Abstract
We report an autopsy case of rapid progressive Waterhouse-Friderichsen syndrome (WFS) associated with Streptococcus pneumonia infection in a previously healthy man. Although he once visited a hospital about 6 hours before death, the both physical and serological examination did not show any sign of overwhelming infection. Autopsy showed massive adrenal hemorrhage without inflammation, and showed proliferation of gram positive cocci and microthrombosis in the vessels of many organs. The pathological change of respiratory tract was extremely minimal. Size and weight of the spleen possible decreased than normal. However, histological examination showed that obscuration of germinal center and decreasing the immunological cells of mantle and marginal zone. Immunohisitochemically, marked decreasing the marginal zone macrophages, which are positive for specific intercellular adhesion molecule grabbing nonintegrin receptor-1 (SIGN-R1) and macrophage receptor with collagenous structure (MARCO), were decreased comparing with age-matched control case. Polymerase chain reaction (PCR) assay using each DNA, extraction from formalin-fixed paraffin-embedded specimen (FFPE) samples of lung, adrenal gland, heart, spleen, and kidney showed positive the ply gene and the lytA gene specific for Streptococcus pneumonia. Present case showed possible acquired atrophy of spleen, especially decreasing marginal zone macrophage may correlate with rapid progression of sepsis of Streptococcus pneumonia with massive adrenal hemorrhage. In addition, present case showed the usefulness of PCR using FFPE for the postmortem diagnosis of WFS.
Keywords: Asplenia/hyposplenia, autopsy, Streptococcus pneumonia, Waterhouse-Friderichsen syndrome
Introduction
Waterhouse-Friderichsen syndrome (WFS) was reported by Waterhouse in 1911 [1] and by Friderichsen in 1918 [2]. The syndrome is known as a potentially fatal disease with massive adrenal hemorrhage, and fulminant sepsis and disseminated intravascular coagulation (DIC) are considerable factors related to WFS occurrence [3]. Streptococcus pneumonia is considered one of the frequent pathogenic causes of WFS [4], and asplenia is a well-known major risk factor for the occurrence of WFS related to S. pneumonia infection [5]. The term ‘asplenia’ describes the absence of a spleen, which is generally because of surgery or congenital origin [6]. The anatomic presence of a spleen with compromised function is referred to as functional asplenia, which seems to be synonymous with hyposplenia, and such acquired asplenia/hyposplenia may also contribute to WFS occurrence related to S. pneumonia infection [7-17]. The incidence of S. pneumonia infection in asplenia/hyposplenia, including all anatomical and functional cases, is 50 to 100 times higher than that of controls [18]. Previous studies have shown that encapsulated bacteria, such as S. pneumonia, are only cleared by the spleen because the polysaccharide capsule of S. pneumonia and other types of bacteria impedes binding of complement or prevents complement assembled on the capsule from interacting with macrophage receptors (5). In the spleen, marginal zone macrophages play a major role in the uptake and clearance of S. pneumonia [19].
Herein, we report an autopsy case of rapid, progressive WFS without any previous clinical history. We show a possible unusual splenic pathology by immunohistochemistry and review of literatures showing possible functional asplenia/hyposplenia, and discuss the mechanism and significance of acquired hyposplenism for WFS and severe S. pneumonia infection.
Case report
Clinical summary
A 56-year-old healthy man, who lived alone and had no past clinical history, presented to a hospital because of mild fever and general fatigue. The body temperature on arrival was 37.0°C, and his laboratory data revealed only mild elevation of C-reactive protein (CRP; Table 1). Both sign of meningeal irritation and cardiac dysfunction was not evident. Symptoms and gross inspection of upper airways did not reveal obvious findings. The attending physician diagnosed a common cold, and allowed the patient to go home. About 6 hours after returning home, the patient’s symptoms worsened, and he called and asked a friend to take him to the hospital. The patient suffered cardiopulmonary arrest when the friend arrived at the patient’s home, 15 minutes after the phone call. The patient was subsequently transferred to the hospital by ambulance; however, cardiopulmonary resuscitation was not successful. The patient’s body temperature on arrival was 38.5°C. Laboratory data immediately after arrival at the hospital mainly showed decreased platelets, hyperkalemia, hypoglycemia, and a marked increase in CRP (Table 1), but hemolysis of sampled blood was also identified. Reexamination of the rapid test for influenza virus was negative.
Table 1.
Laboratory data
| First visiting | Second visiting | |
|---|---|---|
| White cell count (/μL) | 6700 | 3200 |
| Erythrocyte count (/μL) | 464×104 | 571×104 |
| Hemoglobin (g/dL) | 13.6 | 17.1 |
| Hematocrit (%) | 41.5 | 53.7 |
| Platelet count (/μL) | 233×103 | 27×103 |
| Glucose (mg/dL) | 111 | 4 |
| AST (IU/L) | 20 | 134 |
| LDH (IU/L) | 178 | 818 |
| BUN (mg/dL) | 18.4 | 27.0 |
| Creatinine (mg/dL) | 1.3 | 3.7 |
| Sodium (mEq/L) | 141 | 128 |
| Potassium (mEq/L) | 4 | 9.5 |
| Chrolide (mEq/L) | 104 | 89 |
| Calcium (mEq/L) | 9.3 | 9.3 |
| C-reactive protein (mg/dL) | 0.8 | 9.3 |
| Hemolysis | (-) | (+) |
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen.
Autopsy findings
Autopsy was performed about 12 hours after death. Gross examination showed some pethechial hemorrhage of the skin. Abnormal findings, such as abscess or consolidation in the upper or lower airways were not evident. Moreover, the brain and heart did not show the findings of lethal disease. However, both adrenal glands showed massive parenchymal hemorrhage (Figure 1A). The spleen weighed 40 g, and the cut surface showed fibrotic atrophy.
Figure 1.
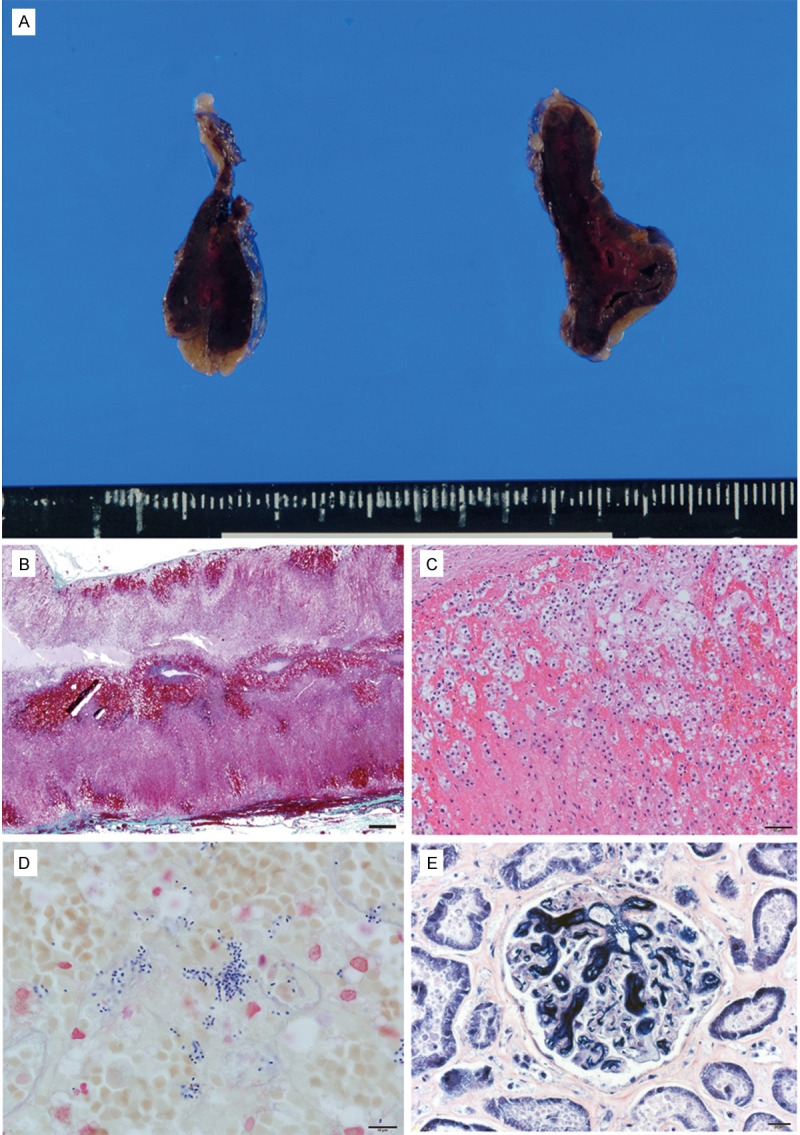
Pathological findings of the Waterhouse-Friderichsen syndrome. (A) Gross appearance of adrenal gland, (B) Low power view of adrenal gland. Massive hemorrhage can be seen. (C) Moderate power view of adrenal hemorrhage, (D) Proliferating gram positive cocci (Gram staining), (E) Fibrin thrombi were found in capillaries of glomeruli (phosphotungstic acid-hematoxylin staining). Scale bar = 1 mm (A); 100 μm (B); 50 μm (C); 20 μm (D, E).
Microscopically, fresh hemorrhage in the adrenal parenchyma without inflammation was observed (Figure 1B, 1C). Gram staining showed positive cocci in pairs and chains, suggestive of S. pneumonia (Figure 1D). In many of the organs, such as brain, heart, liver, and kidney, gram-positive cocci were found in the lumen of numerous vessels. Careful examination of the lung parenchyma showed very minimal inflammatory foci not containing cocci. Meningitis or other inflammatory foci were not found in any organs. Microscopically, multiple fibrin thrombi were evident in the glomerulus in the kidney and gastrointestinal tract (Figure 1E). In spleen, fine subcapsular fibrosis with a lower number white pulp cells were found in the subcapsular area, and loss of germinal center of many follicles in the white pulp was also noted. Follicle atrophy with obscuration of the germinal center can be seen (Figure 2A, 2B).
Figure 2.

Pathological findings of the spleen in Waterhouse-Friderichsen syndrome. (A) Low power view of the spleen shows a decreased volume of white pulp. Germinal center of lymph follicle cannot be seen (hematoxylin and eosin stain). (B) Decreasing number of cells in the subcapsular region with fine fibrosis. (Masson trichrome staining), (C-H) Immunohistochemical analysis of WFS (C-E, G) and the control case (F, H). Fewer CD21 positive follicular dendritic cells (C) and CD5 positive mantle zone lymphocytes (D) are evident. Decreased MARCO (E) and SIGN-R1 (G) positive cells are also evident compared to the control case (F, H). Scale bar = 100 μm (A); 20 μm (B-H).
Serological examination using postmortem blood samples were negative for anti-human immunodeficiency virus (HIV) antibody, Epstein-Barr (EB) virus, anti-nuclear antibody, and anti-RNP antibody.
Immunohistochemistry of spleen
Immunohistochemistry of the spleen was conducted (Figure 2C-E, 2G) and results were compared with control cases (a 55-year-old male who died from a falling accident; Figure 2F, 2H). Specimens were subjected to immunohistochemistry using antibodies CD3, CD8, CD20, CD68, CD79a, CD138, bcl-2, Ki-67 (Dako, Glostorup, Denmark), CD4, CD21 (Novovastra/Leica Biosystems Nussloch GmbH, Germany). In addition, monoclonal antibodies specific for intercellular adhesion molecule grabbing non-integrin receptor 1 (SIGN-R1; Santa Cruz, Santa Cruz, CA, USA) and macrophage receptor with collagenous structure (MARCO; Santa Cruz), which is a type of scavenger receptor, were used as markers of splenic marginal zone macrophage. Metallophilic macrophages-1 (MOMA-1; Santa Cruz) was used as a marker of splenic metallophilic macrophages [19]. Immunohistochemistry showed decreasing mantle zone lymphocytes that were positive for CD5. In addition, marginal zone macrophages, which are positive for SIGN-R1 and MARCO, were markedly decreased compared with the control case. Obscuration of the germinal center that was mostly visualized by immunohistochemistry using CD20 and ki-67 antibodies and marginal zone metallophilic macrophages visualized by anti MOMA-1 were also identified.
PCR analysis using formalin-fixed paraffin-embedded specimen (FFPE)
DNA was extracted and purified using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The extracted DNA of lung and adrenal glands without gram-positive cocci in the microscopic specimens of two autopsy cases were also prepared as controls. For molecular detection of S. pneumoniae, a PCR assay was performed to amplify the ply and lytA genes specific for S. pneumonia using DNA extracted from FFPE samples of the lung, adrenal gland, heart, spleen, and kidney. The oligonucleotide sequences for primer pairs have been previously described in detail [20]. The human β-actin gene was amplified as an internal control for effective DNA isolated from the FFPE specimens. The sequences of the primers were as follows: 5’ GTT GCG TTA CAC CCT TTC TTG 3’ (forward) and 5’ GTC ACC TTC ACC GTT CCA GT 3’ (reverse). The result was that the ply gene and the lytA gene specific for S. pneumoniae were amplified in the DNA from both lung and adrenal glands of the WFS case. All the FFPE specimens from the two controls were negative for the two genes. Samples from all cases were positive for β-actin amplification, indicating that no PCR inhibitors were present (Figure 3). The nucleotide sequence analysis of the amplified fragments was performed as previously reported [21]. Analysis specificity was ascertained by a BLAST search of the detected sequences.
Figure 3.
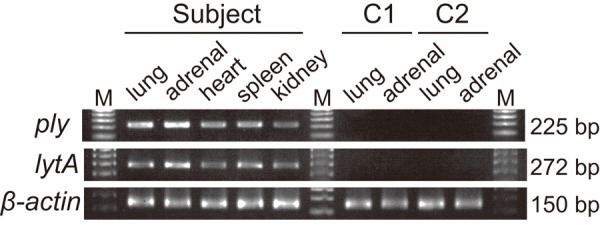
Results of PCR analysis. Presence of Streptococcus pneumoniae is shown in the lung and the adrenal gland of the patient by PCR analyses. Specific targets were amplified by real-time PCR using the ply and the lytA gene from S. pneumonia and β-actin as an internal control, showing detection of the 225-, the 272-, and the 150-bp products, respectively. M; marker, C1; control case 1, C2; control case 2.
Discussion
Pathological features of functional asplenia/hyposplenia for postmortem diagnoses have not yet been established. In our review of literatures, 13 cases of WFS underwent splenic autopsy after sepsis from S. pneumonia (Table 2) [7-17]. In all cases, clinical diagnosis of functional asplenia/hyposplenia was not made during the clinical course. The high incidence of DIC and low incidence of preceding clinical manifestation of local infection are features of S. pneumonia infection related to WFS with asplenia/hyposplenia. In 10 of these 13 cases, the terms of ‘hypoplasty’, ‘atrophy’, or ‘fibrosis’ were used to comment on splenic pathology. Hypoplasty of follicles without germinal centers was additionally reported by Bisno et al [14]. More detailed examinations using immunohistochemistry are few, and only Nanan and colleagues showed a possible defect in B-cell differentiation as a feature of functional hypoplasia of the spleen by immunohistochemistry for lymphocyte heavy chains [17].
Table 2.
Spleen present autopsy cases of WFS with pneumococcal infection
| Age | Sex | dic | Local infection | Spleen | Other | Ref | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Clin | Pathol | ||||||
| 8 mo | F | ND | P | N | 3.4 g, functionally hypoplastic | 21 trisomy | [7] |
| 28 yr | F | P | ND | acute bronchitis | 180 g, normal | [8] | |
| 26 yr | F | P | N | N | 42 g, fibrosis and hypoplasia | systemic lupus erythematosis | [9] |
| 56 yr | F | P | ND | N | 170 g, normal | [10] | |
| 13 mo | M | N | N | N | 60 g, normal | sickle cell disease | [11] |
| 23 mo | M | ND | P | N | 28 g, normal | sickle cell disease | [11] |
| 13 yr | M | P | N | N | 25 g, involution | sickle cell disease | [11] |
| 10 mo | F | ND | P | pneumonia | 1×3 mm, extremely hypoplastic | [12] | |
| 54 yr | F | P | P | very mild in air space | 48 g, suspicious of hypoplasia | [13] | |
| 45 yr | F | P | N | N | 29 g, hypoplastic, infarction, lack of germianl center | [14] | |
| 61 yr | F | ND | P | N | 13 g, fibtotic | [15] | |
| 16 yr | F | P | ND | N | 35 g, small and fibrous | Still’s disease | [16] |
| 18 mo | F | P | P | N | 9 g, hypoplasty | dysgammagloblinemia | [17] |
DIC, disseminated intravascular coagulation; clin, clinical; ref, references; pathol, pathological; yr, year-old; mo, month; ND, not described; P, present; N, none.
The marginal zone is a unique region of the spleen, which is situated at the interface of the red pulp with periarteriolar lymphoid sheath and follicles. In the marginal zone, two kinds of macrophages and B-cells (marginal zone B-cell) are present. One is the metallophilic macrophage located at the inner margin of the marginal zone adjacent to the periarteriolar lymphoid sheath and follicles; their potential function has not been explored. The other is a marginal zone macrophage that is positive for SIGN-R1 and/or MARCO; it plays a major role in filtering and cleaning S. pneumonia. SIGN-R1 binds capsular polysaccharides, such as on S. pneumonia [17,22,23], while MARCO binds to S. aureus and Escherichia coli [24]. In addition, marginal zone macrophages and marginal zone B-cells have a direct intercellular interactions via MARCO expressed on marginal zone macrophages with an undetermined ligand on marginal zone B-cells [25]. The present report is the first description of a significant decrease in marginal zone macrophages shown by immunohistochemistry. We assume that the acquired pathology of the marginal zone, as shown in the present case, is notable for evaluating splenic function in postmortem examination of overwhelming S. pneumonia infection. Various clinical conditions, such as congenital disorder, sickle hemoglobinopathies, gastrointestinal disease, hepatic disorders, autoimmune disorders, hematologic/neoplastic disorders, special form infection, and circulatory disorders of the spleen, can possibly cause functional asplenia/hyposplenia [26]. In addition, we should note that aging has also been considered a cause of functional asplenia/hyposplenia. An autopsy study showed the size and weight of the spleen decreases with age by the sixth decade [27], while other studies fail to show a significant involution of the spleen at older age [28]. In an animal experimental study, the function of marginal zone macrophages was examined by quantitative analysis of SIGN-R1 expression, and function was not significantly decreased in old mice compared with young mice; however, the number of marginal zone macrophages was lower in older mice [29].
PCR is a rapid, specific, and sensitive method for amplifying bacteria genes; therefore, PCR may also be useful for making early diagnoses and to decide therapeutic approaches for WFS patients. Additionally, PCR using FFPE has been used to identify the causative pathogenic organism by some researchers, and it has also been reported that the sensitivity of PCR using FFPE is comparable with using fresh tissue [30]. In the present case, we succeed in amplifying S. pneumonia 16S rDNA using ply and lytA. The amplified products were confirmed to be homologous to S. pneumonia; therefore, a false-positive reaction with other microorganisms did not occur in the present case. A confirmation of the consistency between both pathological and molecular findings is essential to avoid misunderstanding PCR results.
In summary, herein we showed rapid, progressive WFS with overwhelming S. pneumonia infection. The present case showed decreasing marginal zone macrophages in the spleen due to undetermined etiology and may possibly indicate a pathological change leading to life-threatening infection of encapsulated bacteria. PCR analysis using FFPE specimens is useful for postmortem examination of infectious diseases during autopsy.
Acknowledgements
This study was supported in part by a KAKENHI grant from the Japan Society for the Promotion of Science, to Yukiko Hata (24590852) and Presidential Discretionary Funds, University of Toyama 2014, to Naoki Nishida. The authors thank Ms. Syuko Kitora, Ms. Tamae Sasakura, Mr. Noboru Onozuka, and Mr. Osamu Yamamoto for their technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Waterhouse R. A case of suprarenal apoplexy. Lancet. 1911;1:577–578. [Google Scholar]
- 2.Friderichsen C. Nebennierenapoplexie bei kleinen kindern. Jahrb Kinderh. 1918;87:109–125. [Google Scholar]
- 3.Maitra A. The endocrine system. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran pathologic basis of disease. 8th edition. Philaderphia: Saunders-Elsevior; 2010. p. 1155. [Google Scholar]
- 4.Guarner J, Paddock CD, Bartlett J, Zaki SR. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod Pathol. 2008;21:1113–1120. doi: 10.1038/modpathol.2008.98. [DOI] [PubMed] [Google Scholar]
- 5.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic stetes. Lancet. 2011;378:86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- 6.Castagnola E, Fioreuda F. Prevention of life-threatening infections due to encapsulated bacteria in children with hyposplenia or a asplenia: a brief review of current recommendations for practical purposes. Eur J Hematol. 2003;71:319–326. doi: 10.1034/j.1600-0609.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 7.Angelski CL, Mckay E, Blackie B. A case of functional asplenia and pneumococcal sepsis. Pediatr Emerg Care. 2011;27:639–641. doi: 10.1097/PEC.0b013e31822255f4. [DOI] [PubMed] [Google Scholar]
- 8.Doherty S. Fetal pneumococcal Waterhouse-Friderichsen syndrome. Emerg Med. 2001;13:237–239. doi: 10.1046/j.1442-2026.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 9.Guertler AT, Carter CT. Fatal pneumococcal septicemia in a connective tissue disease. J Emerg Med. 1996;14:33–38. doi: 10.1016/0736-4679(95)02048-9. [DOI] [PubMed] [Google Scholar]
- 10.Bramley PN, Shah P, Williams DJ, Losowsky MS. Pneumococcal Waterhouse-Friderichsen syndrome despite a normal spleen. Postgrad Med J. 1989;65:687–688. doi: 10.1136/pgmj.65.767.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobel JS, Bove KE. Clinicopathological characteristics of septicemia in sickle cell disease. Am J Dis Child. 1982;136:543–547. doi: 10.1001/archpedi.1982.03970420067015. [DOI] [PubMed] [Google Scholar]
- 12.Neff JM, Dorst JP, Spear GS. Rapid death in childhood. J Pediatr. 1973;83:679–684. doi: 10.1016/s0022-3476(73)80239-2. [DOI] [PubMed] [Google Scholar]
- 13.Grant JM, Horowitz HI, Lorian V, Brodman HR. Waterhouse-Friderichsen syndrome induced by pneumococcemic shock. JAMA. 1970;212:1373–1374. [PubMed] [Google Scholar]
- 14.Bisno AL, Freeman JC. The syndrome of asplenia, pneumococcal sepsis, and disseminated intravascular coagulation. Ann Intern Med. 1970;72:389–393. doi: 10.7326/0003-4819-72-3-389. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker AN. Infection and the spleen: association between hyposplenism, pneumococcal sepsis and disseminated intravascular coagulation. Med J Aust. 1969;14:1213–1219. doi: 10.5694/j.1326-5377.1969.tb62293.x. [DOI] [PubMed] [Google Scholar]
- 16.Larr LJ, Shipton EA, Holland EH. A fatal case of Still’s disease associated with Waterhouse-Friderichsen syndrome due to pneumococcal septicemia. Med J Aust. 1953;28:300–305. [PubMed] [Google Scholar]
- 17.Nanan R, Peters K, Schrod L, Kreth HW. Lethal pneumococcal infection in an 18-month-old girl with splenic hypoplasia and dysgammagloblinemia. Ann Hematol. 2001;80:674–676. doi: 10.1007/s002770100373. [DOI] [PubMed] [Google Scholar]
- 18.Chidiac C. Pneumococcal infections and adult with risk factors. Med Mal Inf. 2012;42:517–524. doi: 10.1016/j.medmal.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 20.Sakai F, Talekar SJ, Klugman KP, Vidal JE Investigators Group. Expression of Streptococcus pneumoniae Virulence-Related Genes in the Nasopharynx of Healthy Children. PLoS One. 2013;8:e67147. doi: 10.1371/journal.pone.0067147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hata Y, Mori H, Tanaka A, Fujita Y, Shimomura T, Tabata T, Kinoshita K, Yamaguchi Y, Ichida F, Kominato Y, Ikeda N, Nishida N. Identification and characterization of a novel genetic mutation with prolonged QT syndrome in an unexplained postoperative death. Int J Legal Med. 2014;128:105–115. doi: 10.1007/s00414-013-0853-4. [DOI] [PubMed] [Google Scholar]
- 22.Jablonska J, Dittmar K, Kleinke T, Buer J, Weiss S. Essential role of CCL2 in clustering of splenic ERTR-9+ macrophages during infection of BALB/c mice by Listeria monocytogenesis. Infect Immun. 2007;75:462–470. doi: 10.1128/IAI.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koppel EA, Litjens M, van der Berg VC, van Kooyk Y, Geijtenbeek TB. Interaction of SIGNR1 expressed by marginal zones macrophages with marginal zone B cells is essential to early IgM responses against Streptococcus pneumonia. Mol Immunol. 2008;45:2881–2887. doi: 10.1016/j.molimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 26.William BM, Corozza GR. Hyposplenism: a comprehensive review. Part I: Basic concepts and causes. Hematology. 2007;12:1–13. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
- 27.Deland FH. Normal spleen size. Radiology. 1970;97:589–592. doi: 10.1148/97.3.589. [DOI] [PubMed] [Google Scholar]
- 28.van Krieken JHJM, Te Velde J, Hermans J, Cornelisse CJ, Welvaart C, Ferrari M. The amount of white pulp in the spleen a morphometrical study done in methacrylate-embeded splenectromy specimens. Histopathol. 1983;7:767–782. doi: 10.1111/j.1365-2559.1983.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 29.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cell in old mice. J Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guarner J, Packard MM, Nolte KB, Paddock CD, Shieh WJ, Tondella ML, McGee L, Zaki SR. Usefulness of immunohistochemical diagnosis of streptococcus pneumonia in formalin-fixed paraffin-embedded specimens compared with culture and gram stain techniques. Am J Clin Pathol. 2007;127:612–618. doi: 10.1309/J3LD0RBP788W1TM8. [DOI] [PubMed] [Google Scholar]


