Abstract
TGF-β1 is a key factor in the process of wound healing, which is regulated by TGF-β/Smad pathway. We previously demonstrated that TGF-β1 contributed to pathological scar formation. And previous studies also suggested Wnt/β-catenin pathway might be involved in wound healing. However, their role and relation in pathological scar formation remains not very clear. For evaluating TGF-β1 and β-catenin, key factors of the two signal pathways, immunohistochemistry, western blot analysis and RT-PCR were used. Simultaneously, immunohistochemistry were used to evaluate Smad2, Smad3 and Wnt-1, which were also the important factors. We found that they all significantly accumulated in pathological scars compared with normal skins (P<0.05), that implied the two signal pathways both contributed to pathological scar formation. Meanwhile, β-catenin expression showed a tendency to increase first and then decrease under the influence of different concentrations of TGF-β1 (P<0.01). It is possible that there is a complicated interaction between the two signal pathways in pathological scar formation (both synergy and antagonism).
Keywords: TGF-β/Smad pathway, Wnt/β-catenin pathway, pathological scar
Introduction
Pathological scars are the product of excessive wound healing, which are divided into keloids (K) and hypertrophic scars (HS). Pathological scars are characterized as an abnormal hyperplastic skin fiber disease, because that excessive extracellular matrix (ECM) deposition, especially type I and III collagen, due to persistent activation of fibroblast cells [1]. With the increasing hope of health and beauty, treatment of pathological scars is more and more urgent. But we still have not an ideal treatment, through the medical technology is developing rapidly. First of all, it must be known that the pathogenesis of pathological scars. Many scholars have studied the pathogenesis of pathological scars from many aspects, such as skin tension, location, age, infection, genetic mechanism, and so on [2]. But none of them can explain completely. Therefore, it must be combined effect of many factors. Therefore, we try to explain the pathogenesis of pathological scars with many reasons from the perspective of molecular biology.
TGF-β1 expression increases in wound, exogenous TGF-β1 can increase the amount of collagenous fibers, proteins and inflammatory cells [3,4]. Wnt signaling pathway is an important pathway, which regulates various cellular functions including proliferation, differentiation, apoptosis, survival, migration, and polarity, by regulating multiple intracellular signaling cascades [5]. And it is closely related to tumorigenesis, especially fibromatosis [6,7].
Previous studies demonstrated that there were synergistic effects between the TGF-β/Smad signal pathway and the Wnt/β-catenin signal pathwayin various cellular functions, such as tumorigenesis and stem cell differentiation [8], especially wound healing [9,10]. Furthermore, phenotypic effect of TGF-β signaling dysregulation on wound healing is mediated by β-catenin [11]. We previously demonstrated that TGF-β1 contributed to pathological scar formation [12], and further aimed to explore the role of the two signal pathways above in the pathogenesis of pathological scars, also the correlation of the two signal pathways.
Materials and methods
Samples
We randomly selected 30 samples of keloids as Group K, 30 samples of hypertrophic scars as Group HS, 30 samples of normal skins (NS) as Group NS (Table 1). They were all obtained with consent from surgical procedures at the First Hospital of China Medical University between 2008 and 2012. The criteria for diagnosis of scar are based on definitions suggested by international clinical recommendations on scar management [13].
Table 1.
Patient demographic and medical background
| Variable | Group K | Group HS | Group NS |
|---|---|---|---|
| Sex (n) | |||
| Male | 15 | 14 | 13 |
| Female | 15 | 16 | 17 |
| Age (years) | |||
| Mean | 35 | 33 | 39 |
| Range | 20-58 | 17-60 | 16-72 |
| Location (n) | |||
| Scalp | 0 | 0 | 2 |
| Forehead | 0 | 0 | 2 |
| Eyelid | 0 | 0 | 4 |
| Cheek | 0 | 0 | 3 |
| Ear lobe | 6 | 4 | 0 |
| Chin | 0 | 1 | 0 |
| Neck | 2 | 3 | 2 |
| Chest | 12 | 9 | 3 |
| Back | 5 | 4 | 4 |
| Abdomen | 1 | 2 | 3 |
| Shoulder | 2 | 5 | 1 |
| Arm | 1 | 0 | 4 |
| Wrist | 0 | 1 | 0 |
| Hip | 0 | 0 | 1 |
| Knee | 1 | 0 | 0 |
| Leg | 0 | 1 | 1 |
| Areas (cm2) | |||
| Range | 1.0×0.5-10.0×8.0 | 0.5×0.5-11.0×8.0 | 3.0×2.5-1.3×0.6 |
| Previous treatment | Nil | Nil | - |
Primary cell culture
Keloid cell lines, hypertrophic scar cell lines and normal skin cell lines were established from three samples, respectively. After removing subcutaneous tissue, samples were incised into small pieces in aseptic environment. Then, they were digested by trypsin (Hyclone, UT, USA) in Dulbecco’s Modified Eagle Medium (DMEM, Hyclone, UT, USA) prior to centrifugation. After that, fibroblasts could be collected, then seeded to cell culture dishes and allowed to adhere and proliferate in a humidified incubator with 5% CO2 at 37°C. DMEM was supplemented with 10% Fetal Bovine Serum (FBS, Hyclone, UT, USA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Each cell population was maintained until confluence, and then trypsinized for seeding. After three or four passages, fibroblasts were used for experiments.
Immunohistochemistry in tissues
The tissue sections (K n=30, HS n=30, NS n=30) are obtained from wax embedded whole tissue blocks. They were stained with primary antibody (rabbit anti-human Smad2+Smad3 polyclonal antibody 1:100, Abcam, Cambridge, UK; Goat anti-human Wnt-1 monoclonal antibody 1:50, Santa Cruz, TX, USA; mouse anti-human β-catenin monoclonal antibody 1:100, Zymed, CA, USA). Cover slips were incubated overnight at 4°C, and then stained with secondary antibodies and incubated at 37°C for 30 min, followed by incubation with third set of antibodies at 37°C for 30 min. It is necessary to wash with PBS three times for 5 min between each step. DAB (diaminobenzidine) coloration was used for detecting TGF-β1 and Wnt-1, and it was terminated until brown color appeared. Meanwhile, AEC (3-amino-9-ethylcarbazole) coloration was used for detecting Smad2, Smad3 and β-catenin, and it was terminated until red color appeared. After that, cover slips were counterstained with hematoxylin for 5-10 sec, submerged in hydrochloric acid ethanol, and then, mounted to slides prior to viewing. PBS (phosphate-buffered saline) was used as a negative control for comparing within each group and further validating the accuracy of the experiment. Tissue stained sections were viewed and imaged on an Olympus CH-2 upright microscope. Immunostaining results were interpreted independently by two pathologists. 10 views were chosen from each sample for counting the percentage of stained cells. We divided the samples into two groups based on results of the percentage of stained cells: positive cases (≥10%) and negative cases (<10%) 6.
Immunohistochemistry in cells
Fibroblasts (K n=30, HS n=30, NS n=30) were seeded into 24 well plates containing cover slips, and left for 72 h to attach and proliferate, meanwhile, treated with 0%, 20%, 40%, 60%, 80%, 100% TGF-β1 cell factor (10 mg/L as 100%, PeproTech, NJ, USA), respectively. Cover slips were stained with primary antibody (mouse anti-human β-catenin monoclonal antibody, negative control: PBS). After incubation at 37°C for 60 min, cover slips were stained with secondary antibodies and incubated at 37°C for 30 min, and then, third antibodies, incubating at 37°C for 30 min. It is necessary to wash with PBS three times for 5 min between each step. After DAB coloration, cover slips were counterstained with hematoxylin, and then, mounted to slides prior to viewing. PBS was used as a negative control. Cell stained coverslips (200x) were viewed and imaged on an Olympus CH-2 upright microscope. Immunostaining results were interpreted independently by two pathologists. 5 views were chosen for counting the number of stained cells randomly.
Western blotting
Samples (K n=30, HS n=30, NS n=30) were incised into small pieces. For obtaining tissue protein, they were lysed with lysis buffer prior to centrifugation. The concentration of protein in each cell lysate was determined using a BCA protein assay kit (Preice, NJ, USA) with bovine serum albumin (BSA) as the standard. An identical amount of protein (100 μg) from each sample was loaded onto a 10% SDS-PAGE gel and electrophoresed at 80 V for 30 min and then 120 V for 30 min. Thereafter, membranes were blocked for non-specific protein with 5% non-fat dry milk in PBS and then probed for 2 h at room temperature. The blots were incubated with primary antibodies (mouse anti-human β-catenin monoclonal antibody 1:400 and β-actin 1:500) at a respective dilution of 1:400 and 1:500 overnight at 4°C. The membranes were washed (10 min per wash) with TTBS (TBS added with 0.05% Tween-20), and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (rabbit anti-mouse IgG 1:5000, Jackson ImmunoResearch Laboratories, PA, USA) for 2 h at room temperature. All blots were developed using enhanced chemiluminescence reagents (ECL, Zhong Shan -Golden Bridge, Beijing, CHN) according to the manufacturer’s instructions. The signals were captured, and the density of the bands was estimated using Quantity-One software (Bio-Rad Laboratories).
Real-time PCR (RT-PCR)
Total RNA was extracted from 100 mg of each sample (K n=30, HS n=30, NS n=30) with Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The quantity and purity of RNA were assessed by optical density measurements at 260 nm and 280 nm using UV spectroscopy, and the integrity of the RNA was confirmed by 2% agarose gel electrophoresis. There were primers (Sangon, Shanghai, CHN) as follows: human TGF-β1 forward, 5’-CCC ACA ACG AAA TCT ATG ACA AG-3’, reverse, 5’-GCC ATG AGA AGC AGG AAA GG-3’; human β-actin forward, 5’-AGC GAG CAT CCC CCA AAG TT-3’, reverse, 5’-GGG CAC GAA GGC TCA TCA TT-3’; human GAPDH forward, 5’-AGG TCG GAG TCA ACG GAT TTG-3’, reverse, 5’-GTG ATG GCA TGG ACT GTG GT-3’. The RT-PCR was performed in an AMV reverse transcriptase reaction system under the following conditions: an initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 56°C for 45 s, and 72°C for 1 min, with a final10-min extension at 72°C. Amplified PCR products were separated on 5 μL 2% agarose gels and visualized using gel imaging analysis system Tanon4500.
Statistical analysis
All the experiments were performed at least three times. Data were analyzed with the SPSS 13.0 software. The data were presented as mean ± SD. Comparisons were performed using the SNK (Student-Newman-Keuls) test. Significance was assumed for P<0.05.
Results
More expression of TGF-β1, Smad2 and Smad3 in K and HS tissue compared with NS tissue
Immunohistochemical analysis of expression of Smad2 and Smad3 were performed in K, HS and NS tissue (Figure 1). They were stained more in nucleus, and accumulated in nucleus more obviously in K and HS tissue than in NS tissue (P<0.05, Table 2). However, their expression in K tissue was not significantly different from that in HS tissue (P>0.05).
Figure 1.

Immunohistochemical analysis of expression of Smad2, Smad3, Wnt-1 and β-catenin were performed in K , HS and NS tissue. Magnification=200 μm.
Table 2.
Percentage of positive cases expressed Smad2+Smad3, Wnt-1 and β-catenin in pathological scars and normal skin
| Group | Percentage of positive cases (%) | |||
|---|---|---|---|---|
|
| ||||
| Smad2+Smad3 | Smad2+Smad3 (intranuclear) | Wnt-1 | β-catenin | |
| K | 76.67 | 70.00 | 93.33 | 83.33 |
| HS | 66.67a | 63.33a | 86.67a | 76.67a |
| NS | 46.67a | 26.67b | 25.00b | 26.67c |
P>0.05;
P<0.05;
P<0.01.
RT-PCR indicated that TGF-β1 expression appeared greater in K and HS tissue than that in NS tissue (P<0.01, Table 3, Figure 2). And the difference in expression between K and HS tissue was not significant (P>0.05).
Table 3.
Western blot analysis of β-catenin in pathological scars and normal skin (mean ± SD)
| Group | Cases | Result | P values |
|---|---|---|---|
| K | 30 | 1.746 ± 0.610 | |
| HS | 30 | 1.429 ± 0.534 | >0.05 |
| NS | 30 | 0.773 ± 0.416 | <0.01 |
Figure 2.
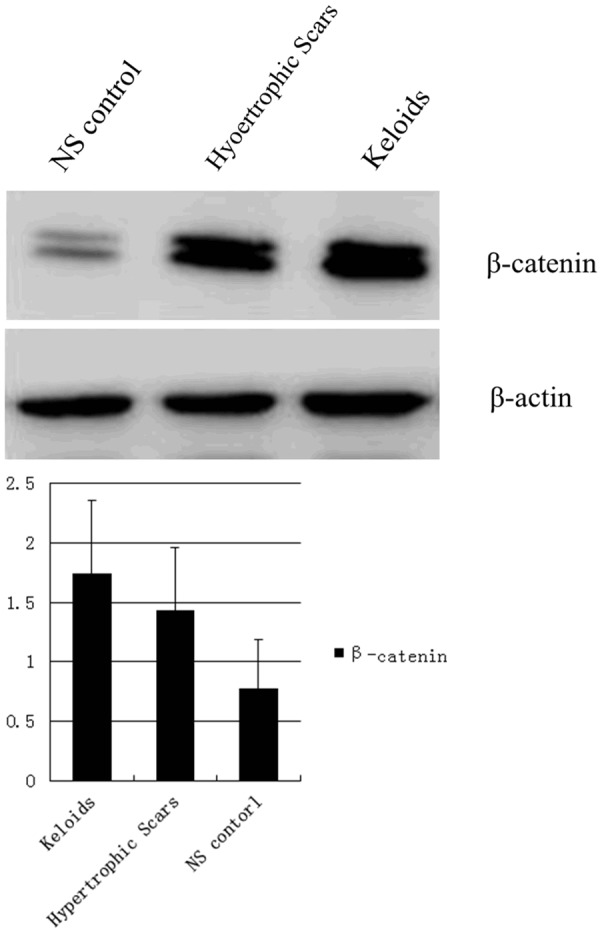
Western blot analysis of β-catenin in pathological scars and normal skin.
All above and our previous finding that TGF-β1 expressed excessively in pathological scar formation using immunohistochemical analysis 6, jointly suggested TGF-β/Smad pathway contributed to pathological scar formation.
More expression of Wnt-1 and β-catenin in K and HS tissue compared with NS tissue
Immunohistochemical analysis of expression of Wnt-1 and β-catenin were performed in K, HS and NS tissue (Figure 1). Wnt-1 was located more in cell cytoplasm. In K and HS tissue, Wnt-1 was expressed almost in every layer of epidermis, but its expression in Stratum basale and stratum spinosum was densest. In NS tissue, Wnt-1 was expressed only in Stratum basale. β-catenin was located in both cell cytoplasm and the nucleus. In NS tissue, β-catenin was almost expressed in epidermis, but in K and HS tissue, β-catenin could also be expressed in papillare demis. Wnt-1 and β-catenin were all more expressed in K and HS tissue, but less expressed or unexpressed in NS tissue (P<0.01: β-catenin, P<0.05: Wnt-1, Table 2). And the difference in expression between K and HS tissue was not significant (P>0.05).
Western blotting and RT-PCR also indicated that β-catenin expression appeared greater in K and HS tissue than in NS tissue (P<0.01, Tables 3, 4; Figures 2, 3). The expression of β-catenin in K tissue is about 2.243 times as much as that in NS tissue, meanwhile, that in HS tissue is about 1.830 times as much as that in NS tissue. But the difference in β-catenin expression between K and HS tissue was not significant (P>0.05).
Table 4.
RT-PCR analysis of TGF-β1 and β-catenin in pathological scars and normal skin (mean ± SD)
| Group | TGF-β1 | β-catenin | P values |
|---|---|---|---|
| K | 79.9 ± 5.1 | 58.9 ± 7.0 | |
| HS | 75.9 ± 3.7 | 52.1 ± 6.2 | >0.05 |
| NS | 51.2 ± 7.0 | 28.4 ± 7.3 | <0.01 |
Figure 3.
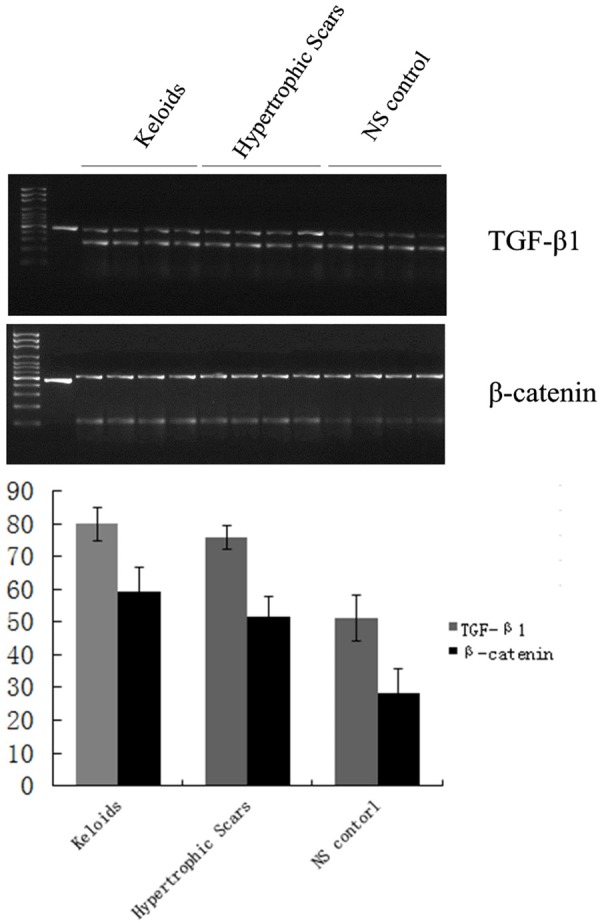
RT-PCR analysis of TGF-β1 and β-catenin mRNA in pathological scars and normal skin.
These observations suggested that Wnt/β-catenin pathway contributed to pathological scar formation.
Change of β-catenin expression by TGF-β1
With treatment of same concentration of TGF-β1, β-catenin expression was significantly higher in K and HS cells than in NS cells (P<0.05, Table 5; Figure 4). Meanwhile, it was found that, with the increasing in concentration of TGF-β1, β-catenin expression firstly increased and then decreased (Figure 5). The amplitude of variation was K<HS<NS. And the change was more significant in NS cells than in K and HS cells (P<0.01), but the difference in change between K and HS tissue was not significant (P>0.05). These suggested TGF-β/Smad pathway could activate and inhibit Wnt/β-catenin pathway in pathological scar formation.
Table 5.
Percentage of positive cells expressed β-catenin with different concentrations of TGF-β1 in pathological scars and normal skin (mean ± SD)
| Group/TGF-β1 | Percentage of positive cells (%) | P values | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0% | 20% | 40% | 60% | 80% | 100% | ||
| K | 43.33 ± 0.23 | 48.49 ± 0.29 | 52.84 ± 0.75 | 51.83 ± 0.29 | 49.34 ± 0.24 | 47.89 ± 0.49 | |
| HS | 38.62 ± 0.16 | 43.75 ± 0.29 | 50.25 ± 0.46 | 47.06 ± 0.25 | 43.62 ± 0.44 | 41.61 ± 0.38 | >0.05 |
| NS | 7.39 ± 0.34 | 17.69 ± 0.20 | 30.09 ± 0.37 | 21.02 ± 0.24 | 15.15 ± 0.61 | 11.39 ± 0.32 | <0.01 |
Figure 4.
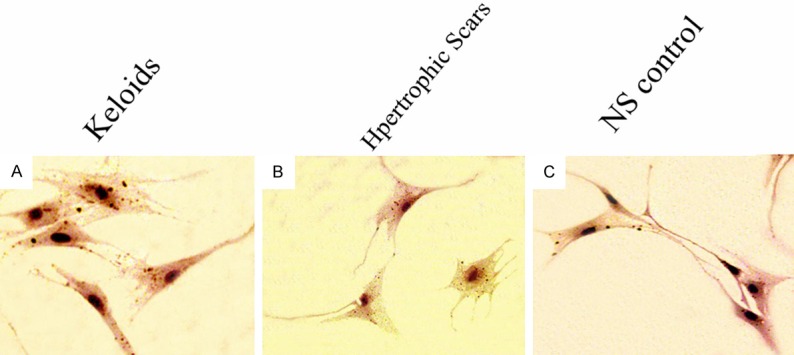
β-catenin expression in fibroblast cells from K (A), HS (B) and NS (C) tissue. Magnification=200 μm.
Figure 5.
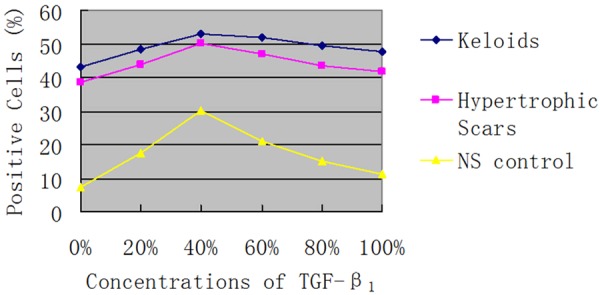
Percentage of positive cells expressed β-catenin with different concentrations of TGF-β1 in in pathological scars and normal skin.
Discussion
As an important intracellular signal molecule for TGF-β1, Smads can pass information from cell membrane to nucleus [14]. On one hand, with the help of Smad2/3, TGF-β1 activates its intranuclear promoter to induct endogenous TGF-β1 expression, which is called positive feedback regulation loop. On the other hand, phosphorylated Smad2/Smad3 (P-Smad2/3) activate Smad7 promoter to up-regulate Smad7 expression, and then Smad7 inhibits TGF-β1 expression, which is called negative feedback regulation loop. While, we found that in pathological scars, TGF-β1 expressed excessively and intranuclear Smad2/3 accumulated significantly, but not in normal skins. In some study, Smad2/3 expression increased, especially in the nucleus, and Smad7 expression was very low in keloid fibroblasts [9,15,16]. Hence, the positive feedback regulation loop must be amplified, which results in endogenous TGF-β1 expression and the formation of scar tissue.
More than 20 kinds of target genes exist in Wnt/β-catenin pathway, including c-myc, survivin, cyclin D1, CD44, metalloproteinases gene and vascular endothelial growth factor (VEGF). They played important roles in cell proliferation, anti-apoptosis, cell migration and maintaining stem cell anti-apoptosis of tumor stem cells [17,18]. Especially, CD44 played an important role in fibroblast growth [19]. In our study, we can see that the main factors, Wnt-1 and β-catenin, were all excessively expressed in K and HS. And researches have proved that expression of target genes, for example, c-myc, survivin and metalloproteinases gene, were increased in pathologic scars [20-22]. Conversely, with decline in β-catenin intranuclear accumulation, expression of target genes, for example, c-myc and CD44, also tapered off [23]. So we can see that Wnt/β-catenin pathway must contribute to pathologic scar formation.
TGF-β/Smad pathway and Wnt/β-catenin pathway have synergistic effects in many aspects, such as tumor formation, stem cells differentiation, and so on [24]. In human dermal fibroblasts, TGF-β1 and β-catenin/TCF (T cell factor) pathway regulate expression of matrix metalloproteinases, collagen and other connective tissue macromolecules [25]. While, Smad2/3 physically bind and modulate the activity of TCF/LEF (lymphoid enhancer factor) transcription factors [24,26]. And intranuclear β-catenin combines with TCF/LEF transcription factors causing fibroblast cells to secrete collagen excessively 21. In our study, we treated K, HS and NS with different concentrations of TGF-β1, and found the relation between β-catenin expression and TGF-β1 concentration: with the increase in the concentration of TGF-β1, β-catenin expression in each group showed a trend of increase first, and then decrease. The peak was at the concentration of TGF-β1 was 40%. Under higher concentration of TGF-β1, β-catenin expression reduced which might be caused by activation of the negative feedback regulation loop of TGF-β1 [28]. And this change of β-catenin expression existed in K, HS and NS at the same time. But only in NS, there was a statistical significance. All above show that in K and HS, the negative feedback regulation loop of TGF-β1 is inhibited compared with NS, and TGF-β/Smad pathway could activate and inhibit Wnt/β-catenin pathway in pathological scar formation.
In conclusion, we believe that both of TGF-β/Smad pathway and Wnt/β-catenin pathway play an important role in pathological scar formation, and there is a complex interaction between the two signal pathways (both synergy and antagonism). However, our study was not a randomized controlled trial, only tested a few factors, and lacked in vivo test. Meanwhile, the sample size was small, and experimental methods were less. Thus it needs further studies to support above points.
Disclosure of conflict of interest
None.
References
- 1.Bayat A, Bock O, Mrowietz U, Ollier WE, Ferguson MW. Genetic susceptibility to keloid disease: Transforming growth factor β receptor gene polymorphisms are not associated with keloid disease. Exp Dermatol. 2004;13:120–124. doi: 10.1111/j.0906-6705.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 2.Naylor MC, Brissett AE. Current concepts in the etiology and treatment of keloids. Facial Plast Surg. 2012;28:504–512. doi: 10.1055/s-0032-1325644. [DOI] [PubMed] [Google Scholar]
- 3.Miragliotta V, Lefebvre-Lavoie J, Lussier JG, Theoret CL. Equine ANXA2 and MMP1 expression analyses in an experimental model of normal and pathological wound repair. J Dermatol Sci. 2008;51:103–12. doi: 10.1016/j.jdermsci.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, de Crombrugghe B, Davidson JM, Bhowmick NA. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98–107. doi: 10.2353/ajpath.2010.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Li Y, Wang Y, Wu J, Yang G, Yang T, Gao Y, Lu Y. Versican gene: Regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J Dermatol Sci. 2012;68:157–163. doi: 10.1016/j.jdermsci.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Ge X, Wang X. Role of Wnt canonical pathway in hematological malignancies. J Hematol Oncol. 2010;3:33. doi: 10.1186/1756-8722-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, He Y, Huang C, Ma TT, Li J. Distinctive microRNA signature associated of neoplasms with the Wnt/beta-catenin signaling pathway. Cell Signal. 2013;25:2805–2811. doi: 10.1016/j.cellsig.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathway. J Biol Chem. 2008;283:9136–45. doi: 10.1074/jbc.M708968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D. Wnt/β-catenin pathway forms a negative feedback loop during TGF-β1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci. 2012;65:38–49. doi: 10.1016/j.jdermsci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Chua AW, Gan SU, Ting Y, Fu Z, Lim CK, Song C, Sabapathy K, Phan TT. Keloid fibroblasts are more sensitive to Wnt3a treatment in terms of elevated cellular growth and fibronectin expression. J Dermatol Sci. 2011;64:199–209. doi: 10.1016/j.jdermsci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Tong S, Jing SF, el al. Expression and significance of TGF-β1 and CTGF in pathological scar. Chinese J Aesth Plast Surg. 2008;4:100–2. [Google Scholar]
- 13.Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, Stella M, Téot L, Wood FM, Ziegler UE. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Abraham DJ. TGF-β and Smad signalling in fibrosis. Exp Dermatol. 2008;17:887–90. [Google Scholar]
- 15.Schiller M, Javelaud D, Mauviel A. TGF-β-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zeng B, Yao H, Xu J. The effect of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in human keloid. Burns. 2013;39:465–72. doi: 10.1016/j.burns.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Su K, Huang L, Li W, Yan X, Li X, Zhang Z, Jin F, Lei J, Ba G, Liu B, Wang X, Wang Y. TC-1 (c8orf4) enhances aggressive biologic behavior in lung cancer through the Wnt/β-catenin pathway. J Surg Res. 2013;185:255–63. doi: 10.1016/j.jss.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 18.Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J. Wnt signaling in prostate development and carcinogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:11–8. doi: 10.5507/bp.2011.016. [DOI] [PubMed] [Google Scholar]
- 19.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Puré E. Fibroblast migration is mediated by CD44- dependent TGF beta activation. J Cell Sci. 2008;121:1393–402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, Zhou KM, Wang Y. Expressions and Significance of CyclinA and C-myc in Skin Scar and Skin Scar Carcinoma. Cancer Research on Prevention and Treatment. 2011;38:1147–50. [Google Scholar]
- 21.Cao Y, Zhang R, Wang X, Huo R, Wang F, Lin L, Li Q, Wang Y. Is survivin a novel pathway for the treatment and pathogenesis of keloid? Med Hypotheses. 2013;81:389–93. doi: 10.1016/j.mehy.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Drymoussi Z, Lemonas P, Myers S, et al. Matrix metalloproteinases 1, 2, 13 and 14 are differentially expressed in keloid scars compared to normal skin. J Invest Dermatol. 2013;33(Suppl 1):S250. [Google Scholar]
- 23.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–50. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 24.Minoo P, Li C. Cross-talk between transforming growth factor-beta and Wingless/Int pathways in lung development and disease. Int J Biochem Cell Biol. 2010;42:809–12. doi: 10.1016/j.biocel.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook BD, Ferrari G, Pintucci G, Mignatti P. TGF-beta1 induces rearrangement of FLK-1-VE-cadherin-beta-catenin complex at the adherens junction through VEGF-mediated signaling. J Cell Biochem. 2008;105:1367–73. doi: 10.1002/jcb.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota M, Watanabe K, Hamada S, Sun Y, Strizzi L, Mancino M, Nagaoka T, Gonzales M, Seno M, Bianco C, Salomon DS. Smad2 functions as a co-activator of canonical Wnt/beta-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cell Signal. 2008;20:1632–41. doi: 10.1016/j.cellsig.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 28.Konikoff CE, Wisotzkey RG, Stinchfield MJ, Newfeld SJ. Distinct Molecular Evolutionary Mechanisms Underlie the Functional Diversification of the Wnt and TGFβ Signaling Pathways. J Mol Evol. 2010;70:303–12. doi: 10.1007/s00239-010-9337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


