Abstract
Pathogenesis of Richter transformation (RT) or Richter syndrome (RS) of chronic lymphocytic leukemia (CLL) is still largely unknown. Increasing evidences show that c-MYC may play a role in the development of RS. Here we report three cases of RS with overexpression of c-MYC. The first case was a 78-year-old male who initially presented with CLL and then developed diffuse lymphadenopathy and ascites shortly after. Ascites cytology showed a population of large lymphoid cells positive for MYC (8q24) rearrangement by fluorescence in situ hybridization (FISH) and overexpression of c-MYC by immunohistochemistry (IHC). The second case was a 66-year-old male presented with rapidly enlarging lymph nodes and pleural effusion after a long history of CLL. Biopsy showed large B-cells positive for c-MYC overexpression and high Ki-67 proliferation index (80-90%). The third case was a 62-year-old female with CLL who presented for lobectomy for lung adenocarcinoma. Interestingly, along with the carcinoma, large B-cell lymphoma was incidentally found which had the same immunophenotype as the CLL. FISH analysis revealed gain of c-MYC at 8q and IHC showed increased c-MYC expression. This study supports that c-MYC plays a critical role in RS.
Keywords: Chronic lymphocytic leukemia/small lymphocytic lymphoma, Richter syndrome, MYC, diffuse large B-cell lymphoma
Introduction
Chronic lymphocytic leukemia (CLL) is a mature B-cell neoplasm characterized by overproduction of monotypic B cells in the peripheral blood and bone marrow [1]. CLL is generally a slowly progressive disease that is monitored without therapy until symptoms develop; however, Richter transformation (RT) [also known as Richter syndrome (RS) to aggressive lymphoma occurs rarely, and diffuse large B-cell lymphoma (DLBCL) is the most common type of RS [2]. The incidence of RS has been reported between 2% and 10% and RS involves most frequently lymph nodes. Extranodal organs are also commonly involved, which include gastrointestinal tract, skin, liver, tonsil, and bone marrow, etc. RS is usually heralded by sudden clinical deterioration and a rapid increase in the size of a lymphoid mass. The median time for the development of RS is from 21.9 [3] to 44.4 months [4]. The exact molecular mechanism of RS is unclear, but risk factors include trisomy 12, deletion of 11q23, microsatellite instability, mutations in tumor suppressor genes, and Epstein-Barr virus infections [5,6]. Recently MYC pathway has been suggested to be involved in RS [6-8]. Here we describe three cases of RS with overexpression of c-MYC, two of which involve body cavities.
Report of cases
Case 1
A 78-year-old man with past medical history of coronary artery disease, prostate cancer status post cryotherapy, and abdominal aortic aneurysm, presented to our Emergency Department in October 2011 with a painless, firm left submandibular mass. A computerized tomography (CT) scan with contrast showed several enlarged lymph nodes that scattered throughout the neck, mediastinum, and hila, with the largest one measuring 3.4 cm. His complete blood count (CBC) showed a normal white blood cell (WBC) count (7.1 × 106/L) with a normal differential. Core needle biopsy of the left submandibular mass showed complete effacement of the nodal architecture by predominantly small lymphoid cells, paraimmunoblasts, and focally increased large cells (single and small clusters) with readily identified mitotic figures. Flow cytometry revealed a population of monotypic B-cells that were positive for CD19, CD20 (dim), CD23, and CD38 with aberrant coexpression of CD5 and surface lambda light chain restriction, consistent with CLL/SLL. Immunohis-tochemistry (IHC) for cyclin D1 was negative. Cytogenetic study showed massive hyperdiploidy; however, an accurate karyotype was not obtained due to the poor viability of the lymphoma cells. The patient was left untreated until May 2012 when PET/CT scan showed significant interval worsening/progression of hypermetabolic lymphadenopathy throughout the visualized body. The patient was admitted to start first cycle of fludarabin, cyclophosphamide and rituximab (FCR). In August, after 4 rounds of chemotherapy, the patient presented to the Emergency Department for evaluation of right lower quadrant discomfort. CT of the abdomen/pelvis showed multiple enlarged lymph nodes and a large 6.8 × 5 cm necrotic mass in the right hemipelvis. Starting from September 2012, the patient complained abdominal distension with nausea and vomiting. CT confirmed rapidly accumulating ascites. Laboratory tests showed rapidly elevating uric acid (11.2) and lactate dehydrogenase (LDH) levels (1092), and rapidly worsening renal function. Cytology of the peritoneal fluid showed a monotonous population of large atypical lymphoid cells with abundant basophilic and vacuolated cytoplasm, round or irregular vacuolated nuclei and inconspicuous nucleoli, with numerous mitotic figures (Figure 1A). IHC of the cell block from the ascites showed the atypical large lymphoid cells were positive for c-MYC in approximately 30-40% of the cells (Figure 1B). Flow cytometry of the ascites showed a similar immunophenotype as before except for the loss of CD23 (Figure 1C). Based on the cytology and flow cytometry, RS was diagnosed. FISH showed c-MYC (8q24) rearrangement in 96% of the cells examined. Considering the poor prognosis of RS, the patient elected to defer treatment and expired at the end of the month.
Figure 1.
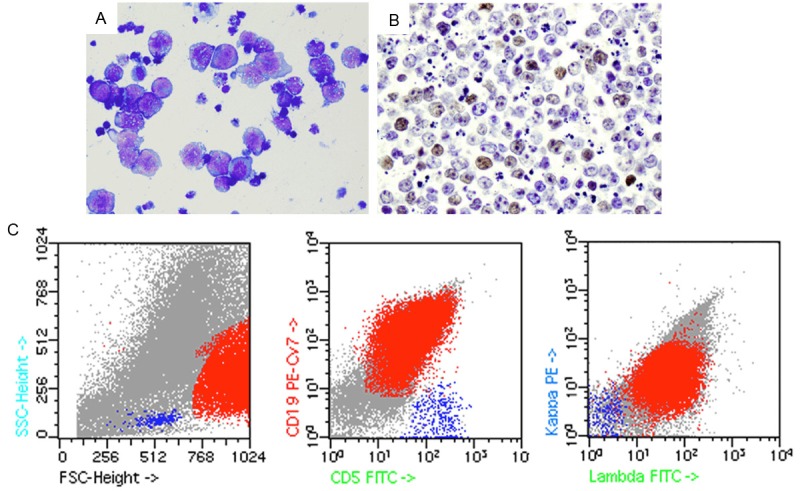
(Case 1) Richter transformation in ascites. A. Shows scattered atypical large lymphoid cells with abundant basophilic and vacuolated cytoplasm, round or irregular nuclei and inconspicuous nucleoli (Diff-Quick, × 500). Nuclear vacuolation is also present. B. Shows approximately 30-40% of the neoplastic cells are positive for c-MYC (× 400). C. Shows a predominant population of lambda-restricted large lymphoid cells that are CD19+/CD5+ by flow cytometry.
Case 2
A 66-year-old male was diagnosed CLL in 1993 and required intermittent therapy since 1996. His CLL progressed in 2006 but remained stable for approximately 3 years after FCR treatment. A flow cytometry in 2010 revealed only 1% residual CLL cells in the marrow. His disease progressed in August 2011 with thrombocytopenia and leukopenia, and bone marrow biopsy showed 10-20% CLL cells. He was treated with Benadmustine and rituximab and 3 cycles of FCR. FISH of the marrow showed trisomy 12 (+12) and deletion of short arm of chromosome 17 [del(17p)] in 15.5% and 12.5% of the metaphase cells, respectively. A left inguinal lymph node core biopsy in 2013 showed infiltration of large atypical lymphoid cells with abundant cytoplasm, vesicular nuclei, and prominent nucleoli (Figure 2A). These cells were positive for CD20 (Figure 2B), negative for CD3 (Figure 2C), but weakly positive for CD5 (Figure 2D). In addition, approximately 50% of these cells were also positive for cyclin D1 (Figure 2E) and c-MYC (Figure 2F), with a high proliferation index at 80-90% by Ki-67. However, cytogenetics showed no evidence of c-MYC rearrangement. In addition to the previously detected +12 (now 30%) and del(17p) (now 66%), a new gain of distal 13q (52%) was detected. Due to poor marrow function and cytopenias, the patient elected to be treated with HDMP+rituximab instead of chemoimmunotherapy with the plan of marrow transplant. Due to lack of response after cycle #2, he was started with HDMP-Gazyva, and then switched to Ibrutinib and GA101. In June 2014 the patient presented with progressive lymphadenopathy and large malignant right pleural effusion. He soon expired of respiratory distress.
Figure 2.
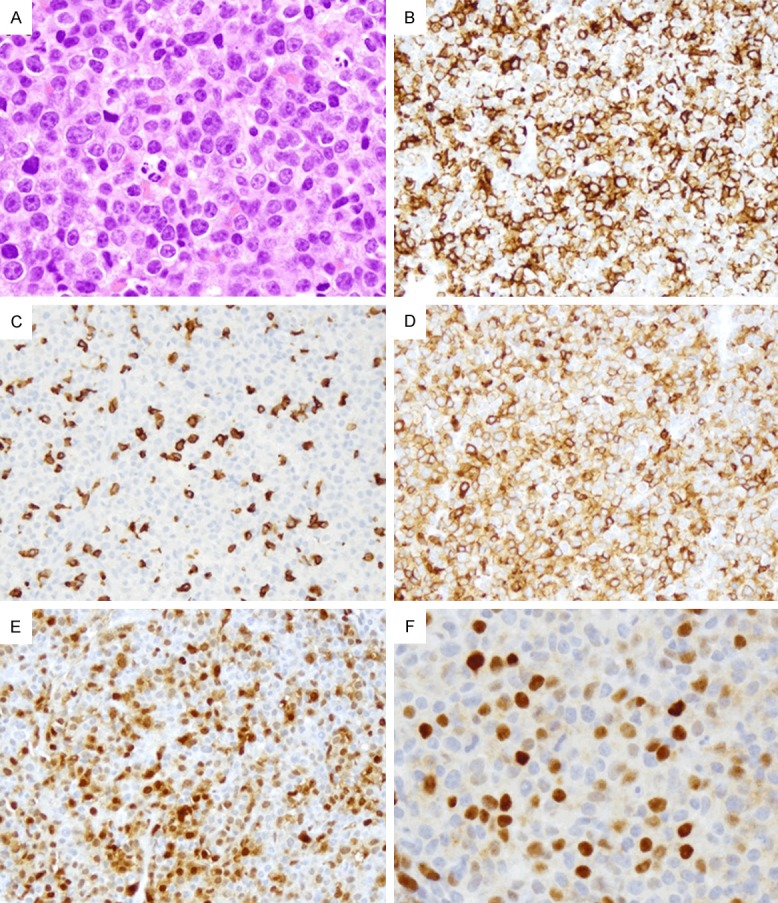
(Case 2) Richter transformation in right axillary lymph node. Histology shows infiltration of atypical large lymphoid cells with abundant cytoplasm, vesicular nuclei, and prominent nucleoli (A × 400). Large neoplastic cells are positive for CD20 (B × 200), negative for CD3 (C × 200), but weakly positive for CD5 (D × 200). Approximately 50% of the cells are positive for cyclin D1 (E × 200) and c-MYC (F × 400).
Case 3
This was a 62-year-old white female who was diagnosed CLL in May 2010, with del(13q14) and del(14q32) by FISH and a complex karyotype with del(3p), add(3q29), add(5q35), and add(11p15), del(4p), del(14q) and del(17p). She was treated with 1 day of FCR in June 2010, complicated with tumor lysis syndrome, and then the treatment was switched to 5 cycles of Bendamustine and rituximab. She received 3 cycles of rituximab and HDMP from March to May 2013, ofatumumab in October, and ibrutinib from Dec 2013 to October 2014 because of her progressive disease with symptomatic lymphadenopathy and splenomegaly. In October 2014, she was evaluated for shortness of breath and CT showed a partially solid, partially ground-glass lesion in the posterior apical segment of the left upper lobe measuring approximately 1.6 × 1.2 cm in maximum dimension. Left upper lobectomy specimen showed a moderately differentiated adenocarcinoma in the lung parenchyma. Interestingly, intermixed with the carcinoma cells within the pulmonary parenchyma and within the accompanying lymph nodes are diffuse infiltrate of medium to large atypical lymphoid cells with slightly condensed chromatin and conspicuous nucleoli (Figure 3A-C). IHC showed the atypical lymphoid cells are positive for CD20, CD23 (partial and weak), CD79a, MUM1 (data not shown), and c-MYC (Figure 3D) with focal dim positivity for CD5. Ki-67 staining showed a 90% proliferation index in the lymphoma cells. The atypical lymphoid cells were negative for cyclin D1, BCL6, CD3, CD10, or EBV (EBER). FISH of the lymph node showed trisomy 8 [+8q] in 16% of the neoplastic cells. Microarray analysis showed complex copy number abnormalities including del(14q24), -3p, -4p14-p15, -5q, t(13q14;X), and +8q24.
Figure 3.
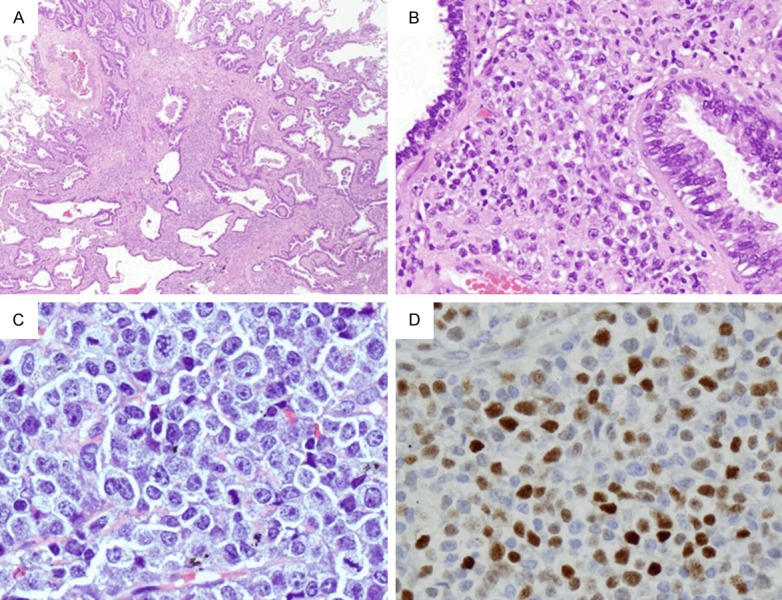
(Case 3) Richter transformation in the lung and a lymph node from the neck. A and B. Show collision tumor of adenocarcinoma and diffuse large B-cell lymphoma in the lung parenchyma (A × 20; B × 200). Note the lymphoid infiltration is composed of large cells with prominent nucleoli. C. Shows lymph node with infiltration of similar atypical large lymphoid cells with slightly condensed chromatin and conspicuous nucleoli (× 400). D. Shows approximately 60% of these cells are positive for c-MYC (× 200).
Discussion
Deregulation of the MYC oncogene, located on chromosome 8q24, has been shown to play a critical role in lymphomagenesis. MYC translocation has been identified in various aggressive lymphomas including Burkitt lymphoma, de novo DLBCL, B-cell prolymphocytic leukemia (PLL), blastoid variant of mantle cell lymphoma, and DLBCL transformed from follicular lymphoma [9-13]. MYC translocation is extremely rare in CLL. However, recent evidence suggested that MYC has a critical, albeit incompletely understood, role in priming the development of RS [6-8].
The first case is unusual for its clinical course and RS diagnosis made from the ascites. Different from typical CLL, no lymphocytosis was observed when the patient first presented with enlarged lymph nodes. Conventional cytogenetics showed an abnormal karyotype with massive hyperdiploidy. In less than one year, the patient’s condition deteriorated rapidly with significant lymphadenopathy throughout his body and presented with large amount of ascites. While flow cytometry of the large neoplastic cells revealed an immunophenotype similar to the previously diagnosed CLL/SLL, the morphology and high proliferation index led us to pursue c-MYC abnormalities. As we expected, both MYC (8q24) rearrangement and c-MYC protein were detected by FISH and IHC, respectively, in the large lymphoma cells. Huh el al. reported eight patients with CLL with increased prolymphocytes and MYC rearrangement, and suggested that MYC rearrangements were often associated with other cytogenetic abnormalities during transformation of CLL [8]. In our case, massive hyperdiploidy was also found. It is known that CLL is associated with certain cytogenetic abnormalities, but the newly acquired MYC (8q24) translocation may further prime the transformation of CLL.
In addition to rearrangements, MYC can also be deregulated by amplifications, mutations, or microRNA-dependent mechanisms. It has recently been reported that tumors with increased MYC protein production have coordinated up-regulation of MYC target genes. In a cohort study of patients with DLBCL, Johnson et al. found MYC translocations, high MYC mRNA, and MYC protein expression were in 11%, 11%, and 33% of the samples, respectively [14]. It is thus not surprising that in our third case, gain of 8q24 was identified by both FISH and Microarray analysis, which may correspond to the MYC amplification and the overproduction of MYC protein.
In our second case, MYC protein was detected in the large lymphoma cells with no detectable MYC rearrangement by FISH. It is noteworthy that although MYC rearrangements can be detected by FISH, MYC deregulation caused by other mechanisms than rearrangements or copy number increase can be failed by FISH analysis. In the second and third cases, cytogenetics also revealed TP53 deletion due to deletion of short arm of chromosome 17 [del(17p)] before the patients presented with rapidly enlarging PET positive lymph nodes. TP53 deletion due to del(17p) is well known for its correlation with unfavorable outcomes for CLL [15], it seems that activation of MYC oncogene and deletion of TP53 tumor suppressor gene played a perfect duo in the RS cases described here. Although del (13q14) is common in CLL, gain of distal 13q (52%) or translocation at 13q14 in the second and third case, respectively, is unusual. Both patients had long history of CLL and had been treated multiple times with chemotherapy. The function of the newly gained distal 13q/translocation at 13q14 in our cases remains unknown. Studies by Scandurra et al showed that 13q13.3-qter region containing MIRHG1 (MIR-17-92), a cluster of microRNA interacting with c-MYC, was acquired at the time of RS and was absent in the antecedent CLL phase. The 13q gain was coupled with the gain of c-MYC and loss of TP53 in RS cases. More interestingly, Scandurra et al. also observed that c-MYC rearrangement acquired at transformation appeared mutually exclusive with gain of MIRHG1 [7]. Thus, c-MYC in the second case might be activated by the microRNA dependent mechanism through the gain of 13q and further primed the transformation of CLL.
In addition to c-MYC, cyclin D1 was also positive in the second case. Although CCND1 overexpression has been detected in a subset of atypical and clinically aggressive CLL [16], it has rarely been reported in CLL evolving into RS [17,18]. This event may occur alone or in association with other abnormalities, such as p53 mutations. However, no t(11;14) involving CCND1 was detected in our RS case. Moreover, certain features associated with CLL with CCND1 overexpression such as leukocytosis, massively enlarged spleen, and atypical morphology with a CD5+/CD23-/FMC7+ immunophenotype [16], were not seen in our case. Thus, the relationship between cyclin D1 and the RS is not clear.
In summary, we reported three cases of RS with c-MYC overexpression. Two patients presented with body cavity involvement and expired in 1 month and 6 months, respectively, after the diagnosis. Due to the poor prognosis of RS with c-MYC overexpression, it is important to have high clinical suspicion for this entity with any sudden patient deterioration. Whether patients of RS with c-MYC overexpression will benefit from therapeutic regimens for high grade B-cell lymphoma (such as hyper-CVAD) is not known. Identification of c-MYC overexpression will likely provide another piece of evidence to support targeting c-MYC for appropriate and timely treatment of RS.
Disclosure of conflict of interest
None.
References
- 1.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 2.Rossi D, Gaidano G. Richter syndrome: molecular insights and clinical perspectives. Hematol Oncol. 2009;27:1–10. doi: 10.1002/hon.880. [DOI] [PubMed] [Google Scholar]
- 3.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103:216–228. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 4.Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, Arcaini L, Lucioni M, Rocque GB, Xu-Monette ZY, Visco C, Chang J, Chigrinova E, Forconi F, Marasca R, Besson C, Papadaki T, Paulli M, Larocca LM, Pileri SA, Gattei V, Bertoni F, Foà R, Young KH, Gaidano G. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117:3391–3401. doi: 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 5.Swords R, Bruzzi J, Giles F. Recent advances in the diagnosis and therapy of Richter’s syndrome. Med Oncol. 2007;24:17–32. doi: 10.1007/BF02685899. [DOI] [PubMed] [Google Scholar]
- 6.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123:1647–57. doi: 10.1182/blood-2013-11-516229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scandurra M, Rossi D, Deambrogi C, Rancoita PM, Chigrinova E, Mian M, Cerri M, Rasi S, Sozzi E, Forconi F, Ponzoni M, Moreno SM, Piris MA, Inghirami G, Zucca E, Gattei V, Rinaldi A, Kwee I, Gaidano G, Bertoni F. Genomic profiling of Richter’s syndrome: recurrent lesions and differences with de novo diffuse large B-cell lymphomas. Hematol Oncol. 2010;28:62–67. doi: 10.1002/hon.932. [DOI] [PubMed] [Google Scholar]
- 8.Huh YO, Lin KI, Vega F, Schlette E, Yin CC, Keating MJ, Luthra R, Medeiros LJ, Abruzzo LV. MYC translocation in chronic lymphocytic leukaemia is associated with increased prolymphocytes and a poor prognosis. Brit J Haematol. 2008;142:36–44. doi: 10.1111/j.1365-2141.2008.07152.x. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92:1297–1301. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 11.Hao S, Sanger W, Onciu M, Lai R, Schlette EJ, Medeiros LJ. Mantle cell lymphoma with 8q24 chromosomal abnormalities: a report of 5 cases with blastoid features. Mod Pathol. 2002;15:1266–1272. doi: 10.1097/01.MP.0000037310.82136.99. [DOI] [PubMed] [Google Scholar]
- 12.Lossos IS, Alizadeh AA, Diehn M, Warnke R, Thorstenson Y, Oefner PJ, Brown PO, Botstein D, Levy R. Transformation of follicular lymphoma to diffuse large-cell lymphoma: alternative patterns with increased or decreased expression of c-myc and its regulated genes. Proc Natl Acad Sci U S A. 2002;99:8886–8891. doi: 10.1073/pnas.132253599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuriakose P, Perveen N, Maeda K, Wiktor A, Van Dyke DL. Translocation (8;14)(q24;q32) as the sole cytogenetic abnormality in B-cell prolymphocytic leukemia. Cancer Genet Cytogenet. 2004;150:156–158. doi: 10.1016/j.cancergencyto.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel RM, Cook JR, Tubbs RR, Weisenburger DD, Campo E, Rosenwald A, Ott G, Delabie J, Holcroft C, Jaffe ES, Staudt LM, Gascoyne RD. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiler T, Dohner H, Stilgenbauer S. Risk stratification in chronic lymphocytic leukemia. Semin Oncol. 2006;33:186–194. doi: 10.1053/j.seminoncol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Cuneo A, Bigoni R, Negrini M, Bullrich F, Veronese ML, Roberti MG, Bardi A, Rigolin GM, Cavazzini P, Croce CM, Castoldi G. Cytogenetic and interphase cytogenetic characterization of atypical chronic lymphocytic leukemia carrying BCL1 translocation. Cancer Res. 1997;57:1144–1150. [PubMed] [Google Scholar]
- 17.Cobo F, Martínez A, Pinyol M, Hernández L, Gómez M, Beá S, Esteve J, Rozman M, Bosch F, López-Guillermo A, Montserrat E, Campo E. Multiple cell cycle regulator alterations in Richter’s transformation of chronic lymphocytic leukemia. Leukemia. 2002;16:1028–34. doi: 10.1038/sj.leu.2402529. [DOI] [PubMed] [Google Scholar]
- 18.Vela-Chavez T, Adam P, Kremer M, Bink K, Bacon CM, Menon G, Ferry JA, Fend F, Jaffe ES, Quintanilla-Martínez L. Cyclin D1 positive diffuse large B-cell lymphoma is a post-germinal center-type lymphoma without alterations in the CCND1 gene locus. Leuk Lymph. 2011;52:458–466. doi: 10.3109/10428194.2010.540361. [DOI] [PMC free article] [PubMed] [Google Scholar]


