular sequence joined to a sequence incorporating the entire putative TM domain and C-terminal intracellular tag sequences (Myc and 6His) (Figure 1). Plasmids were checked by sequencing (Eurogentec, Seraing, Belgium, or at the IGBMC, Illkirch, France).
Figure 1.
Schematic diagram of minigenes used to encode the TM sequences. (A) Each box represents a section of the constructions made within the Invitrogen pSecTag A plasmid. (B) Oligonucleotide sequences used for cloning are indicated. (C) Amino acid sequence was as follows, using one-letter codes: signal sequence peptide (METDTLLLWVLLLWVPGSTG, 1-20), artificial extracellular sequence (extracellular tag, ET) from the multiple cloning site (DAAQPARRAVRSF, 21-33), different TM regions as indicated, including the stop transfer tribasic sequence (as indicated, 34-60; except for the EGF receptor sequence that is one amino acid longer), followed by the intracellular domain (HPAQWRPLESRGPEQKLISEEDLNSAVDHHHHHH, 61-94) containing the myc and 6His tags. Mutations introduced to decrease peptide dimerization are underlined in each sequence (see DISCUSSION), and the C-terminal tribasic stop sequence is italicized.
Antibodies
The extracellular tag (ET) antibody was produced after immunization of rabbits with a synthetic amino acid sequence (DAAQPARRAVRSFC) conjugated to keyhole limpet hemocyanin (Eurogentec). This sequence corresponds to the remaining 5′ part of the pSecTag plasmid multiple cloning site and has no homology with any sequence found in databases. The polyclonal antibodies were purified before use (CAP-8 IgG purification kit, Sterogene, Carlsbad, CA).
Transfection and Western Blotting
A431 and SK-OV3 cells were plated in a six-well culture plate and transiently transfected at 40-50% confluence by Lipofectamine (Invitrogen) by using 2-4 μg of plasmid DNA encoding for the TM peptides. A431cells were deprived of serum for 24 h before sample preparation. After incubation under different experimental conditions, cells were washed with ice-cold phosphate-buffered saline (PBS) and harvested in lysis buffer [30 mM HEPES, pH 7.6, 30 mM NaCl, 1% Nonidet P-40 (vol/vol), 10% glycerol (vol/vol), 50 mM NaF, 10 mM Na pyrophosphate) supplemented with protease inhibitors (Roche Diagnostics, Indianapolis, IN) and 5 mM Na orthovanadate. Cell lysates were cleared by centrifugation at 14,000 × g for 5 min at 4°C. Protein in total lysates was assayed before SDS-PAGE, by using the BCA protein assay kit (Pierce Chemical, Rockford, IL) with bovine serum albumin as a standard. Protein aliquots (5 μg) were applied to a 4-20% SDS-PAGE gradient. After being transferred onto nitrocellulose membrane (Whatman, Maidstone, United Kingdom), blots were blocked overnight and then incubated for 2 h with primary antibody: anti-ErbB2 antibody (0.125 μg/ml, clone 42; BD Transduction Laboratories, Lexington, KY), anti-EGFR antibody (0.05 μg/ml, clone 6F1; Immunotech, Beckman Coulter, Roissy, France; or 1:1000 dilution; reference 2232; Cell Signaling Technology, Beverly, MA), anti-phosphotyrosine antibody (0.2 μg/ml, clone 4G10; Upstate Biotechnology, Lake Placid, NY), anti-phosphorylated ERK1/2 antibody (1:5000 dilution, reference 9101; Cell Signaling Technology), anti-myc antibody (0.04 μg/ml, clone 9E10.3; NeoMarkers Lab Vision, Newmarket Sulfolk, United Kingdom), or anti-beta actin antibodies (1:10,000 dilution, clone AC-15; Sigma-Aldrich, St. Louis, MO). The membranes were then washed and incubated with peroxidase-labeled recombinant A/G protein (Pierce Chemical) diluted to 1:100,000 for 1 h. In some experiments, membranes were stripped of antibody (Restore Western blot stripping buffer; Pierce Chemical), and reprobed with a different one. The bands were visualized using a substrate kit (Supersignal West Dura; Pierce Chemical), according to the manufacturer's instructions, registered and analyzed thanks to a computerized GeneGnome imager (Syngene, Cambridge, United Kingdom).
Double-labeling Immunocytochemistry
SK-OV3 or A431 cells were transiently transfected as described above. Cells were grown on polylysine-coated glass coverslips for 3 d. Cells were then rinsed with PBS and subsequently fixed with 4% (wt/vol) paraformaldehyde for 15 min. Permeabilization was performed by incubation with 4% (wt/vol) paraformaldehyde for 10 min followed by 4% (wt/vol) paraformaldehyde supplemented with 0.1% (vol/vol) Triton X-100 for 10 min. After six rinses in PBS, cells were incubated with 3% bovine serum albumin (wt/vol), 10% goat serum (vol/vol) in PBS at 37°C for 45 min to reduce nonspecific staining. Cells were then incubated at room temperature for 2 h with primary antibody: ET antibody (20 μg/ml) or ErbB2 antibody (1 μg/ml, clone 9G6.10, NeoMarkers) or EGFR antibody (5 μg/ml, clone LA 1; Upstate Biotechnology). After six washes, cells were incubated for 30 min at 23°C with Alexa488-conjugated anti-rabbit (1:1000 dilution; Molecular Probes, Eugene, OR) or anti-mouse Cy3-conjugated antibody (1:2000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA). After extensive washing, the coverslips were mounted in Mowiol 4-88 (France Biochem, Meudon, France).
Synthesis, Purification, and Cell Incorporation of Hydrophobic Peptides
Peptides corresponding to the TM domains of the EGF and insulin receptors (sequences SIATGMVGALLLLLVVALGIGLFMR and KIIIGPLIFVFLFSVVIGSIYLFLR, respectively) were synthesized and purified by Neosystems (Strasbourg, France) by using 9-fluorenylmethoxycarbonyl chemistry. High-performance liquid chromatography (HPLC) analysis (reverse phase C4 column) demonstrated that the peptides were ∼98% pure, and they were checked by sequencing. For cellular incorporation, stock solution of peptide (1 mg/ml) was prepared in hexafluoropropanol. The desired amount was dried and resuspended in 10 mM octylglucoside buffer to obtain a 10-5 M final concentration. Peptide was incorporated by addition of this solution to the cells. In all cases, the added volume never exceeded 1% of the total volume to obtain a final detergent concentration much lower than its critical micellar concentration. We verified that, for each tested concentration of detergent alone, no effect on cell viability was observed. Cells were incubated with peptide for 4 h at 37°C before stimulation with 10-8 M human recombinant EGF for 5 min, followed by cell lysis. Receptor autophosphorylation was determined by Western blotting as described above. Incorporation of the peptide into cellular membranes under these conditions was checked thanks to a rhodamine-labeled EGFR TM peptide synthesized on a 432A peptide synthesizer (Applied Biosystems, Foster City, CA). Briefly, carboxytetramethylrhodamine [5(6)-TAMRA; Molecular Probes] was coupled at the N terminus of the EGFR TM peptide as the last step of synthesis, and the fluorescent peptide was purified by HPLC (reverse phase C18 column). Glycine-rhodamine was prepared from rhodamine-isothiocyanate in glycine buffer and used as control. Cell incorporation was performed as for the unlabeled peptides and followed by confocal microscopy as described above, in Lab-Tek sterile culture chambers (Nunc, Naperville, IL) without cell fixation. Under these conditions, cells remained viable for at least 2-3 h at 25°C.
Confocal Laser Scanning Microscopy
Immunofluorescence staining and peptide-rhodamine fluorescence were monitored with a laser scanning microscope (LSM 510; Carl Zeiss, Thorn-wood, NY) equipped with a Plan-Apochromat 63× oil immersion lens (numerical aperture 1.4) or a C-Apochromat 40× lens (numerical aperture 1.2). Alexa 488 emission was excited using the 488-nm ray of the argon laser, whereas Cy3 or rhodamine was excited using the 543-nm line of the helium/neon laser. Emission signals of Alexa 488 and Cy3 or rhodamine were filtered with a BP 505-530 and a LP 560 filter, respectively.
Sucrose Gradient Density Centrifugation and Cross-Linking of Receptors
To study the dimerization status of EGF receptors in the presence or absence of transfected fusion peptides, we used a velocity sedimentation method adapted for the Beckman Coulter microultracentrifuge TL100, as described previously (Leray et al., 1992). A 1 M sucrose cushion (100 μl) was layered at the bottom of each tube and overlaid with 400 μl each of 25, 17, 10, and 3% sucrose solutions. A quasilinear gradient was obtained by simple diffusion for 10 min at room temperature, as checked by refractometry. Transfected and nontransfected A431 cells were stimulated or not with 10-8 M EGF for 5 min and lysed for 45 min at 4°C in 30 mM HEPES buffer, 30 mM NaCl, pH 7.6, containing 0.2% Triton X-100 (vol/vol) and protease inhibitors. After centrifugation, the detergent extract (50 μl) was layered onto the top of the gradient. Centrifugation was performed for 2 h 30 min at 100,000 × g and 20°C, in a Beckman Coulter TST 55 rotor. Fractions of ∼120 μl were collected and analyzed by electrophoresis and immunoblotting with antireceptor antibodies as described above.
We also used covalent chemical cross-linking to assess the effects of transfected peptides upon EGF receptor dimerization. Briefly, A431 cells were incubated with 10-8 M EGF for 5 min. Cross-linking was initiated by the addition of 2 mM disuccinimidyl suberate (Pierce Chemical) for 30 min, and quenched in 50 mM Tris, pH 7.4. Cells were then lysed for 45 min at 4°C in 30 mM HEPES buffer, 30 mM NaCl, pH 7.6, containing 0.2% Triton X-100 (vol/vol) and protease inhibitors. After centrifugation, the detergent extract was immunoprecipitated overnight with anti-EGFR antibodies (mAb-13; Neosystems) and protein A-agarose (Pierce Chemical). Samples were analyzed by electrophoresis and immunoblotting with antireceptor antibodies as described above.
RESULTS
Expression of Hydrophobic TM Sequences
To test the potentially inhibitory effects of TM peptides on the activation of the EGF receptor and HER2/ErbB2 tyrosine kinases, we took advantage of the characteristics of the pSecTag expression plasmid. In this plasmid, we constructed minigenes comprising a sequence signal at the N terminus, which should direct the protein to the cell surface, a short antigenic extracellular portion termed ET, the different TM sequences of interest, a basic short stop-sequence to anchor the peptide in the proper orientation, and two C-terminal intracellular tags (myc and 6His) (Figure 1).
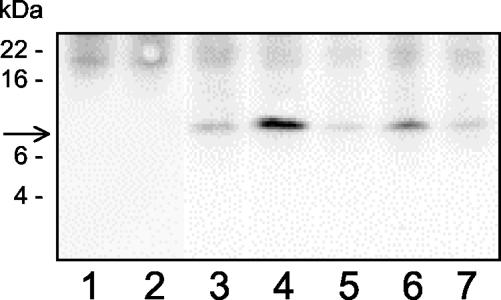
Anti-myc tag antibodies were used in Western blotting experiments to control the synthesis of a peptide with correct apparent molecular mass (∼9 kDa), after transient transfection in cell lines (Figure 2). This peptide was not detectable in nontransfected control cells. The 9-kDa fusion peptide expression was maximal at day 2 after transfection (our unpublished data) and decreased rapidly thereafter. Transfection efficiency was estimated as 85-90% by counting fluorescent cells either after transfection with a control green fluorescent protein-encoding plasmid, or cells transfected with our pSecTag-modified plasmids and labeled for immunocytochemistry as detailed below.
Figure 2.
Western blotting analysis of recombinant peptide expression in A431 cells. A431 cells were transfected with pSecTag plasmids encoding for the TM domain of EGFR (column 3), ErbB2 TM (column 4), insulin receptor TM (column 5), a mutant EGFR TM (EGFR-1C, column 6), a mutant ErbB2 TM (ErbB2-2N/C, column 7), or without plasmid (columns 1 and 2). Cells were lysed in detergent 2 d after transfection and solubilized proteins were submitted to PAGE-electrophoresis and Western blotting with anti-myc tag antibodies. The arrow shows the localization of an ∼9-kDa peptide. Apparent molecular masses of protein standard are indicated at left (SeeBlue Plus2; Invitrogen). The figure represents one of several similar experiments. Identical results were obtained in other cell types, namely, SK-OV 3 as well as CHO cells.
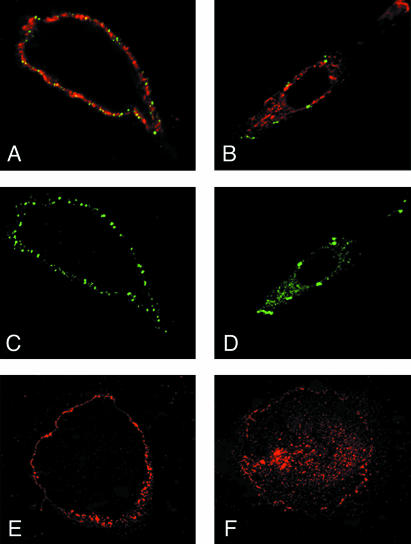
Immunolocalization of the fusion peptides via confocal laser microscopy and the anti-extracellular tag antibodies demonstrated localization on the cell membrane for all tested peptides, in the two cell lines, A431 and SK-OV3. Figure 3 shows the labeling patterns in A431 cells with both anti-ET (green) and anti-EGFR (red) antibodies (Figure 3A) and with anti-ET antibodies alone (Figure 3C). These pictures were recorded in nonpermeabilized cells and demonstrate a distinct bright outline labeling for all antibodies, indicating the peripheral membrane localization of antigens. This was confirmed thanks to analysis of pictures taken at different scanning depths. The membrane staining was not visible when the anti-myc tag antibody was used, indicating that essentially all TM fusion peptides were oriented with their N terminus outside the cells (our unpublished data). EGF receptors seemed to be very evenly distributed around cells, but a striking feature of the anti-ET labeling was its patched aspect (Figure 3C), possibly related to the tendency of the TM peptides to self-associate or aggregate (see DISCUSSION).
Figure 3.
Immunofluorescence analysis of whole A431 cells expressing the TM construct by using anti-EGFR and anti-ET tag antibodies. Staining of intact (A, C, and E) and permeabilized (B, D, and F) cells with anti-ET (antiexternal tag, green; A-D) and/or anti-EGFR (red; A, B, E, and F). A and C show intense membrane staining, indicating expression of the TM peptides at the cell surface, together with the receptors. B and D show membrane and vesicular labeling for both peptide and EGFR in permeabilized cells. E and F depict receptor localization in nontransfected cells.
The same immunolabeling experiments were performed on Triton X-100-permeabilized cells (Figure 3, B and D), by using anti-ET antibodies to detect recombinant peptides. Both peptide and receptor were found again localized in the cell membrane and also in a diffuse punctuated pattern in the cytoplasm, most likely corresponding to intracellular membrane vesicles.
Finally, comparison of the labeling pattern of the receptors in the presence (Figure 3, A and B) or absence of recombinant peptides (nontransfected cells, Figures 3, E and F) showed that expression of the peptides per se did not induce any apparent alteration in their biosynthesis and localization.
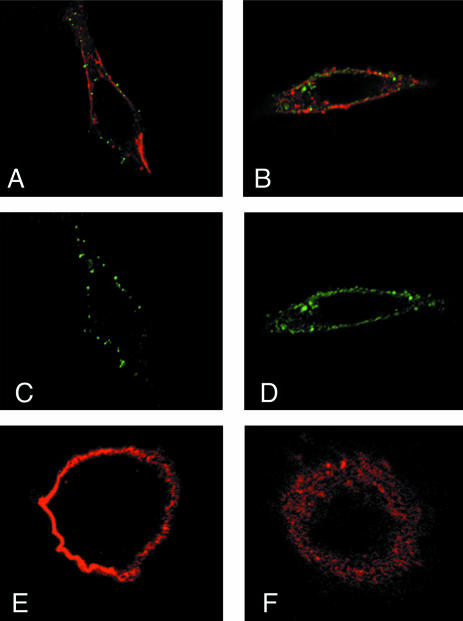
Figure 4 depicts the results of the same immunolocalization experiments of fusion peptides (green fluorescence) and ErbB2 receptors (red) in SK-OV3 cells. Again, in nonpermeabilized cells, peptides and receptor were clearly localized at the cell surface (Figure 4, A and C), with a patched appearance for the peptide (Figure 4C). In permeabilized cells, peptides and receptor also were found in intracellular vesicular structures (Figure 4, B and D). Localization of the receptor in nontransfected cells (Figure 4, E and F) was again identical to that in transfected cells (Figure 4, A and B).
Figure 4.
Immunofluorescence analysis of whole SK-OV3 cells expressing the TM construct by using anti-ErbB2 and anti-ET tag antibodies. Staining of intact (A, C, and E) and permeabilized (B, D, and F) cells with anti-ET (antiexternal tag, green; A-D) and/or anti-ErbB2 (red; A and B, E and F). A and C show intense membrane staining, indicating expression of the TM peptides at the cell surface, together with the receptors. B and D show membrane and vesicular labeling for both peptide and ErbB2 in permeabilized cells. E and F depict receptor localization in nontransfected cells.
Effects of TM Peptides on Receptor Expression, Phosphorylation, and Signaling
Previous experiments established that the expression plasmids were encoding peptides with the correct apparent molecular mass and expected membrane localization and orientation. We next assayed the effects of the expression of these fusion peptides on different functional aspects of the tyrosine-kinase receptors overexpressed in cell lines. To this end, we studied expression of the receptors and peptides together with autophosphorylation of the receptors and phosphorylation of one major kinase in their signaling path-ways, namely, MAPK/ERK 1/2. This was done by Western blot analysis of cell extracts at day 2 after transfection, by using antibodies directed against EGFR, ErbB2, or the myc tag, and antibodies specific to phosphotyrosine and the phosphorylated forms of ERK 1/2, respectively. Equivalent amounts of protein were used to allow for comparison between samples, and this was further checked by controlling the amounts of actin in each sample. Peptide expression was also checked using anti-myc tag antibody (myc-labeled panel in Figures 5 and 6).
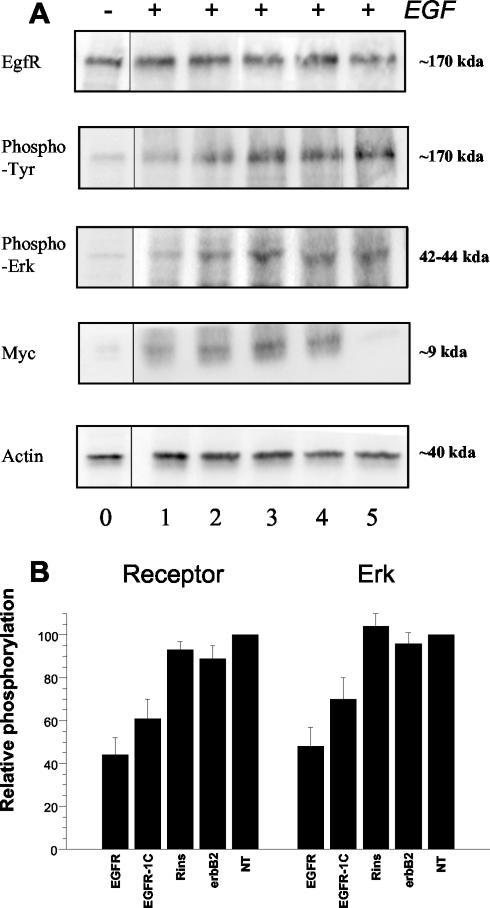
Figure 5.
Expression, autophosphorylation, and signaling of EGF receptors in transfected A431 cells. Experiments were performed at day 2 after transfection with pSecTag plasmids encoding for the TM domain of EGFR (column 1), a mutant EGFR TM (EGFR-1C, column 2), insulin receptor TM (column 3), ErbB2 TM (column 4) or without plasmid (column 5). Column 0 represents the phosphorylation status of non-EGF-stimulated cells. Identical protein amounts of A431 cell lysates were subjected to SDS-PAGE and Western blotting with the indicated antibodies. (A) Results of one of four similar experiments. (B) Combined results after computer analysis of the blots, as histograms (mean ± SEM), for EGFR (left) and ERK 1/2 (right) phosphorylation. Values in nontransfected cells are taken as 100%.
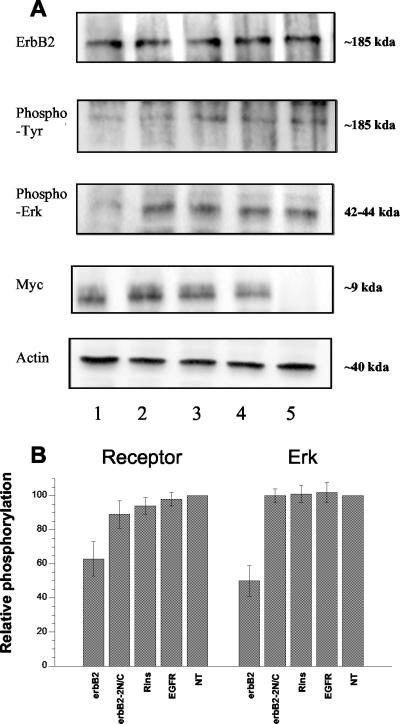
Figure 6.
Expression, autophosphorylation, and signaling of ErbB2 receptors in transfected SK-OV3 cells. Experiments were performed at day 2 after transfection with pSecTag plasmids encoding for the TM domain of ErbB2 (column 1), a mutant ErbB2 TM (ErbB2-2N/C, column 2), insulin receptor TM (column 3), EGFR TM (column 4), or without plasmid (column 5). Identical protein amounts of SK-OV3 cell lysates were subjected to SDS-PAGE and Western blotting with the indicated antibodies. (A) Results of one of four similar experiments. (B) Combined results after computer analysis of the blots, as histograms (mean ± SEM), for ErbB2 (left) and ERK 1/2 (right) phosphorylation. Values in nontransfected cells are taken as 100%.
Figure 5A depicts the activation status of the EGF pathway in A431 cells after transfection with plasmids encoding an EGFR TM peptide (column 1), a mutant less-dimerizing EGFR TM peptide (column 2), an insulin receptor TM peptide (column 3), an ErbB2 TM peptide (column 4), or in the absence of transfection (column 5), and stimulation with 10-8 M EGF for 5 min. EGFR and actin expression levels were not affected by transfection, because they were identical to the nontransfected control. On the contrary, phosphorylation of both EGFR and ERK was strongly inhibited by the expression of the cognate EGFR TM fusion peptide (column 1), but it was not affected by the unrelated insulin receptor and ErbB2 TM peptides (column 3), compared with nontransfected cells (column 5). The mutated EGFR TM sequence provoked a significant decreased of both EGFR and ERK phosphorylation (column 2), but it was less efficient than the native peptide. Quantification of independent experiments is summarized in Figure 5B. It confirms the decrease in phosphorylation of EGFR and ERK 1/2 by the EGFR TM peptide and to a lesser extent by the mutated EGFR TM sequence. Control peptides clearly have no effect.
Essentially identical results were obtained in SK-OV3 cells, which overexpress the ErbB2 receptor, as shown in Figure 6A. These cells were transfected with plasmids encoding an ErbB2 TM peptide (column 1), a mutant nondimerizing ErbB2 TM peptide (column 2), an insulin receptor TM peptide (column 3), an EGFR TM peptide (column 4), or in the absence of transfection (column 5). Again, receptor expression was not affected by transfection. Phosphorylation of ErbB2 receptor and ERK 1/2 was strongly decreased in the presence of the ErbB2 receptor TM peptide, whereas the double-mutant ErbB2 sequence and control peptides had no effect. Figure 6B summarizes the results of the analysis of several (3-8) independent experiments.
All these experiments on the two cell lines demonstrate that TM peptides can inhibit receptor phosphorylation and a downstream signaling enzyme, in a strongly specific manner because inhibition was only observed for a given native peptide on its cognate receptor
Effects of Synthetic TM Peptides
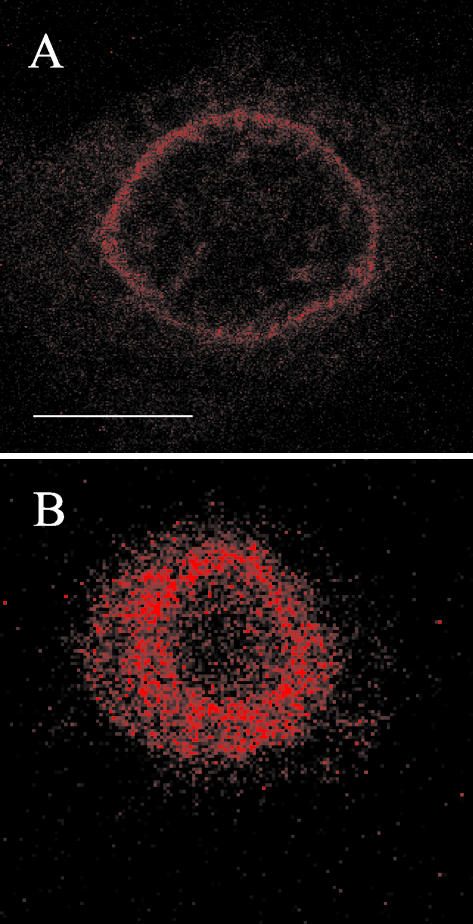
Our results demonstrate so far that membrane expression of EGFR and ErbB2 TM fusion peptides provoke a specific inhibition of the autophosphorylation and subsequent signaling of their respective receptor. Although the expression of the transfected peptides did slightly vary between experiments, the observed inhibitory effects were roughly proportional to the amount of detectable peptide. Notably, time-course experiments showed inhibition to be maximal at day 2 after transfection, the day at which maximal expression of the peptide was observed (our unpublished data). Nevertheless, our experimental design with transient transfection did not allow for measurement of the amount of effective peptide. In an attempt to gain more quantitative information, we thus ran similar experiments with synthetic peptides. Shorter peptides corresponding to the hydrophobic core of the TM domains of EGF and insulin receptors, without tags, were chemically synthesized and purified by reverse phase HPLC. Peptides were then incorporated into detergent micelles and administered to A431 cells by supplementation of the incubation medium (see MATERIALS AND METHODS). Control of membrane incorporation under these conditions was performed using a fluorescent rhodamine-labeled EGFR TM peptide. Confocal microscopy showed the fluorescent peptide to be localized on the cell surface (Figure 7A). No fluorescence was detectable inside the cells. The membrane localization of the peptide was confirmed by the analysis of pictures taken at different scanning depths. Maximal membrane incorporation of the fluorescent peptide was achieved after ∼40 min. We also checked that this membrane localization was not due to rhodamine itself. Indeed, incubation of A431 cells with a glycine-rhodamine derivative demonstrated that the fluorescent molecule was rapidly taken up into the cytoplasm (within a few minutes), which suggests a nonspecific mechanism (Figure 7B). Finally, to ascertain further the membrane localization of the rhodamine-conjugated peptide we used the fluorescence recovery after photobleaching technique, which showed progressive recovery of rhodamine-peptide fluorescence in the plane of the membrane after laser bleaching of a small cell surface (our unpublished data).
Figure 7.
Confocal fluorescence microscopy of cells in the presence of rhodamine-labeled EGFR TM peptide (A), or a glycine-rhodamine conjugate (B). Live A431 cells were grown in Lab-Tek chambers, incubated in the presence of rhodamine peptide (5 × 10-7 M) for 40 min, or glycine-rhodamine (3.5 × 10-7 M) for 3 min at 20°C before images were acquired (40× lens). White bar (A), 10 μm.
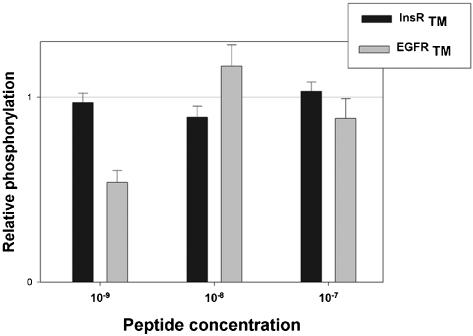
Effect of the incorporated peptide on EGFR phosphorylation was measured after 4 h of incubation and stimulation by 10-8 M EGF for 5 min, by Western blotting with anti-phosphotyrosine antibodies. Figure 8 presents the results of the scanner quantitation of four experiments as histograms. No or very little effect was observed at the higher concentrations (10-8-10-7 M) of EGFR peptide tested. In contrast, a striking decrease by >50% of receptor autophosphorylation is seen at 10-9 M of EGFR peptide. The insulin receptor peptide had no effect at all concentrations tested, and no effect on EGF binding was observed under these conditions.
Figure 8.
Autophosphorylation of EGF receptors in A431 cells incubated with synthetic TM peptides maintained in solution in detergent. Peptides corresponding to the hydrophobic core of the TM domains of EGF (gray areas) and insulin (black areas) receptors, were chemically synthesized and purified, incorporated in detergent micelles, and administered to A431 cells by supplementation of the incubation medium for 4 h. After 5 min of incubation in the presence of 10-8 M EGF, cells were lysed, and equal amounts of protein were submitted to SDS-PAGE and Western blot with anti-EGFR and anti-phosphotyrosine antibodies. Densitometric analysis of the blots was performed, and results were normalized according to the amount of immunoreactive EGFR in each sample, and expressed as percentage of the observed autophosphorylation in the absence of peptides (taken as 100%). The figure represents the results (mean ± SEM) of four similar experiments.
Effect of TM Peptides on Receptor Dimerization
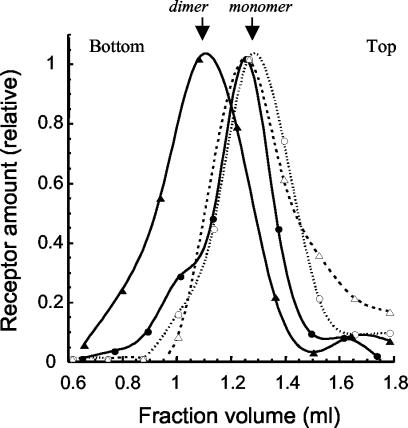
Together with published data about interactions between TM sequences, especially those of the ErbB receptor family (see DISCUSSION), our results imply that the specific inhibitory effects observed in our cellular assays are due to a direct interaction of the exogenous peptide with the cell endogenous receptor. By preventing dimerization of the receptors, the peptides would thus prevent trans-autophosphorylation inside receptor dimers. To attempt the demonstration of such a mechanism, we first used a sucrose gradient centrifugation method. Briefly, A431-transfected cells were lysed with a minimal amount of neutral detergent, and solubilized proteins were layered on top of a sucrose gradient. After centrifugation, 13-15 fractions were separated and submitted to electrophoresis and Western blotting with anti-EGF receptor antibodies. Using this technique, it is possible to distinguish light and EGF-induced heavy forms of the receptor, most likely corresponding to monomers and dimers (Northwood and Davis, 1988). Figure 9 shows that the distribution of EGF receptors solubilized from untransfected and EGF-stimulated A431 cells displays an important shift from a light, monomeric form (open triangles) to a heavier dimeric one (closed triangles). This distribution is strongly affected when A431 cells are transfected with the EGF receptor TM. In this case, the EGF-induced shift to a dimeric receptor is much reduced and occurs as a shoulder (open and closed circles). Transfection of A431 cells with insulin receptor TM peptides showed a distribution of receptors identical to the nontransfected cells, confirming the specificity of the effect (our unpublished data).
Figure 9.
Sucrose gradient analysis of EGF receptor dimerization in the presence or absence of TM peptide. A431 cells were transfected or not with the pSecTag plasmid encoding for the EGFR TM peptide. At day 2 after transfection, cells were stimulated or not with EGF (10-8 M, 5 min), lysed with neutral detergent, and solubilized proteins were layered on top of a sucrose gradient. After centrifugation, fractions were separated and submitted to SDS-PAGE electrophoresis and Western blotting with anti-EGF receptor antibodies. The figure shows the densitometric quantification of a typical blot out of three similar experiments. Values were normalized relatively to the highest peak, to account for differences in total receptor content. Symbols are as follow: triangles, untransfected A431 cells; circles, cells transfected with the pSecTag plasmid encoding for the EGFR TM peptide. Open symbols and dotted lines are for unstimulated cells; filled symbols and full lines are for cells stimulated with EGF. Top and bottom of the gradient are indicated.
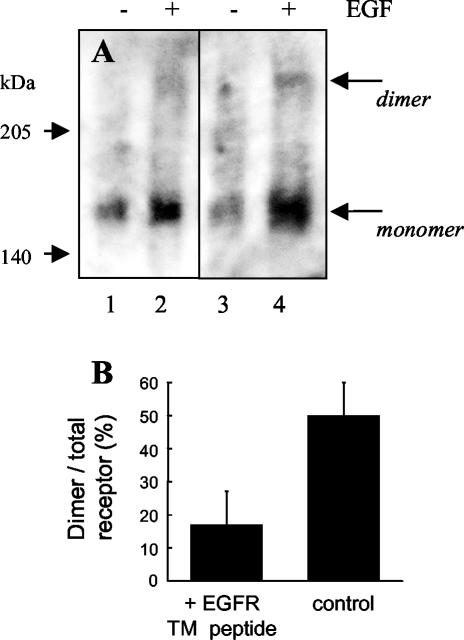
Because it is difficult to derive quantitative information on the dimer/monomer ratio from sucrose gradient profiles, we also performed covalent cross-linking experiments (Figure 10). Again, it is clear that EGF induces receptor dimerization in nontransfected cells (Figure 10A, columns 3 and 4). In contrast, receptor dimerization is much reduced in cells transfected with the EGFR TM peptide (columns 1 and 2). Densitometric quantitation of four different experiments is shown as histograms in Figure 10B. The percentage ratio of dimeric form/total receptor (dimer/dimer + monomer) varies from 50% in EGF-stimulated control cells to 17% in cells transfected with the EGFR TM peptide. This corresponds to an inhibition of dimerization of ∼63% by the TM peptide, in good agreement with the observed effect on other parameters.
Figure 10.
Analysis of EGF receptor dimerization in the presence or absence of TM peptide by using a cross-linking agent. A431 cells were transfected or not with the pSecTag plasmid encoding for the EGFR TM peptide. At day 2 after transfection, cells were stimulated or not with EGF (10-8 M, 5 min), cross-linked with disuccinimidyl suberate, and lysed. Samples were immunoprecipitated and submitted to SDS-PAGE electrophoresis and Western blotting with anti-EGF receptor antibodies. (A) Typical blot out of four experiments. The first two columns represent cells transfected with the EGFR TM peptide without (-) and with (+) stimulation with EGF. Columns 3 and 4 represent control untransfected cells. (B) Mean ratio (± SEM) of dimer versus total receptor (dimer/monomer + dimer) in EGF-stimulated cells after densitometric analysis of the blots.
DISCUSSION
The purpose of this study was to examine the hypothesis that TM domains of receptor tyrosine kinases play an active role in the activation of these receptors. More and more data show 1) that such TM peptides tend to dimerize in detergent solution and in membranes (Smith et al., 1996; Sharpe et al., 2001, 2002a; Mendrola et al., 2002), 2) that some TM mutations can activate constitutively or inhibit their kinase activity (Weiner et al., 1989; Cheatham et al., 1993; Webster and Donoghue, 1996; Burke et al., 1997; Chen et al., 1997; Li et al., 1997; Gardin et al., 1999; Moriki et al., 2001), and 3) there exists a limited knowledge of the specific amino acid patterns that govern these interactions (Sternberg and Gullick, 1990; Senes et al., 2000; Mendrola et al., 2002). If interactions between TM peptides occur during the dimerization of the whole receptors, then introduction in the membrane of homologous sequences corresponding to the TM domain should act as competitors of the dimerization and thus inhibit the kinase activity. To test the validity of this idea, and the eventual specificity of such an effect, we used either expression plasmids encoding TM domains together with extra- and intracellular tags and the required addressing sequences, or chemically synthesized hydrophobic peptides. These were introduced in the plasma membrane of two human cancer cell lines that overexpress either the EGF receptor (ErbB1/HER1 and A431 cells) or ErbB2 (HER2 and SK-OV3 cells) through transfection or detergent micelles.
Control experiments demonstrated that both procedures did efficiently target the exogenous TM peptides to the cell membranes. Both the fusion peptide and fluorescent synthetic peptide occurred with an irregular, patched distribution on the cell surface. This is most probably due to the known tendency of such short alpha-helical hydrophobic peptides to self-assembly in oligomeric structures. For example, Sharpe et al. (2002a) have shown in NMR experiments that ErbB2 TM domains tend to form large oligomers, depending on their concentration. This phenomenon is also the probable reason why synthetic peptides were inhibitory at a 10-9-10-8 M concentration. At higher concentrations, they likely exist mostly as inefficient dimers or oligomers.
We used a detergent dilution technique for the incorporation of the synthetic peptides rather than the more widely used liposome technique. Preliminary experiments had shown that both techniques were equivalent. However, we chose the detergent method for its ease in sample preparation and good reproducibility. It is equivalent to the classical detergent dilution or removal technique, widely used for proteoliposome preparation. We found that delivery of the detergent-peptide micelles was not detrimental to cell viability, provided that final concentration of detergent was <1/100th of its critical micellar concentration. This low detergent concentration allows for micelle disruption and partitioning of the hydrophobic peptide into the cell membrane.
In both cells lines, we found that introduction of the peptides inhibited specifically the autophosphorylation and signaling pathway (phosphorylation of MAPK/ERK 1/2) of their cognate receptor. These results extend, and confirm, a previous report by Lofts et al. (1993) that demonstrated that a plasmid-encoded mutated TM sequence (V664E) of the rat neu/ErbB2 receptor could inhibit cell growth of mutant neu-transformed NIH3T3 cells in monolayers, in soft agar colonies and as tumors in nude mice. Although this work did not include characterization of receptor autophosphorylation and signaling, the studies could demonstrate that the wild-type sequence was also effective in inhibiting cell growth, albeit with a lesser potency than the mutated sequence. In our experimental system, we also have obtained preliminary evidence that the effect of TM peptide expression on receptor autophosphorylation was accompanied by a decrease in cell proliferation as judged by cell viability measurements [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium tests; our unpublished data]. Cell viability in low serum was markedly decreased (by ∼40%) at days 2 and 3 after transfection with EGFR fusion peptide in A431 cells, and erbB2 peptide in SK-OV3 cells. Again, this effect was specific, because expression of peptides other than the cognate TM sequence did not alter cell growth.
It may seem surprising that the average maximal effect of TM peptides reached no more than 50-60%, for the inhibition of receptor phosphorylation, ERK phosphorylation, and other parameters. This partial inhibition is most probably due to our experimental setting. If transient transfection did yield reasonable expression levels (∼90% of cells expressed the peptides as estimated from immunocytochemistry), it should be stressed that the individual expression levels were heterogenous among cells. Thus, the observed ∼50% effect may simply reflect the heterogeneity in peptide levels as an average value. In addition, transmembrane domain interactions certainly do not represent the unique driving force for receptor dimerization and activation. Recent structural studies have confirmed in detail that ligand-induced extracellular domain interactions play an important role. It is thus conceivable that interfering with transmembrane domain interactions is not sufficient to completely overcome ligand-induced conformational changes in the erbB receptors.
Nevertheless, in our experiments, the measured effects were highly selective for homolog TM sequences, i.e., one TM peptide was only inhibitory for its “parental” receptor. This indicates clearly that the inhibition is due to interactions at the TM level and not to possible effects of the extramembrane portions of our fusion peptides. Also, the shorter synthetic peptides, encompassing the TM domain only, were as effective as the fusion peptides.
Furthermore, by using mutant sequences, we also could confirm the importance of a short pattern, called the GxxxG motif, which was found to be most important for glycophorin A dimerization (Senes et al., 2000). This pattern consists of the association of two small amino acids separated by three indifferent residues. It is present in two copies in the TM sequences of EGFR (TgmvG and AlgiG) and ErbB2 (SavvG and GvvfG). Indeed, Mendrola et al. (2002)), by using a bacterial gene reporter assay, named Toxcat (Russ and Engelman, 1999), have shown this short sequence to be important in the dimerization of the TM domains of all four ErbB receptors. We have been able to confirm independently their results by using the same Toxcat system (our unpublished data). Mendrola et al. (2002) also reported that mutations (Gly→Val) of the fifth amino acid in the C-terminal motif of EGFR TM, and of both N- and C-terminal motifs of ErbB2 TM were the most efficient in reducing the dimerization of these receptor TM sequences. We therefore tested the ability of these plasmid-encoded mutant sequences (EGFR-1C and ErbB2-2N/C) to inhibit their corresponding receptors in our cellular assay. Both behaved according to prediction in that the ErbB2 double mutant, which displays very weak dimerization in Toxcat assay, was totally ineffective in inhibiting ErbB2 phosphorylation and signaling. In contrast, the EGFR-1C mutant, which has an ∼50% reduced dimerizing potency in Toxcat assay, was still partly inhibitory for EGFR signaling.
The colocalization of receptors and peptides at the cell surface, as evidenced by immunocytochemistry, is not enough to prove that peptides and receptors are physically associated, nor give insight into the mechanisms involved. Demonstration of such a specific association of the plasmid-encoded TM peptide and its cognate receptor proved to be difficult. This might be due in part to the low apparent affinity of TM domain interactions, as has been established in several systems. For example, by using fluorescence resonance energy transfer to measure dimerization of synthetically labeled glycophorin A TM domains, the dissociation constant has been estimated to range from ∼0.1 μM to ∼0.2 μM in zwitterionic detergent micelles (Fisher et al., 1999). This apparent Kd depends on both detergent type and detergent concentration. By using the same approach, the apparent dissociation constant of the mutant ErbB2 TM peptide has been found to be ∼10 times higher (Duneau and Sturgis, Centre National de la Recherche Scientifique, Marseille, France, personal communication). Thus, the unavoidable use of detergents for receptor solubilization, and subsequent washing steps in coimmunoprecipitation experiments, made it impractical to show receptor-peptide interactions in this way. We rather chose to use sucrose gradient centrifugation and chemical cross-linking experiments to demonstrate that the mechanism of action of the TM peptide was through inhibition of EGF receptor dimerization. Such a mechanism was already indicated by the inability of the less-dimerizing mutated peptide EGFR-1C to totally mimic the effects of the native sequence. In the case of the EGF receptor, these approaches revealed that the presence of the EGFR TM peptide did inhibit the EGF-induced dimerization, whereas other peptides were completely inefficient. The same mechanism is probably also operative for ErbB2, with the major difference that in this case it is heterodimerization with other ErbB receptors that would be inhibited. It has recently been shown that the TM domains of ErbB2 and the EGFR receptor interact in liposomes (Sharpe et al., 2002b).
In conclusion, together with the previous demonstration that addition of the transmembrane segment greatly enhances the dimerization of the extracellular domain of the EGF receptor (Tanner and Kyte, 1999) and the known effects of some mutations in this segment (Weiner et al., 1989), our findings strongly argue in favor of an important role for hydrophobic TM domain interactions in ErbB receptor function. These interactions could act in concert with the ligand-induced association of the extracellular domains, which already contain one or more dimerizing subdomains. Coordinated association of the TM domains could then favor productive dimerization at the intracellular kinase domains to promote trans-autophosphorylation. Further studies should address the question of the relative contribution of interface interactions in the different domains of these receptors. The present work demonstrates that full length transmembrane domains can be used as dimerization and activation inhibitors for some RTKs. It will be of prime interest to study whether similar inhibitory approaches also may be applicable to other RTKs, most notably FGF receptors (Webster and Donoghue, 1996) or other single-spanning membrane proteins.
Acknowledgments
We thank Gérard Nullans for expertise in the synthesis and purification of the rhodamine-labeled peptide and Dr. Jean-Luc Popot for helpful discussions. Confocal fluorescence microscopy was performed using the equipment of the IFR 37 “Neurosciences” in vitro imaging platform. We also thank Drs. Russ and Engelman (Yale University, New Haven, CT) for the gift of the TOXCAT membrane protein interactions reporter system, and Patrice Gadroy and Maria Meira for running the TOXCAT experiments. This work was supported by Institut National de la Santé et de la Recherche Médicale, and grants from the Centre National de la Recherche Scientifique (Physico-Chimie du Vivant program), the French Association pour la Recherche contre le Cancer, the French Ligue contre le Cancer (Bas-Rhin, Haut-Rhin and Montbéliard committees), and a research grant from the Slovak Grant Agency (VEGA2/1098/21).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-10-0753. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-10-0753.
Abbreviations used: EGF(R), epidermal growth factor (receptor); ET, extracellular tag; RTK, receptor tyrosine kinase; TM, transmembrane.
References
- Bargmann, C.I., Hung, M.C., and Weinberg, R.A. (1986). Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 45, 649-657. [DOI] [PubMed] [Google Scholar]
- Bell, C.A., Tynan, J.A., Hart, K.C., Meyer, A.N., Robertson, S.C., and Donoghue, D.J. (2000). Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Mol. Biol. Cell 11, 3589-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355-365. [DOI] [PubMed] [Google Scholar]
- Burke, C.L., Lemmon, M.A., Coren, B.A., Engelman, D.M., and Stern, D.F. (1997). Dimerization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene 14, 687-696. [DOI] [PubMed] [Google Scholar]
- Cheatham, B., Shoelson, S.E., Yamada, K., Goncalves, E., and Kahn, C.R. (1993). Substitution of the erbB-2 oncoprotein transmembrane domain activates the insulin receptor and modulates the action of insulin and insulin-receptor substrate 1. Proc. Natl. Acad. Sci. USA 90, 7336-7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.I., Webster, M.K., Meyer, A.N., and Donoghue, D.J. (1997). Transmembrane domain sequence requirements for activation of the p185(c-neu) receptor tyrosine kinase. J. Cell Biol. 137, 619-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, L.E., Engelman, D.M., and Sturgis, J.N. (1999). Detergents modulate dimerization, but not helicity, of the glycophorin A transmembrane domain. J. Mol. Biol. 293, 639-651. [DOI] [PubMed] [Google Scholar]
- Gardin, A., Auzan, C., Clauser, E., Malherbe, T., Aunis, D., Crémel, G., and Hubert, P. (1999). Substitution of the insulin receptor transmembrane domain with that of glycophorin A inhibits insulin action. FASEB J. 13, 1347-1357. [DOI] [PubMed] [Google Scholar]
- Heldin, C.H. (1995). Dimerization of cell surface receptors in signal transduction. Cell 80, 213-223. [DOI] [PubMed] [Google Scholar]
- Hubbard, S.R. (1999). Structural analysis of receptor tyrosine kinases. Prog. Biophys. Mol. Biol. 71, 343-358. [DOI] [PubMed] [Google Scholar]
- Kolibaba, K.S., and Druker, B.J. (1997). Protein tyrosine kinases and cancer. Biochim. Biophys. Acta 1333, F217-F248. [DOI] [PubMed] [Google Scholar]
- Lemmon, M.A., Bu, Z.M., Ladbury, J.E., Zhou, M., Pinchasi, D., Lax, I., Engelman, D.M., and Schlessinger, J. (1997). Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 16, 281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray, V., Hubert, P., Crémel, G., and C.S. (1992). Detergents affect insulin binding, tyrosine kinase activity and oligomeric structure of partially purified insulin receptors. Arch. Biochem. Biophys. 294, 22-29. [DOI] [PubMed] [Google Scholar]
- Li, Y., Mangasarian, K., Mansukhani, A., and Basilico, C. (1997). Activation of FGF receptors by mutations in the transmembrane domain. Oncogene 14, 1397-1406. [DOI] [PubMed] [Google Scholar]
- Lofts, F.J., Hurst, H.C., Sternberg, M.J.E., and Gullick, W.J. (1993). Specific short transmembrane sequences can inhibit transformation by the mutant neu growth factor receptor in vitro and in vivo. Oncogene 8, 2813-2820. [PubMed] [Google Scholar]
- Mendelsohn, J., and Baselga, J. (2000). The EGF receptor family as targets for cancer therapy. Oncogene 19, 6550-6565. [DOI] [PubMed] [Google Scholar]
- Mendrola, J.M., Berger, M.B., King, M.C., and Lemmon, M.A. (2002). The single transmembrane domains of ErbB receptors self-associate in cell membranes. J. Biol. Chem. 277, 4704-4712. [DOI] [PubMed] [Google Scholar]
- Moriki, T., Maruyama, H., and Maruyama, I.N. (2001). Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J. Mol. Biol. 311, 1011-1026. [DOI] [PubMed] [Google Scholar]
- Northwood, I.C., and Davis, R.J. (1988). Activation of the epidermal growth factor receptor tyrosine protein kinase in the absence of receptor oligomerization. J. Biol. Chem. 263, 7450-7453. [PubMed] [Google Scholar]
- Robertson, S.C., Tynan, J.A., and Donoghue, D.J. (2000). RTK mutations and human syndromes: when good receptors turn bad. Trends Genet 16, 265-271. [DOI] [PubMed] [Google Scholar]
- Russ, W.P., and Engelman, D.M. (1999). TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc. Natl. Acad. Sci. USA 96, 863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, G., Akita, R.W., and Sliwkowski, M.X. (1999). A discrete three-amino acid segment (LVI) at the C-terminal end of kinase-impaired ErbB3 is required for transactivation of ErbB2. J. Biol. Chem. 274, 859-866. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J. (2000). Cell signaling by receptor tyrosine kinases. Cell 103, 211-225. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J. (2002). Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110, 669-672. [DOI] [PubMed] [Google Scholar]
- Senes, A., Gerstein, M., and Engelman, D.M. (2000). Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 296, 921-936. [DOI] [PubMed] [Google Scholar]
- Sharpe, S., Barber, K.R., and Grant, C.W. (2002a). Evidence of a tendency to self-association of the transmembrane domain of ErbB-2 in fluid phospholipid bilayers. Biochemistry 41, 2341-2352. [DOI] [PubMed] [Google Scholar]
- Sharpe, S., Barber, K.R., and Grant, C.W. (2002b). Interaction between ErbB-1 and ErbB-2 transmembrane domains in bilayer membranes. FEBS Lett. 519, 103-107. [DOI] [PubMed] [Google Scholar]
- Sharpe, S., Grant, C.W., Barber, K.R., Giusti, J., and Morrow, M.R. (2001). Structural implications of a Val→Glu mutation in transmembrane peptides from the EGF receptor. Biophys. J. 81, 3231-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon, D.J., et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707-712. [DOI] [PubMed] [Google Scholar]
- Smith, S.O., Smith, C.S., and Bormann, B.J. (1996). Strong hydrogen bonding interactions involving a buried glutamic acid in the transmembrane sequence of the neu/erbB-2 receptor. Nat. Struct. Biol. 3, 252-258. [DOI] [PubMed] [Google Scholar]
- Sternberg, M.J.E., and Gullick, W.J. (1990). A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein Eng. 3, 245-248. [DOI] [PubMed] [Google Scholar]
- Tanner, K.G., and Kyte, J. (1999). Dimerization of the extracellular domain of the receptor for epidermal growth factor containing the membrane-spanning segment in response to treatment with epidermal growth factor. J. Biol. Chem. 274, 35985-35990. [DOI] [PubMed] [Google Scholar]
- Ullrich, A., and Schlessinger, J. (1990). Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203-212. [DOI] [PubMed] [Google Scholar]
- Webster, M.K., and Donoghue, D.J. (1996). Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J. 15, 520-527. [PMC free article] [PubMed] [Google Scholar]
- Weiner, D.B., Liu, J., Cohen, J.A., Williams, W.V., and Greene, M.I. (1989). A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature 339, 230-231. [DOI] [PubMed] [Google Scholar]
- Weiss, A., and Schlessinger, J. (1998). Switching signals on or off by receptor dimerization. Cell 94, 277-280. [DOI] [PubMed] [Google Scholar]