Abstract
A limited number of pulmonary adenocarcinoma cases with morule-like components have been described to date, and the most frequent histological subtype is papillary-predominant adenocarcinoma. Occasionally, this type of adenocarcinoma is associated with solid-predominant adenocarcinoma. EGFR mutations are predominant in adenocarcinoma with morule-like components, followed by ALK rearrangements. Herein, we present 2 cases of solid-predominant adenocarcinoma with morule-like components harboring either an EGFR or KRAS mutation. This KRAS-mutant case is the first to be associated with morule-like components, to the best of our knowledge. Both cases showed transition between micropapillary and morule-like components. Transition between morule-like and solid components was also observed in both cases. Although a few cases of solid-predominant adenocarcinoma have been shown to harbor morule-like components, this type of transition has not been previously well described. We surmised that the solid components of some EGFR-mutant adenocarcinomas might be derived from morule-like components.
Keywords: Lung, adenocarcinoma, morule, solid, EGFR, KRAS
Introduction
Morules are small buds of ovoid-cell proliferation lacking significant nuclear atypia and are typically observed in endometrioid adenocarcinoma [1]. While they have been detected in several tumors such as thyroid carcinoma [2] and colonic adenoma [3], they have also been found in lung tumors such as pulmonary blastoma and low-grade adenocarcinoma of the fetal lung type [4,5].
Bronchioloalveolar carcinoma with morule-like features was first reported in 2003 [6]. Subsequently, several cases of pulmonary adenocarcinoma with morule-like components have been described [1,7]. In 2014, Tsuta et al. [8] and Matsukuma et al. [9] reported 17 cases of adenocarcinoma with morule-like components and 7 cases of adenocarcinoma with non-sarcomatous spindle-cell foci (N-SSCF) or morule-like foci, respectively. The most frequent histological subtype of adenocarcinoma with morule-like components was papillary-predominant adenocarcinoma [1,6-9]. Occasionally, this type of adenocarcinoma was associated with solid-predominant adenocarcinoma [8].
EGFR mutations were predominant in adenocarcinoma with morule-like components, followed by ALK rearrangements, in a study by Tsuta et al. [8]. However, very few cases were examined, and the involvement of other gene mutations could not be excluded.
Herein, we present 2 cases of adenocarcinoma with morule-like components, both of which showed solid-predominant adenocarcinoma. One had an EGFR mutation and the other, a KRAS mutation. In this report, we consider the relationship between morule-like and solid components, and the possibility that there could be transition from the former to the latter type.
Clinical summary
Case 1
A 60-year-old man without any significant past medical history was referred to our hospital because of an abnormal chest radiograph. He was a non-smoker. Chest computed tomography (CT) showed a cavitated mass that measured 33 × 30 × 28 mm in the S8 segment of the right lower lobe (Figure 1A). Although the cavitated mass was difficult to diagnose using bronchoscopy, significant accumulation of F-18-fluorodeoxyglucose in the mass during positron emission tomography (PET) strongly suggested malignancy. Subsequently, surgery was performed and as the intraoperative aspiration biopsy cytology revealed the presence of carcinoma, a right lower lobectomy was performed. A histopathological examination confirmed the diagnosis of solid-predominant adenocarcinoma. The patient’s post-operative course was uneventful, and 31 months after surgery, the patient was free of recurrent disease.
Figure 1.
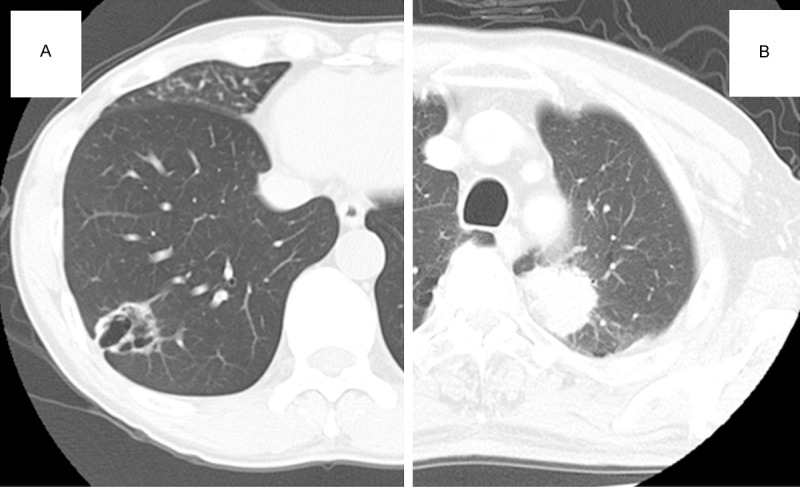
Computed tomography findings. A. A chest computed tomography scan showing a cavitated mass, 33 × 30 × 28 mm, in the right lower lobe. The wall of the mass is relatively thicker than that of a typical benign cyst. B. A chest computed tomography scan showing a mass, 42 × 40 × 35 mm, with a serrated margin in the left upper lobe.
Case 2
A 72-year-old man attended our hospital for a routine check-up after surgery for bladder cancer. He had quit smoking 15 years previously, but prior to this, he had smoked 15 cigarettes a day for 35 years. A chest CT scan revealed a mass, 42 × 40 × 35 mm, with a serrated margin in the S1+2 segments of the left upper lobe (Figure 1B); no significant lymph node swelling was observed. Although the mass was difficult to diagnose on bronchoscopy, PET showed significant accumulation of F-18-fluorodeoxyglucose in the mass. As this was strongly suggestive of malignancy, a partial resection of the left upper lobe was performed. The histopathological diagnosis of the mass was solid-predominant adenocarcinoma. The patient’s post-operative course was uneventful and 12 months after surgery, the patient is free of recurrent disease.
Pathological findings
Case 1
The surgically resected tumor was off-white with a central cavity. Some necrotic material was attached to the cavitary wall (Figure 2A). On histopathological examination, the tumor was a solid-predominant adenocarcinoma with peripheral lepidic or papillary growth. A morule-like component was observed between the papillary and solid growth areas (Figure 3A). Transition between the micropapillary and morule-like components was observed at the periphery (Figure 3B). Transition between the morule-like and solid components was also observed more inward than that of the micropapillary and morule-like components. The cellular composition of the solid component seemed to be similar to the morule-like component (Figure 3C). The constituent cells of the morule-like cellular aggregate showed slightly less nuclear atypia than the cuboidal to columnar adenocarcinoma cells. Some of the tumor cells in the cellular aggregate showed ovoid to short-spindled morphology (Figure 3D). Mutational analysis of EGFR exons 18, 19, 20, and 21 revealed a deletion mutation at exon 19: del L747_E749, A750P.
Figure 2.
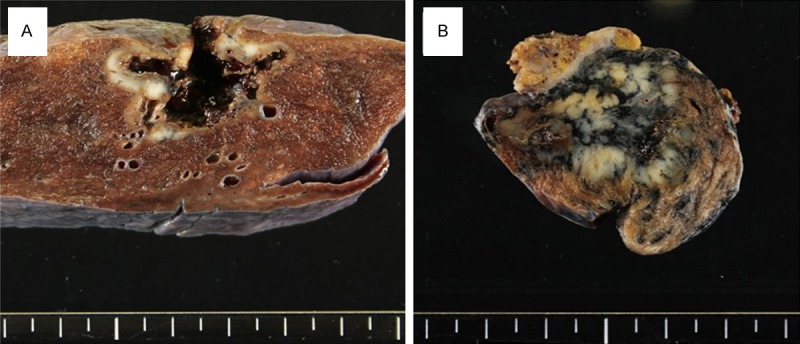
Macroscopic findings. A. An off-white tumor with a central cavity is observed. Some necrotic material is attached to the cavitary wall. B. An off-white to yellowish-white tumor is observed. It is accompanied by heavy anthracosis.
Figure 3.
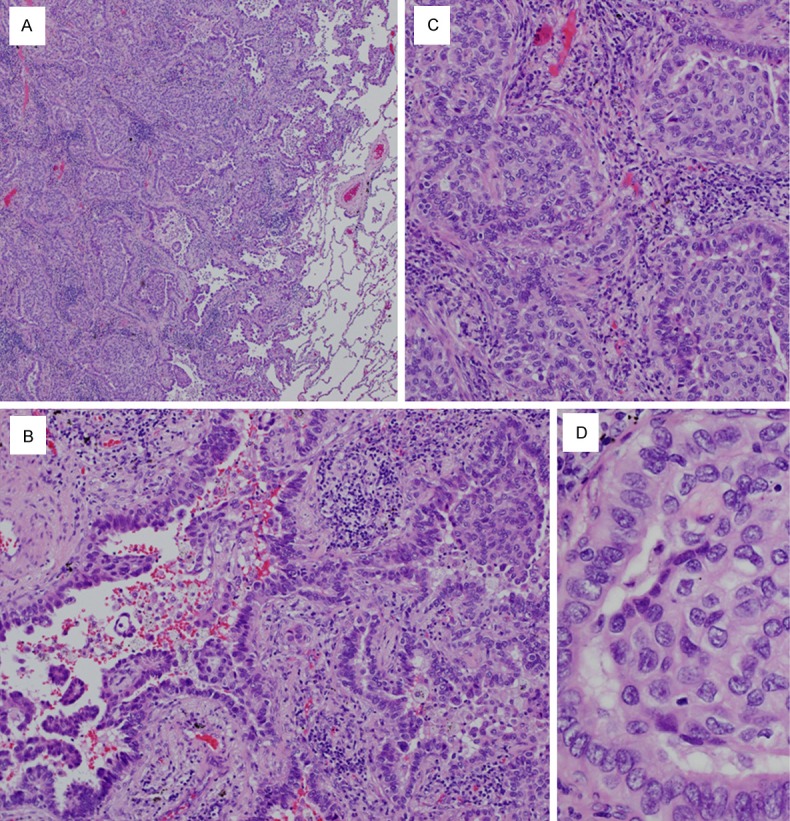
Microscopic findings of Case 1. A. The tumor is a solid-predominant adenocarcinoma with lepidic growth or papillary growth at the periphery. A morule-like component can be observed between the papillary and solid growth areas (× 20). B. Transition between the micropapillary (lower left) and morule-like components (upper right) is observed (× 200). C. Transition between the morule-like (right half) and solid components (left half) is observed. The cellular composition of the solid component seems to be similar to the morule-like component (× 200). D. The constituent cells of the morule-like cellular aggregate show slightly less nuclear atypia than the cuboidal to columnar adenocarcinoma cells. Some of the tumor cells in the cellular aggregate show ovoid to short-spindled morphology (× 400).
Case 2
The surgically resected tumor was off-white to yellowish-white and was accompanied by heavy anthracosis (Figure 2B). On histopathological examination, the tumor was a solid-predominant adenocarcinoma with papillary growth in some places. A morule-like component was observed between the papillary and solid growth areas (Figure 4A). Transition between the micropapillary and morule-like components was observed (Figure 4B). Transition between the morule-like and solid components was also observed; the cellular composition of the solid component seemed to be similar to the morule-like component (Figure 4C). The constituent cells of the morule-like cellular aggregate showed slightly less nuclear atypia than the cuboidal to columnar adenocarcinoma cells. Some of the tumor cells in the cellular aggregate showed ovoid to short-spindled morphology (Figure 4D). Mutational analysis of EGFR exons 18, 19, 20, and 21 revealed no mutations; however, analysis of KRAS codons 12 and 13 identified a substitution mutation at codon 12: G12C.
Figure 4.
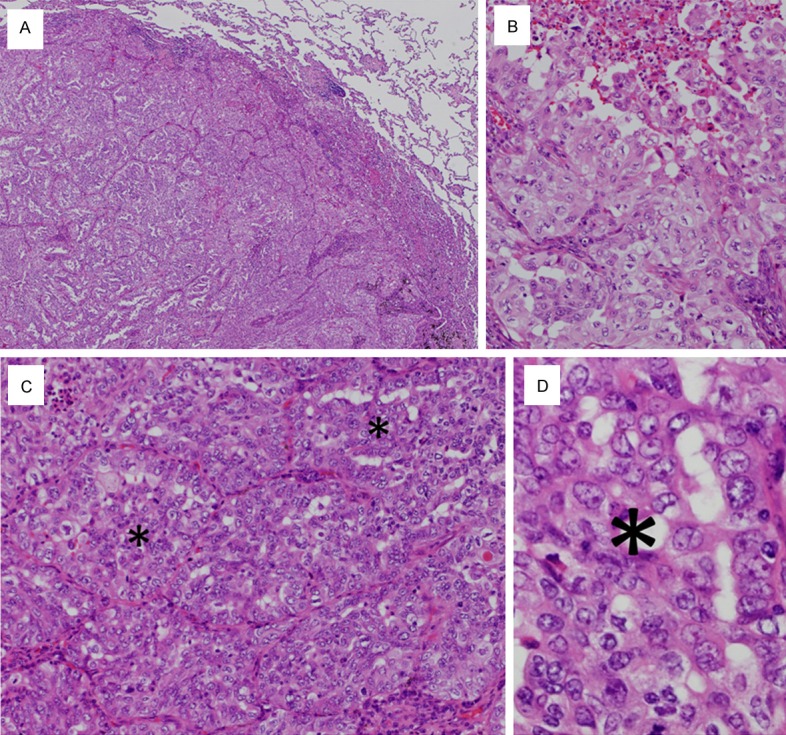
Microscopic findings of Case 2. A. The tumor is a solid-predominant adenocarcinoma with papillary growth in some places (× 20). B. Transition between the micropapillary (upper half) and morule-like components (lower left) is observed in some places (× 200). C. Transition between the morule-like and solid components is observed; the cellular composition of the solid component seems to be similar to the morule-like component. Asterisks indicate morule-like components (× 200). D. The constituent cells of the morule-like cellular aggregate show slightly less nuclear atypia than the cuboidal to columnar adenocarcinoma cells. Some of the tumor cells in the cellular aggregate show ovoid to short-spindled morphology. An asterisk indicates the morule-like cellular aggregate (× 400).
Discussion
The incidence of adenocarcinoma with morule-like components (or N-SSCF) ranges from 1.9% (17 of 904 cases) to 4% (7 of 173 cases) [8,9]. It has been determined that morule-like components are different from the morules identified in pulmonary blastoma and low-grade adenocarcinoma of the fetal lung type [8,9]. The morule-like components consisted of tightly packed spindle-or round-shaped cells occupying 5-50% of the entire tumor [8,9]. They were observed within the air spaces of the tumor nests, or, in cases of insular N-SSCF, they seemed to connect several tumor nests [9]. Tsuta et al. speculated that the morule-like components represented a form of stromal invasion in spite of the apparent desmoplastic reaction [8]; however, Matsukuma et al. surmised that they were probably not aggressive, invasive components [9].
In a study by Tsuta et al. [8], papillary-predominant adenocarcinoma was the most frequent histological subtype of adenocarcinoma with morule-like components (53%; 9 of 17 cases). The remaining subtypes were acinar-predominant (17.6%; 3 of 17 cases), micropapillary-predominant (17.6%; 3 of 17 cases), solid-predominant (5.9%; 1 of 17 cases), and lepidic-predominant (5.9%; 1 of 17 cases) adenocarcinomas. Moreover, in a report by Matsukuma et al. [9], all of the cases (total, 7) had papillary-predominant adenocarcinoma. Similar to our investigation, transition between the micropapillary and morule-like components was observed by Tsuta et al. [8], and was at least focally identified in 88% of cases (15 of 17 cases).
Tsuta et al. suggested that morule-like components could be caused by overgrowth of the micropapillary component as analysis using serial sections revealed that most micropapillary component tufts were connected with other tufts and did not truly float in the air spaces [8,10]. However, while Matsukuma et al. recognized some similarities between N-SSCF and the micropapillary component, they thought that these two morphological features were distinct [9]. These different views make it difficult to determine whether the morule-like component represents a true prognostic indicator, whereas it has been confirmed that the micropapillary component predicts poor prognosis [11]. Tsuta et al. demonstrated that morule-like components were one of the indicators of poor prognosis for lung adenocarcinoma; in contrast, the results of Matsukuma et al. did not agree with this finding.
It has been suggested that there are 2 types of morule-like components: micropapillary-associated and non-micropapillary-associated. The former shows more atypia and is associated with an unfavorable prognosis. Most of the cases reported by Tsuta et al. [8] may have corresponded to the former, while those of Matsukuma et al. [9] may have corresponded to the latter. The 2 cases in our study had micropapillary-associated morule-like components.
It was proposed by Matsukuma et al. that N-SSCF should be regarded as a solid component, different from the traditional solid component because of its non-high-grade cytology containing indolent-like spindle cells [9]. As both of our cases had solid components probably derived from morule-like components (or N-SSCF), this proposition seems reasonable. However, the constituent cells of the solid components and the morule-like components in our case were not as bland looking as those described by Matsukuma et al.
EGFR mutations (70.6%; 12 of 17 cases), especially deletion mutations, occurred more frequently in adenocarcinoma patients with morule-like components than in the total adenocarcinoma patient group (40.5%; 356 of 880 cases), as reported by Tsuta et al. [8]. This mutation rate ranges from 46% to 64% in the few representative studies of Japanese patients [12-14]; we restricted our analysis to Japanese patients to ensure consistency with the cohort studied by Tsuta et al. ALK rearrangements were also associated with adenocarcinoma with morule-like components in the study by Tsuta et al. (5.9%; 1 of 17 cases) [8]. In our study, one patient had an EGFR-deletion mutation and the other, a KRAS-point mutation.
It is known that KRAS mutations are more likely to be present in adenocarcinomas with solid components than other gene mutations [15]. KRAS mutants are more likely to have ≥ 20% solid components (44%; 28 of 63 cases) compared with EGFR (6%; 2 of 35 cases) and non-KRAS/EGFR mutants (26%; 21 of 82 cases) [15]. Based on both of our solid-predominant adenocarcinoma cases with either an EGFR or KRAS mutation and the study conducted by Tsuta et al [8], we suggest that the solid component of some EGFR mutants may be derived from morule-like components, and the solid component of KRAS mutants may only occasionally be derived from morule-like components.
In conclusion, we presented 2 cases of solid-predominant adenocarcinoma with morule-like components: one was an EGFR mutant and the other, a KRAS mutant. To the best of our knowledge, this KRAS-mutant case is the first reported case, which is associated with a morule-like component. As previously described, both of our cases showed transition between micropapillary and morule-like components. Transition between morule-like components and solid components as shown in our cases has not been previously well described, although a few cases of solid-predominant adenocarcinoma have been reported to harbor morule-like components. We surmise that the solid components especially of some EGFR-mutant adenocarcinomas may be derived from morule-like components.
Disclosure of conflict of interest
None.
References
- 1.Makishi S, Kinjo T, Sawada S, Chinen K, Hirayasu T, Hamada T, Saito K, Iwamasa T. Morules and morule-like features associated with carcinomas in various organs: report with immunohistochemical and molecular studies. J Clin Pathol. 2006;59:95–100. doi: 10.1136/jcp.2005.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto Y, Yokoyama S, Sasaki A, Kashima K, Daa T, Nakayama I, Noguchi S. Oncofetal expression of blood group-related antigen on morules in thyroid carcinoma. Pathol Int. 1996;46:867–873. doi: 10.1111/j.1440-1827.1996.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki A, Yokoyama S, Arita T, Inomata M, Kashima K, Nakayama I. Morules with biotin-containing optically clear nuclei in colonic tubular adenoma. Am J Surg Pathol. 1999;23:336–341. doi: 10.1097/00000478-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Nakatani Y, Dickersin GR, Mark EJ. Pulmonary endodermal tumor resembling fetal lung: a clinicopathologic study of five cases with immunohistochemical and ultrastructural characterization. Hum Pathol. 1990;21:1097–1107. doi: 10.1016/0046-8177(90)90145-u. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani Y, Kitamura H, Inayama Y, Kamijo S, Nagashima Y, Shimoyama K, Nakamura N, Sano J, Ogawa N, Shibagaki T, Resl M, Mark EJ. Pulmonary adenocarcinomas of the fetal lung type: a clinicopathologic study indicating differences in histology, epidemiology and natural history of low-grade and high-grade forms. Am J Surg Pathol. 1998;22:399–411. doi: 10.1097/00000478-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Fornelli A, Cavazza A, Cancellieri A, Rossi G, De Marco L. Bronchioloalveolar carcinoma with nodular (“morule-like”) features. Virchows Arch. 2003;442:407–408. doi: 10.1007/s00428-003-0767-5. [DOI] [PubMed] [Google Scholar]
- 7.Moran CA, Jagirdar J, Suster S. Papillary lung carcinoma with prominent “morular” component. Am J Clin Pathol. 2004;122:106–109. doi: 10.1309/C8KP-65RN-UF03-UNM1. [DOI] [PubMed] [Google Scholar]
- 8.Tsuta K, Kawago M, Yoshida A, Sekine S, Asamura H, Furuta K, Kushima R. Primary lung adenocarcinoma with morule-like components: a unique histologic hallmark of aggressive behavior and EGFR mutation. Lung Cancer. 2014;85:12–18. doi: 10.1016/j.lungcan.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Matsukuma S, Obara K, Kato K, Ohshika Y, Takeo H, Tsuchiya S, Sato K. Non-sarcomatous spindle cell morphology in conventional lung adenocarcinoma: a clinicopathological study. Virchows Arch. 2014;465:165–172. doi: 10.1007/s00428-014-1598-2. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, Izumi Y, Watanabe M, Kawamura M, Horinouchi H, Shimada N, Kobayashi K, Sakamoto M. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Lee HY, Jeong JY, Han J, Cha MJ, Lee KS, Kim J, Shim YM. Clinical Impact of Minimal Micropapillary Pattern in Invasive Lung Adenocarcinoma: Prognostic Significance and Survival Outcomes. Am J Surg Pathol. 2015;39:660–6. doi: 10.1097/PAS.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 12.Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J. Clin. Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 13.Haneda H, Sasaki H, Lindeman N, Kawano O, Endo K, Suzuki E, Shimizu S, Yukiue H, Kobayashi Y, Yano M, Fujii Y. A correlation between EGFR gene mutation status and bronchioloalveolar carcinoma features in Japanese patients with adenocarcinoma. Jpn J Clin Oncol. 2006;36:69–75. doi: 10.1093/jjco/hyi228. [DOI] [PubMed] [Google Scholar]
- 14.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22–29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 15.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26:1307–1319. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]


