Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) is one of the most common heritable causes of stroke and dementia in adults. The gene involved in the pathogenesis of CADASIL is Notch3; in which mutations affect the number of cysteine residues in its extracellular domain, causing its accumulation in small arteries and arterioles of the affected individuals. Besides the usual neurological and vascular findings that have been well-documented in CADASIL patients, this paper additionally reports multiple neoplastic lesions that were observed in an autopsy case of CADASIL patient; that could be related to Notch3 mutation. The patient was a 62 years old male, presented with a past history of neurological manifestations, including gait disturbance and frequent convulsive attacks. He was diagnosed as CADASIL syndrome with Notch3 Arg133Cys mutation. He eventually developed hemiplegia and died of systemic convulsions. Autopsy examination revealed-besides the vascular and neurological lesions characteristic of CADASIL- multiple neoplastic lesions in the body; carcinoid tumorlet and diffuse idiopathic pulmonary neuro-endocrine cell hyperplasia (DIPNECH) in the lungs, renal cell carcinoma (RCC), prostatic adenocarcinoma (ADC) and adenomatoid tumor of the epididymis. This report describes a spectrum of neoplastic lesions that were found in a case of CADASIL patient that could be related to Notch3 gene mutations.
Keywords: Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy, autopsy case, neoplastic lesions
Introduction
CADASIL is the acronym for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. It is an autosomal dominant arteriopathy of small arteries and arterioles of the brain and other organs that affects middle aged adults [1,2]. Clinical manifestations include recurrent cerebral ischemic episodes, progressive cognitive deficit, migraine with aura, dementia, and psychiatric symptoms [3,4]. It is associated with mutations in the Notch3 gene on chromosome 19 [1]. This gene encodes a single-pass transmembrane receptor with an extracellular domain (ECD) containing 34 epidermal growth factor repeats (EGFR); each includes six cysteine residues, a single transmembrane domain, and an intracellular domain [5]. In an adult brain, Notch3 is expressed by vascular smooth muscle cells (VSMCs), especially in small caliber arteries, and by pericytes, to maintain the normal vascular structure [6]. Moreover, Notch3 signaling functions in endothelium-pericyte interactions to maintain the microcirculatory networks, disruption of which may result in microcirculatory dysfunction [7]. In CADASIL syndrome, mutations occur within Notch3 exons 2-24, which encode the 34 EGFR, resulting in removing or inserting cysteine residues, leading to conformational changes and accumulation of Notch3 ECD in the cytoplasmic membrane of VSMCs [6,8,9]. Over 95% of mutations are missense mutations; others are small in-frame deletions or splice-site mutations [10,11].
Genetic testing is the gold standard for the diagnosis of CADASIL. It is based on screening the 23 exons of Notch3 that encode the 34 EGFR, in search for a mutation leading to an odd number of cysteine residues [12]. In addition, skin biopsy could be examined by electron microscopy for detection of glomerular osmiophilic material (GOM)- which is thought to be derived from the ECD of Notch3- in the VSMCs of arterioles, or by immunohistochemistry (IHC) for detection of the deposited Notch3 ECD [13-15]. In addition, magnetic resonance imaging (MRI) is considered the most relevant tool for monitoring the cerebral pathology in CADASIL [16].
CADASIL syndrome is distributed worldwide and the spectrum of Notch3 mutations as well as the clinical features and the pathological neurological and vascular lesions have been studied and widely reported [10,17-21], including among Japanese patients [15,22-29]. However, to our knowledge there has been no report of describing neoplastic lesions in CADASIL patients. We report herein multiple neoplastic lesions that were observed in an autopsied case with CADASIL syndrome.
Case report
Clinical presentation
A 62-year-old-man was admitted for hemiplegia and systemic spastic convulsions. Detailed patient’s past history and results of investigation were previously reported by Ueda et al. [15] and by Uyama et al. [23] (patient 1). In brief, the symptoms started at the age of 38, when he developed ophthalmoplegia, and after 10 years, he developed systemic spastic convulsions. One year later, he developed gait disturbance and frequent convulsive attacks. At the age of 52, he was diagnosed as CADASIL syndrome; with Notch3 Arg133Cys mutation. MRI examination showed diffuse white matter hyper-intensities. Eight years later, MR angiogram showed multiple arterial stenosis, and the patient developed hemiplegia. By the time of admission, the hemiplegia progressed and the patient developed systemic convulsions and died. A systemic autopsy examination was done with permission of the family of the deceased.
Materials and methods
Autopsy specimens were fixed in 10% formalin and embedded in paraffin. Serial sections, 5 µm thick, were processed for hematoxylin and eosin (H&E) staining. Immunohistochemical (IHC) staining was carried out with the streptavidin-biotin method. List of primary antibodies used are listed in Table 1. This was followed by sequential 60 min incubations with secondary antibodies (EnVision+System-HRP Labeled Polymer, Dako, Glostrup, Denmark) and visualization with the Liquid DAB+Substrate Chromogen System (Dako). All slides are lightly counterstained with hematoxylin for 30s prior to dehydration and mounting.
Table 1.
Antibodies for immunohistochemistry. References, lot and working dilutions of antibodies are indicated
| Primary antibodies | Reference | Lot | Working dilution |
|---|---|---|---|
| CD56 | Leica, Wetzlar, Germany | NCL-CD56-504 | 1:100 |
| CGA | Dako, Glostrup, Denmark, | A0430 | 1:100 |
| SYP | Leica | NCL-SYNAP-299 | 1:50 |
| Notch3 | Epitomics, Burlingame, CA, US | ab60087 | 1:200 |
| CK7 | Dako | OV-TL 12/30 | 1:50 |
CGA, chromogranin A; SYP, synaptophysin; CK, cytokeratin.
Results
Pathological findings
Postmortem examination revealed several neoplastic lesions in body. In the upper lobe of the left lung, a well circumscribed nodule; 0.5 × 0.3 cm; with grey white cut section, was observed (Figure 1A). Histologically, observed by H&E staining, the mass consisted of sheets of spindle and round cells, with uniform nuclei, salt and pepper chromatin and eosinophilic cytoplasm, situated within mucosa of bronchiolar gland (Figure 1B). By IHC staining, the neuro-endocrine (NE) nature of cells was confirmed, by positive cytoplasmic staining for CD56, chromogranin A (CGA) and synaptophysin (SYP) (Figure 1C and 1D respectively). In addition, weak cytoplasmic reactivity for Notch3 was observed in the cells (Figure 1E). The diagnosis of carcinoid tumorlet was established. In addition, we observed scattered foci of NE cells proliferation, within bronchial and bronchiolar epithelium, which were diagnosed as diffuse idiopathic pulmonary neuro-endocrine cell hyperplasia (DIPNECH) (Figure 1F).
Figure 1.
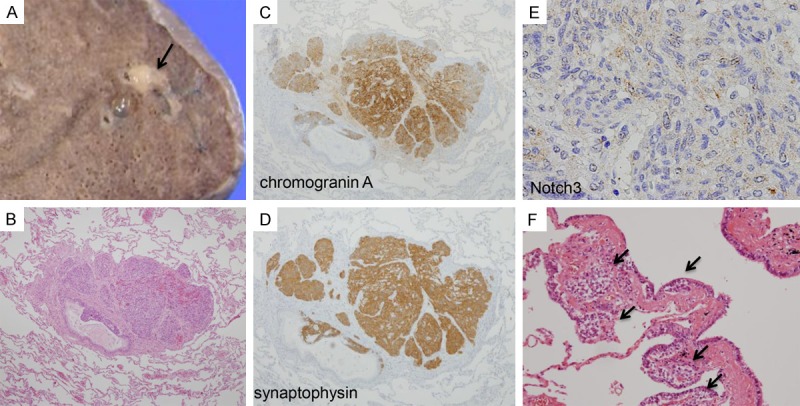
Gross, histological and immunohistochemical (IHC) features of lung neoplasms. A. A well-defined nodule, 0.5 × 0.3 cm in size, with grey white cut section (Arrow). The rest of lung cut section shows no remarkable gross changes. B. Representative hematoxylin and eosin (H&E) staining photo for lung nodule. Sheets of spindle and round cells, with uniform nuclei, salt and pepper chromatin and eosinophilic cytoplasm, situated within mucosa of bronchiolar gland (4 ×). C and D. Tumor cells show strong cytoplasmic positivity for neuroendocrine markers; chromogranin A and synaptophysin (4 ×). E. Tumor cells show cytoplasmic reactivity for Notch3 (10 ×). F. Proliferation of NE cells within the terminal bronchiolar epithelia (Arrows) (20 ×).
In right kidney, a well-circumscribed cortical mass, 1 × 0.5 cm, with grey white cut section was observed. By H&E staining, the mass consisted of cystic, papillary and tubular proliferation of epithelial cells; with eosinophilic cytoplasm and mild atypical nuclei and indistinct nucleoli (Figure 2A). Papillae were lined with single layer of cells, of low-grade nuclear features and scant cytoplasm. The cells showed positive staining for CK7. In addition, weak cytoplasmic reactivity for Notch3 was observed. The diagnosis of papillary renal cell carcinoma (RCC); type I, nuclear fuhrman grade 2 was established. In addition, another cortical mass in left kidney, 1.7 × 1.2 cm, was seen, with hemorrhagic and cystic cut surface. The mass consisted of alveolar nests and sheets of clear cells interspersed by delicate vascular network. The cells had uniform nuclei with indistinct nucleoli (Figure 2B). The mass was diagnosed as RCC; clear cell type, nuclear fuhrman grade 2.
Figure 2.
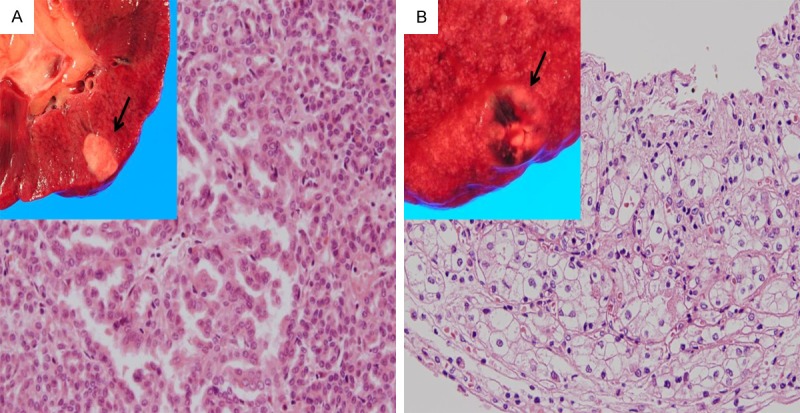
Gross, histological and IHC features of renal neoplasms. A. A well-defined cortical mass, 1 × 0.5 cm, with grey white cut section (Arrow). The rest of kidney cut section showed congestion. With H&E staining, the mass was formed from papillary and tubular proliferation of epithelial cells; with eosinophilic cytoplasm and mild atypical nuclei and indistinct nucleoli (10 ×). B. Another cortical mass, 1.7 × 1.2 cm, was seen with hemorrhagic ulcerated cut surface. With H&E staining, the mass was formed from alveolar nests of cells with uniform nuclei and clear cytoplasm, interspersed by delicate vascular network (10 ×).
Although no detected gross findings were observed in the prostate, yet sections from it stained with H&E staining showed a well circumscribed focus of proliferated crowded acini with variation in size and shape and irregular contours (Figure 3A). Acini were lined by a single layer of cuboidal or columnar epithelial cells with eosinophilic cytoplasm and enlarged pleomorphic nuclei (Figure 3B). The diagnosis of prostatic adenocarcinoma (ADC), acinar type, Gleason score 6 (3+3) was established.
Figure 3.
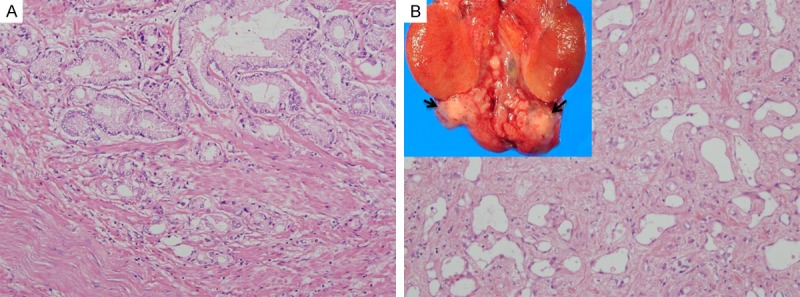
Gross and histological features of genital neoplasms. A. H&E staining photo for sections from prostate revealed a circumscribed proliferation of variable sized irregular acini, lined by a single layer of columnar epithelial cells with eosinophilic cytoplasm and atypical nuclei (20 ×). B. A well-circumscribed firm grey white nodule (Arrow), 1.2 × 1 cm, was seen attached to the lower end of the right bisected testicle. With H&E staining, the nodule was composed of tubular and cystic structures, lined with flat uniform epithelial cells, in fibrous stroma (4 ×).
On sectioning the right testis, a well-circumscribed firm grey white nodule, 1.2 × 1 cm was seen, at the lower end of the testis. The nodule was composed of round and oval tubular and cystic structures, lined with flat and cuboidal epithelial cells with round to oval nuclei and eosinophilic cytoplasm, and embedded in fibrous stroma (Figure 3B). The nodule was diagnosed as adenomatoid tumor of the epididymis.
In addition to the previously reported neoplasms, other pathological findings were observed: multiple infarcts in cerebral hemispheres, thick-walled cerebral blood vessels with dilated perivascular spaces, atherosclerosis of the aorta and coronaries, old myocardial infarction in the sub-endocardial region of the left ventricular posterior wall with hypertrophy of the left ventricle, mild renal sclerosis, mild acute tubular necrosis, and congestion of the lungs, liver and spleen.
Discussion
CADASIL is a hereditary vasculopathy affecting small arteries and arterioles of the brain and other tissues, in young and middle-aged adults [2]. Clinically, CADASIL is characterized by five main symptoms: migraine with aura, subcortical ischemic events, mood disturbances, apathy, and cognitive impairment [10]. The disease develops due to mutations in Notch3 gene, resulting in aberrant cysteine residues interaction on Notch3 ECD protein, and thus its deposition in vascular wall in the affected vessels [1]. Notch3 mutations of CADASIL patients are most frequently detected in exons 3 and 4 [10,12]. Diagnosis of CADASIL is based mainly on genetic detection of Notch3 mutations and microscopic and ultra-structural examination of peripheral skin and/or muscle biopsies for detection of characteristic vascular changes [13,14]. The clinical manifestations and investigation results of the present case has been previously reported as a Japanese case of CADASIL syndrome with Argl33Cys mutation of Notch3 [15,23]. The present report describes the results of autopsy examination of that case. Several studies in patients with CADASIL have documented the characteristic pathological vascular and neurological changes [22,30-32], and thus the present report focused mainly on the multiple neoplastic lesions that were observed during autopsy examination. Based on the hypothesis that the gain of function for the mutant Notch3 protein is likely the mechanism for the CADASIL mutations [10,33], thus it was not surprising to find multiple neoplastic lesions in the present case, which could be related to Notch3 activation. In the lungs, carcinoid tumorlet and foci of NE cell proliferation were seen. In the kidneys, RCC; both clear cell and papillary types were observed. In addition, prostatic adenocarcinoma and epididymis adenomatoid tumor were detected. All of the observed neoplastic lesions-except for adenomatoid tumor- have been previously reported with association of Notch3 signaling pathway [34-38], which could possibly explain their concomitant appearance in such case of CADASIL syndrome. In addition, in another autopsied case of CADASIL syndrome, with Arg449Cys Notch3 mutation, we observed pancreatic intraepithelial neoplasia (PanIN-2) (data not shown). Although we couldn’t detect other neoplastic lesions in that second case, yet the observed PanIN lesion further support the association between pre/neoplastic lesions in CADASIL with Notch3 activation, which has been previously linked to pancreatic neoplasms as well [39,40]. More studies are needed to elucidate the differences in Notch3 mutations and their effect in CADASIL syndrome.
Acknowledgements
The authors would like to thank Dr. Yoji Nagashima at Department of Pathology, Tokyo Women’s Medical University Hospital, for his advice on the diagnosis of renal tumors.
Disclosure of conflict of interest
None.
References
- 1.Tournier-Lasserve E, Joutel A, Melki J, Weissenbach J, Lathrop GM, Chabriat H, Mas JL, Cabanis EA, Baudrimont M, Maciazek J. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy maps to chromosome 19q12. Nat Genet. 1993;3:256–9. doi: 10.1038/ng0393-256. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG, Tournier-Lasserve E. Summary of the proceedings of the First International Workshop on CADASIL. Paris, May 19-21, 1993. Stroke. 1994;25:704–07. doi: 10.1161/01.str.25.3.704. [DOI] [PubMed] [Google Scholar]
- 3.Caeiro L, Ferro JM. Cognitive profile in CADASIL patients. J Neurol Neurosurg Psychiatry. 2006;77:144–145. doi: 10.1136/jnnp.2005.074583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, Breuning MH, van Buchem MA, Middelkoop HA, Lesnik Oberstein SA. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007;38:923–8. doi: 10.1161/01.STR.0000257968.24015.bf. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol. 2008;96:499–509. doi: 10.1016/j.pbiomolbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, LI Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG, Bach JF, Tournier-Lasserve E. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- 9.Dichgans M, Ludwig H, Muller-Hocker J, Messerschmidt A, Gasser T. Small in-frame deletions and missense mutations in CADASIL: 3D models predict misfolding of Notch3 EGF-like repeat domains. Eur J Hum Genet. 2000;8:280–285. doi: 10.1038/sj.ejhg.5200460. [DOI] [PubMed] [Google Scholar]
- 10.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 11.Ayata C. CADASIL: experimental insights from animal models. Stroke. 2010;41:S129–34. doi: 10.1161/STROKEAHA.110.595207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters N, Opherk C, Bergmann T, Castro M, Herzog J, Dichgans M. Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies. Arch Neurol. 2005;62:1091–94. doi: 10.1001/archneur.62.7.1091. [DOI] [PubMed] [Google Scholar]
- 13.Markus HS, Martin RJ, Simpson MA, Dong YB, Ali N, Crosby AH, Powell JF. Diagnostic strategies in CADASIL. Neurology. 2002;59:1134–1138. doi: 10.1212/wnl.59.8.1134. [DOI] [PubMed] [Google Scholar]
- 14.Joutel A, Favrole P, Labauge P, Chabriat H, Lescoat C, Andreux F, Domenga V, Cécillon M, Vahedi K, Ducros A, Cave-Riant F, Bousser MG, Tournier-Lasserve E. Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet. 2001;358:2049–2051. doi: 10.1016/S0140-6736(01)07142-2. [DOI] [PubMed] [Google Scholar]
- 15.Ueda A, Hirano T, Takahashi K, Kurisaki R, Hino H, Uyama E, Uchino M. Detection of granular osmiophilic material of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy by light microscopy in frozen sections. Neuropathol Appl Neurobiol. 2009;35:618–22. doi: 10.1111/j.1365-2990.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T CADASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226:35–9. doi: 10.1016/j.jns.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Utku U, Celik Y, Uyguner O, Yüksel-Apak M, Wollnik B. CADASIL syndrome in a large Turkish kindred caused by the R90C mutation in the Notch3 receptor. Eur J Neurol. 2002;9:23–8. doi: 10.1046/j.1468-1331.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 18.Delibas S, Guven H, Comoglu SS. A Case report about CADASIL: mutation in the NOTCH 3 receptor. Acta Neurologica Taiwanica. 2009;18:262–266. [PubMed] [Google Scholar]
- 19.Shahien R, Bianchi S, Bowirrat A. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy in an Israeli family. Neuropsychiatr Dis Treat. 2011;7:383–90. doi: 10.2147/NDT.S19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tikka S, Baumann M, Siitonen M, Pasanen P, Pöyhönen M, Myllykangas L, Viitanen M, Fukutake T, Cognat E, Joutel A, Kalimo H. CADASIL and CARASIL. Brain Pathol. 2014;24:525–44. doi: 10.1111/bpa.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojanov D, Grozdanović D, Petrović S, Benedeto-Stojanov D, Stefanović I, Stojanović N, Ilić DN. De novo mutation in the NOTCH3 gene causing CADASIL. Bosn J Basic Med Sci. 2014;14:48–50. doi: 10.17305/bjbms.2014.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio T, Arima K, Eto K, Ogawa M, Sunohara M. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: report of an autopsied Japanese case. Clin Neural (Tokyo) 1997;37:910–916. [PubMed] [Google Scholar]
- 23.Uyama E, Tokunaga M, Suenaga A, Kotorii S, Kamimura K, Takahashi K, Tabira T, Uchino M. Arg133Cys mutation of Notch3 in two unrelated Japanese families with CADASIL. Intern Med. 2000;39:732–7. doi: 10.2169/internalmedicine.39.732. [DOI] [PubMed] [Google Scholar]
- 24.Uchino M, Uyama E, Maeda Y, Hirano T, Suenaga A, Yamada H, Hashimoto Y, Kotorii S, Takahashi K, Tabira T. CADASIL: clinical analysis of CADASIL and CADASIL-like disorders in Japan. Rinsho Shinkeigaku. 2000;40:1247–50. [PubMed] [Google Scholar]
- 25.Kotorii S, Takahashi K, Kamimura K, Nishio T, Arima K, Yamada H, Uyama E, Uchino M, Suenaga A, Matsumoto M, Kuchel G, Rouleau GA, Tabira T. Mutations of the Notch3 gene in non-Caucasian patients with suspected CADASIL syndrome. Dement Geriatr Cogn Disord. 2001;12:185–193. doi: 10.1159/000051256. [DOI] [PubMed] [Google Scholar]
- 26.Uchino M, Hirano T, Uyama E, Hashimoto Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and CADASIL-like disorders in Japan. Ann N Y Acad Sci. 2002;977:273–8. doi: 10.1111/j.1749-6632.2002.tb04826.x. [DOI] [PubMed] [Google Scholar]
- 27.Santa Y, Uyama E, Chui DH, Arima M, Kotorii S, Takahashi K, Tabira T. Genetic, clinical and pathological studies of CADASIL in Japan: a partial contribution of Notch3 mutations and implications of smooth muscle cell degeneration for the pathogenesis. J Neurol Sci. 2003;212:79–84. doi: 10.1016/s0022-510x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 28.Oki K, Nagata E, Ishiko A, Shimizu A, Tanaka K, Takahashi K, Tabira T, Katayama T, Suzuki N. Novel mutation of the Notch3 gene in a Japanese patient with CADASIL. Eur J Neurol. 2007;14:464–6. doi: 10.1111/j.1468-1331.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki K, Irioka T, Ishikawa K, Mizusawa H. CADASIL with a Novel NOTCH3 Mutation (Cys478Tyr) J Stroke Cerebrovasc Dis. 2015;24:e61–2. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser MG. Autosomal dominant leukoencephalopathy and subcortical ischemic stroke. A clinicopathological study. Stroke. 1993;24:122–25. doi: 10.1161/01.str.24.1.122. [DOI] [PubMed] [Google Scholar]
- 31.Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case. Stroke. 2002;33:2565–69. doi: 10.1161/01.str.0000032620.91848.1c. [DOI] [PubMed] [Google Scholar]
- 32.Miao Q, Paloneva T, Tuominen S, Pöyhönen M, Tuisku S, Viitanen M, Kalimo H. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–64. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donahue CP, Kosik KS. Distribution pattern of Notch3 mutations suggests a gain-of-function mechanism for CADASIL. Genomics. 2004;83:59–65. doi: 10.1016/s0888-7543(03)00206-4. [DOI] [PubMed] [Google Scholar]
- 34.Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Olson S, Gonzalez A, Carbone DP, Dang TP. Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res. 2005;65:3555–61. doi: 10.1158/0008-5472.CAN-04-3132. [DOI] [PubMed] [Google Scholar]
- 35.Sun S, Du R, Gao J, Ning X, Xie H, Lin X, Liu J, Fan D. Expression and clinical significance of Notch receptors in human renal cell carcinoma. Pathology. 2009;41:335–41. doi: 10.1080/00313020902885003. [DOI] [PubMed] [Google Scholar]
- 36.Aparicio LM, Villaamil VM, Gallego GA, Caínzos IS, Campelo RG, Rubira LV, Estévez SV, Mateos LL, Perez JL, Vázquez MR, Calvo OF, Bolós MV. Expression of Notch1 to -4 and their ligands in renal cell carcinoma: a tissue microarray study. Cancer Genomics Proteomics. 2011;8:93–101. [PubMed] [Google Scholar]
- 37.Danza G, Di Serio C, Ambrosio MR, Sturli N, Lonetto G, Rosati F, Rocca BJ, Ventimiglia G, del Vecchio MT, Prudovsky I, Marchionni N, Tarantini F. Notch3 is activated by chronic hypoxia and contributes to the progression of human prostate cancer. Int J Cancer. 2013;133:2577–86. doi: 10.1002/ijc.28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: a moving target. Prostate. 2014;74:933–45. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao J, Qian C. Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol. 2010;27:1017–22. doi: 10.1007/s12032-009-9326-5. [DOI] [PubMed] [Google Scholar]
- 40.Mann CD, Bastianpillai C, Neal CP, Masood MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP, Manson MM. Notch3 and HEY-1 as prognostic biomarkers in pancreatic adenocarcinoma. PLoS One. 2012;7:e51119. doi: 10.1371/journal.pone.0051119. [DOI] [PMC free article] [PubMed] [Google Scholar]


