Abstract
Objective: To explore the expression of SIRT1 with oxidative stress and observe physiological and pathological changes in the corneas as well as the association between SIRT1 and oxidative stress of diabetic dry eyes in mice. Method: Forty-eight C57BL/6Jdb/db mice at eight weeks of age were divided randomly into two groups: the diabetic dry eye group and the diabetic group. An additional forty-eight C57BL/6J mice at eight weeks of age were divided randomly into two groups: the dry eye group and the control group. Every mouse in the dry eye groups (diabetic and normal) was injected with scopolamine hydrobromide three times daily, combined with low humidity to establish a dry eye model. After the intervention, phenol red cotton string tests and corneal fluorescein staining were performed. In addition, HE staining and immunofluorescence were done. Expression of SIRT1 in the cornea was examined by real-time PCR and Western Blot and expression of FOXO3 and MnSOD proteins was detected by Western Blot. Results: At one, four, and eight weeks post intervention, all of the groups except the controls showed significant decreases in tear production and increases in the corneal fluorescein stain (P<0.05 vs control). Between the experimental groups, the diabetic dry eye group had the least tear production and the highest corneal fluorescein stain score (P<0.05). As the disease progressed, all of the experimental groups showed obviously pathological changes in HE staining, particularly the diabetic dry eye group. In the 1st and 4th week, the expression of SIRT1, FOXO3, and MnSOD were significantly higher in the diabetic DE and DM groups but lower in the DE group compared to the controls (P<0.05). In the 8th week, the expression of SIRT1, FOXO3, and MnSOD was significantly down-regulated in the diabetic DE group and the DM group (P<0.05). Immunofluorescence showed similar results. Conclusion: In the condition of diabetic dry eye, tear production declined markedly coupled with seriously wounded corneal epithelium. Oxidative stress in the cornea was enhanced significantly and the expression of SIRT1 was decreased.
Keywords: Dry eye, diabetes mellitus, SIRT1, FOXO3, MnSOD, cornea
Introduction
Dry eye (DE) is a common ocular disease described by the 2007 World Dry Eye Workshop as disorders of the tear film caused by reduced tear production, poor tear quality, or excessive tear evaporation [1]. This disorder is associated with symptoms of ocular discomfort such as dryness, irritation, foreign body sensation, redness and itching [2]. It is estimated that almost 5 million Americans beyond 50 years old have DE, and additional millions experience the symptoms of DE [3]. There are many risk factors for the development of DE including advanced age, female sex, autoimmune disease, infection, ophthalmic surgery, contact lens use, and environmental stress [1,4]. The pathogenesis of DE is not fully understood; however, it is recognized that oxidative stress has a prominent role in the development of DE [5-7].
Diabetic Mellitus (DM) is a systemic disease characterized by chronic hyperglycemia and leading to chronic complications such as neuropathy, nephropathy, and microvascular disease [8]. Several pathophysiological conditions are known to be involved in the development of DM including apoptosis, inflammation, neurotrophic damage, and oxidative stress [9]. The most common ocular complications, including cataracts, glaucoma and retinopathy are well documented [8,10]. In recent years, it has been found that diabetic patients often complain of DE symptoms and have decreased Schirmer test readings [11]. These results are likely due to neuropathy, metabolic dysfunction, or abnormal lacrimal secretion. Many studies have reported different pathological causes for diabetic dry eye [12-14]; nevertheless, the association between DM and DE is complex.
Silent information regulator 2 (Sirt2), first described in yeast, was first discovered in the Sirtuin family. There are seven mammalian members of the Sirt2 family [15,16]; the closest to yeast Sirt2 is SIRT1. SIRT1 functions as a class III histone deacetylase, with its deacetylase activity dependent on intracellular NAD+ concentrations. This protein regulates a wide range of cellular processes through deacetylase activity, including antioxidation, anti-apoptosis, DNA repair, antiaging, and life-span extension [17,18]. SIRT1 promotes cell survival by inhibiting apoptosis or cellular senescence induced by stress, including DNA damage and oxidative stress. SIRT1 is richly distributed in many tissues and organs and has been found in the nucleus and cytoplasm of cells from all of the ocular structures, including the cornea, iris, ciliary body, lens, and retina [19]. In the cornea, SIRT1 is localized in the nucleus and cytoplasm of corneal epithelial cells, and in the nucleus of keratocytes and corneal endothelial cells. No expression of SIRT1 is detected in the acellular part of the corneal stroma [20].
Forkhead box O (FOXO) transcription factors have been identified as substrates of SIRT1. In mammals, there are four evolutionarily conserved FOXO family members (FOXO1, FOXO3, FOXO4 and FOXO6) [21]. FOXOs are involved in mediating the response to oxidative stress and enhancing oxidative stress resistance. The association between SIRT1 and FOXO3 is increased during oxidative stress [22]. SIRT1 regulates the transactivation activity of FOXO by catalyzing its deacetylation in an NAD-dependent manner in response to oxidative stress [23].
As a member of the superoxide dismutase (SOD) family, MnSOD is located in the mitochondrial matrix, catalyzing the dismutation of the superoxide radicals and significantly decreasing oxidative stress [24]. Active FOXO3 protects cells from oxidative stress by directly increasing MnSOD mRNA and protein [21].
Many previous studies have investigated the relationship between SIRT1 and DM. However, although oxidative stress has been reported in DE, it remains unclear if SIRT1 is associated with diabetic DE. The pathogenesis of DE in diabetic patients is more complex. In this study, we investigate the role of SIRT1 in diabetic DE and evaluate the relationship between diabetic DE and oxidative stress.
Materials and methods
Animal
Eight-week-old female C57BL/KsJ-db/db mice and eight-week-old female C57BL/6J mice (Slac Laboratory Animal Center, Shanghai, China) were housed in plastic cages with well-ventilated stainless steel grid tops at 22°C±2°C with a 12-hour light-dark cycle (8 AM to 8 PM). In total, 48 C57BL/KsJ-db/db mice and 48 C57BL/6J mice were used in this study. 48 C57BL/KsJ-db/db mice were randomly divided into two groups: the diabetic DE group and the DM group. 48 C57BL/6J mice were randomly divided into two groups: the DE group and the control group. To induce dry eye model, the diabetic DE and the DE groups were injected with 0.1 mg/0.2 ml scopolamine hydrobromide intraperitoneally (Sigma-Aldrich, St. Louis, MO, USA) three times daily. Mice in the diabetic DE and the DE groups were exposed to air drafts for 12 hours every day to maintain the ambient humidity below 40%. Diabetic mice were fed with a high-fat diet. Mice were sacrificed by cervical dislocation at three different time points: 1 week, 4 weeks, and 8 weeks after intervention. All experiments were performed according to the Guidelines of Animal Experiments from the Ethics Committee of Tongji University (Shanghai, China). Animals were cared for in accordance with the Statement for the Use of Animals in Ophthalmology and Vision Research of The Association of Research for Vision and Ophthalmology.
Glucose analysis
Blood samples were obtained from the tail veins of mice after withholding food for 6 hours, then measured with the One Touch Ultra blood glucose meter (Johnson & Johnson, New Brunswik, NJ, USA) at 1, 4, and 8 weeks after induction.
Detection of aqueous tear production
Aqueous tear production was measured using phenol-red impregnated cotton threads (Zone-Quick; Oasis, Glendora, CA, USA). The threads were held with jeweler forceps (Katena Products, Inc., Denville, NJ, USA) and placed in the lateral cantus of the conjunctival fornix of the right eye for 60 seconds. The length of moist thread was observed under a dissecting microscope (model SZX10, Olympus, Tokyo, Japan), using a slide gauge with a precision accuracy of 0.02 mm.
Corneal fluorescein staining
Corneal fluorescence staining was performed to evaluate corneal integrity. Briefly, 0.5 μl of 1% fluorescein (Sigma-Aldrich, St. Louis, MO, USA) was added to the inferior conjunctival sac of the right eye with a micropipette. The cornea was examined 1 min after fluorescein addition under a slit lamp biomicroscope (66 Vision Tech Co Ltd, Suzhou, China) in cobalt blue light. Corneal fluorescein staining was classified using a grading system developed by Park et al [25]. No staining was graded as 0; staining that measured ≤1/8 of the cornea was graded as 1; staining comprising ≤1/4 of the cornea was graded as 2; staining at ≤1/2 of the cornea was graded as 3; and staining that composed >1/2 of the cornea was graded as 4.
Corneal histopathology
Mice were sacrificed by cervical dislocation and the integral eyeball was collected, fixed in 4% paraformaldehyde, and embedded in paraffin. The eyes were sectioned in the vertical plane (4 μm thick). Sections were stained for glycoproteins by using the periodic acid-Schiff (PAS) staining system (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The morphology of the cornea was observed under the microscope (Olympus, Tokyo, Japan) and evaluated by two investigators blinded to the study.
Western blot analysis
The mice were sacrificed and the cornea was carefully dissected. Corneas were sonicated in Tris-buffered saline (TBS) containing protease inhibitors. The processed corneas were centrifuged and the supernatant collected. Proteins of equal concentration were separated on 10% SDS-PAGE and transferred to PVDF transfer membranes (Millipore, Billerica, MA, USA). Nonspecific binding was blocked with 5% non-fat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) overnight at 4°C. Next, the membranes were incubated with mice anti-SIRT1, anti-FOXO3, anti-MnSOD, and mouse anti-β-actin monoclonal primary antibody (1:1000). The membranes were rinsed three times with TBST for 10 min and then incubated with horseradish peroxidase-conjugated rabbit anti-mouse secondary antibodies (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford, IL, USA). The relative densities of target proteins were normalized against β-actin using a Gel-Pro analyzer (Media Cybernetics, Silver Spring, MD, USA).
Quantitative real-time RT-PCR
The mRNA expression of SIRT1 was analyzed using the ABI 7300 Real-Time PCR System (Foster City, CA, USA). The specific primer pairs are shown in Table 1. The reverse transcription reaction was performed with 1 μg total RNA isolated from the cells of each group. Quantitative real-time RT-PCR was performed with pre-designed primers for SIRT1 (forward primer: GCAGATTAGTAAGCGGCTTGAGG, reverse primer: AGCACATTCGGGCCTCTCCGTA) and GAPDH as a housekeeping gene (forward primer: CCCGTAGACAAAATGGTGAAGGTC, reverse primer: GCCAAAGTTGTCATGGATGACC). For polymerase chain reaction (PCR) amplifications, cDNAs were amplified using the SYBR Green Real-Time PCR Master Mix (Takara) and 0.4 μmol/L of each primer pair. Amplification was carried out with an initial incubation for 30 s at 94°C, followed by 40 cycles of the amplification step (94°C 30 s, 60°C 60 s and 72°C 1 min) for SIRT1. All amplification reactions for each sample were carried out in triplicate and the averages of the threshold cycles were used to interpolate curves using 7300 System SDS Software (ABI, CA, USA). Results were expressed as the ratio of SIRT1 to GAPDH mRNA, and the value of SIRT1 expression level in the group of the control was regarded as 100%.
Table 1.
Blood glucose for every group in every different time point
| Variable | Glucose (mmol/L, n=8) | ||
|---|---|---|---|
|
| |||
| 1st week | 4th week | 8th week | |
| Diabetic DE | 23.68±2.55* | 23.89±2.76* | 22.54±2.55* |
| DE | 7.28±0.72 | 6.21±0.40 | 6.30±0.53 |
| DM | 22.68±3.33* | 23.26±1.81* | 22.88±1.44* |
| Control | 6.47±0.49 | 6.55±0.65 | 6.23±0.58 |
P<0.01 vs. the control group.
Immunofluorescence assays
For immunofluorescence staining on 5 μm thick paraffin sections, the sections were mounted on coated slides and allowed to dry overnight at 37°C, then deparaffinized and rehydrated through a graded alcohol series. Antigen retrieval was performed in a microwave with Epitope Retrieval Solution, pH 6.0 (Novacastra, Newcastle upon Tyne, UK) for 10 minutes (3 min at 100% power and 4 min at 50% power). All of the sections were then incubated in 2% skim milk in TRIS buffer to minimize non-specific binding. All of the tissue sections were then incubated with anti-SIRT1 antibodies (1:200 dilutions; Santa Cruz Biotech Inc., Paso Robles, CA, USA) overnight at 4°C. Sections were washed in buffer (Novacastra, Newcastle upon Tyne, UK) and then incubated with secondary antibodies (Invitrogen, CA, USA). Sections were then treated with 0.03% Sudan black for 2 mins to remove autofluorescence and washed in running water before mounting with Prolong Gold antifade media (BD Pharmingen, San Jose, CA). Images were taken using the Olympus Fluoview program and the original slides were imaged with a confocal microscope (Olympus FV1000 Inverted Laser Scanning Microscope).
Statistical analysis
All results were expressed as the mean ± standard deviation unless otherwise indicated. Statistical evaluations were performed with SPSS 20.0 for Windows (SPSS, Inc., Chicago, IL, USA) using ANOVA with multiple comparisons between groups and Pearson correlation tests. Statistical significance was accepted for P<0.05.
Results
Blood glucose
Blood glucose is shown in Table 1. There was a significant increase in blood glucose for diabetic DE group and DM group when compared with the control group in the 1st, 4th, and 8th weeks after induction (P<0.01). Blood glucose remained stable for every group without significant difference at every time point (P>0.05).
Aqueous tear production
Aqueous tear production is shown in Table 2. Significant decreases in aqueous tear production were found in diabetic DE, DE, and DM groups when compared to controls in the 1st, 4th, and 8th weeks (P>0.05). The lowest aqueous tear production was detected in the diabetic DE group (P>0.05). Aqueous tear production dropped continuously during the induction (P<0.05) for all groups except the control. For the DE group, aqueous tear production decreased significantly when compared to the DM and control groups at every time point (P<0.05). Aqueous tear production didn’t show remarkable change in the 4th week; however, significant decreases were achieved by the 8th week. For the DM group, aqueous tear production decreased significantly when compared to the control group at every time point (P<0.05) and no statistical difference was found in different time points.
Table 2.
Aqueous tear production for every group in every different time point
| Variable | Aqueous tear production (mm, n=8) | ||
|---|---|---|---|
|
| |||
| 1st week | 4th week | 8th week | |
| Diabetic DE | 2.92±0.90*,# | 1.67±0.42*,# | 1.07±0.15*,# |
| DE | 3.59±0.70* | 3.28±0.73* | 2.18±0.61*,# |
| DM | 7.32±1.30* | 6.37±1.07* | 6.30±1.19* |
| Control | 9.40±1.21 | 9.62±0.74 | 9.53±1.40 |
P<0.05 vs. the control group.
P<0.05 vs. the same group in different time point.
Corneal fluorescein staining
The grades of corneal fluorescein staining are shown in Table 3. Significant increases in the corneal fluorescein staining score were found in the diabetic DE, DE, and DM groups when compared with the control group in the 1st, 4th, and 8th week (P>0.05). For the diabetic DE, DE, and DM groups, the score significantly increased in the 4th week compared to groups in the 1st week. The diabetic DE group scored the highest at every time point (P<0.05). For the DE group, the score increased significantly compared to the DM and control groups at every time point (P<0.05). For the DM group, corneal fluorescein staining score increased significantly when compared to the control group at every time point (P<0.05) (Figure 1).
Table 3.
Corneal fluorescein staining for every group at different time points
| Variable | Score (n=8) | ||
|---|---|---|---|
|
| |||
| 1st week | 4th week | 8th week | |
| Diabetic DE | 6.38±1.41* | 10.38±0.74*,# | 11.25±0.89* |
| DE | 4.63±0.92* | 9.13±1.25*,# | 9.75±1.49* |
| DM | 3.63±1.06* | 5.13±1.55*,# | 6.00±1.60* |
| Control | 1.63±0.92 | 1.70±0.93 | 1.75±1.04 |
P<0.05 vs. the control group.
P<0.05 vs. the same group in 4th week.
Figure 1.
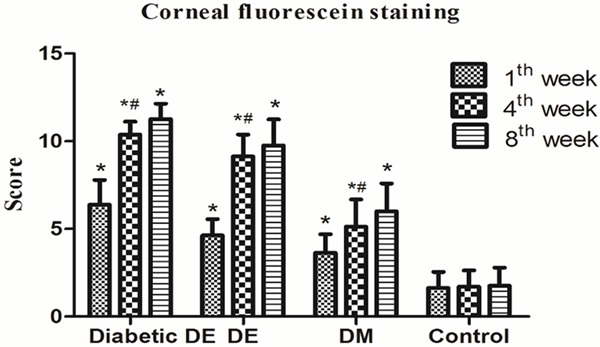
Corneal fluorescein staining for different groups at different time points. *P<0.05 vs. the control group. #P<0.05 vs. the same group in 1st week. Corneal histopathology.
Corneal HE staining
HE staining showed that with the progression of diabetic DE, corneal epithelium became thin and loose, the number of epithelial cells decreased, the corneal basal epithelium became disorderly, and the corneal surface was roughened. In the DE group, the thickness of the corneal epithelium increased and the arrangement of epithelium became loose. In addition, the corneal basal epithelium became disarranged and the corneal surface was rough. In the DM group, the arrangement of the corneal basal epithelium became disordered. The pathological changes in the corneal epithelium in the DM group were less profound than that in the diabetic DE and DM groups (Figure 2).
Figure 2.
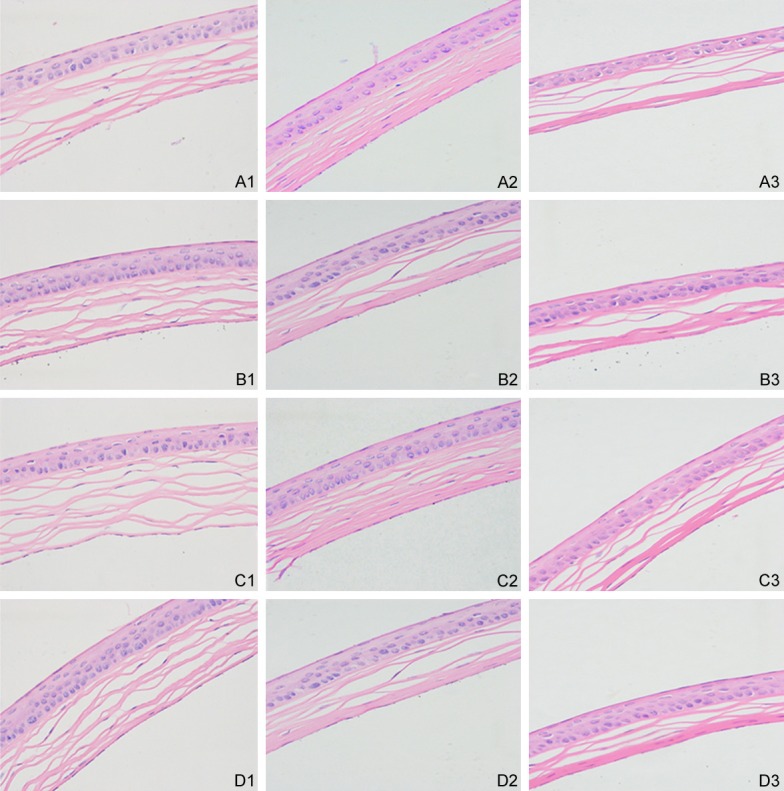
Corneal HE staining for different group in every different time point. A: Diabetic dry eye group; B: Dry eye group; C: Diabetic group; D: Control group. 1: The 1st week after induction. 2: The 4th week after induction. 3: The 8th week after induction.
Western blot analysis of SIRT1, FOXO3, and MnSOD expression
Western blot analysis demonstrated that after 1st week of induction, SIRT1 expression was significantly increased in the diabetic DE group and DM group and decreased in the DE group when compared to controls (P<0.05). The expression of SIRT1 in the diabetic DE group was higher than in the DM group (P<0.05). FOXO3 and MnSOD expression had a similar tend as SIRT1. In the 4th week, SIRT1 protein expression in the diabetic DE group and DM group was still higher than that in the control group (P<0.05). For the DE group, SIRT1 protein maintained decreased expression (P<0.05). The expression of SIRT1 in the diabetic DE group was lower than that of the DM group (P<0.05). FOXO3 and MnSOD expression had a similar tend with SIRT1. In the 8th week, SIRT1 expression significantly decreased in the diabetic DE group and DM group (P<0.05). SIRT1 protein maintained decreased expression in the DE group (P<0.05). The lowest SIRT1 expression occurred in the diabetic groups. The expression of FOXO3 and MnSOD was similar to SIRT1 (Figure 3).
Figure 3.
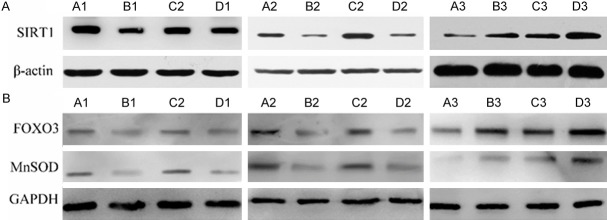
A: The expression of SIRT1 for all groups in the 1st, 4th and 8th week. B: The expression of FOXO3 and MnSOD for all groups in the 1st, 4th and 8th week. A: Diabetic dry eye group; B: Dry eye group; C: Diabetic group; D: Control group. 1: The 1st week after induction. 2: The 4th week after induction. 3: The 8th week after induction.
Quantitative real-time PCR analysis
SIRT1 mRNA expression had a similar tend with SIRT1 protein. In the 1st week, SIRT1 mRNA expression was significantly up-regulated in the diabetic DE group and DM group and down-regulated in the DE group (P<0.05). In the 4th week, SIRT1 mRNA expression in the diabetic DE group and DM group was still higher than that in the control group (P<0.05). SIRT1 mRNA maintained decreased expression (P<0.05). In the 8th week, SIRT1 mRNA was markedly down-regulated in the diabetic DE group and DM group. For the DE group, SIRT1 mRNA still kept decreased expression (P<0.05). SIRT1 mRNA in the diabetic DE group was lower than in any other group (P<0.05) (Figure 4). Immunofluorescence the results showed that fluorescence intensity of SIRT1 for diabetic DE group, DE group, DM group and control group were similar in 1st and 4th week. In the 8th week, fluorescence intensity of SIRT1 for diabetic DE group was significantly weaker than that for DE group, DM group and control group (Figure 5).
Figure 4.
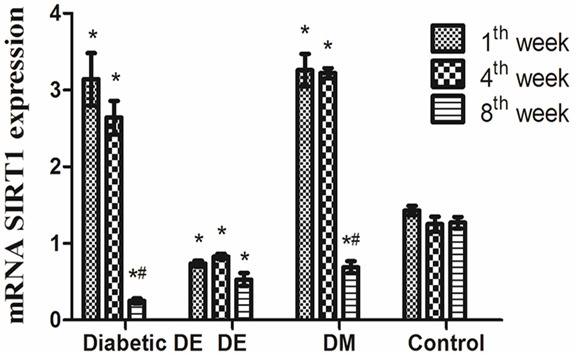
The expression of mRNA SIRT1 for diabetic DE group, DE group, DM group, and control group in different time points. *P<0.05 vs. the control group. #P<0.05 vs. the same group in different time point.
Figure 5.
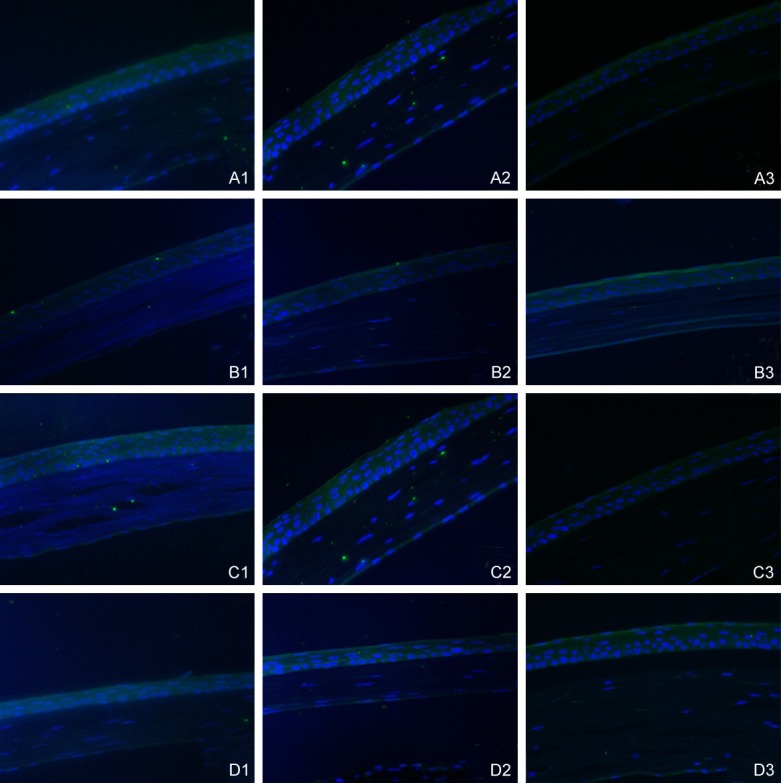
Immunofluorescence with SIRT1 antibody across all groups in different time points. A: Diabetic dry eye group; B: Dry eye group; C: Diabetic group; D: Control group. 1: The 1st after induction. 2: The 4th week after induction. 3: The 8th week after induction.
Discussion
In this study, the dry eye model was established by systemic scopolamine administration, a commonly used model which significantly decreases tear production. The air draft in the blower hood created an increased evaporation environment with humidity maintained at 35% to 40%, further desiccating the ocular surface. Inhibition of tear secretion coupled with desiccating environmental stress significantly worsened the severity of dry eye symptoms and corneal epithelial defects [26]. The db/db mice were used as a diabetic mouse model for stable hyperglycemia and similar diabetic procession with humans [27]. Glucose analysis demonstrated that blood glucose in db/db mice was stable throughout the experiment. Detection of aqueous tear production and corneal fluorescein staining showed that diabetic DE mice and DE mice significantly decreased tear secretion and displayed epithelial defects. The situation for diabetic DE mice is more serious. Mice in the diabetic group also exhibited decreased tear secretion and epithelial defects, similar to previous reports by Zagon IS et al [28]. Through HE staining, we demonstrated that corneal epithelial injury became aggravated with course elongating. More severe injury was caused in diabetic DE mice compared with simple DE mice. Pathological changes were also found in the diabetic group; however, these were not serious. Our model showed that injection of scopolamine hydrobromide combined with low humidity can effectively induce dry eyes in our mouse models.
In recent years, oxidative stress has been recognized as a pathway for many ocular diseases such as keratoconus, age-related macular degeneration, cataracts, and diabetic retinopathy [29]. In the ocular surface, oxidative stress has been linked to injury of the cornea, conjunctiva, and lacrimal glands as associated with certain pathological conditions [30,31]. Nakamura et al has reported increased reactive oxygen species (ROS), anti-oxidative genes, and oxidative stress markers in the corneal epithelium of a blink-suppressed DE model [6]. Augustin et al found that obvious oxidative stress occurred in the tear film of DE patients [5]. Yuichi et al reported that increased oxidative stress was related to decreased tear secretion, leading to DE [7]. These articles strongly suggest that oxidative stress has a direct relationship with DE. DM-related DE is more complex. It has been indicated that oxidative stress is an important role in DM and DM complications.
Western blot, RT-PCR, and immunohistochemistry showed interesting results. After the induction of DE, the expression of the SIRT1 protein and gene significantly increased for the diabetic DE and DM group in the first week and 4th week, and then decreased in the 8th week. We believe that these results are not contradictory. On the contrary, we think these results accurately describe the pathological process of DM and DE. In the first week, the up-regulated expression of SIRT1 in the diabetic DE group and DM group implies that special physiological mechanisms of DM activate a compensatory reaction against oxidative stress. This agrees with previously reported research showing that the expression of SIRT1 may be activated by oxidative stress. Meanwhile, the expression in the DE group was decreased when compared to the control group. This phenomenon may be due to the severe pathological process of DE. The expression of SIRT1 in the diabetic DE group was higher than in the DM group. This situation may due to additional pathogenic factors of DM combined with DE, causing stronger compensatory reactions. In the 4th week, the expression of SIRT1 in the diabetic DE group and DM group maintained up-regulation. However, the expression of SIRT1 in diabetic DE group was lower than that in the DM group. We believe that this may be due to double pathogenic factors leading to a reduced compensatory effect. In the 8th week, SIRT1 expression in the diabetic DE and DM groups was significantly down-regulated. It may be that a long period of pathological stimuli causes an exhausted compensatory effect. We observed that SIRT1 in the DE group maintained a low level of expression during the experiment.
The expression of FOXO3 and MnSOD protein showed similar tends with SIRT1. FOXO3 plays an important role in resistance to oxidative stress by regulating the genetic expression of MnSOD. It is well known that SOD is an antioxidant enzyme and that, to some extent, the level of SOD represents the level of oxidative stress. In the first week, the up-regulation of FOXO and MnSOD in diabetic DE and DM groups indicated a low level of oxidative stress and the down-regulation of FOXO; however, the MnSOD expression in the DE group indicated a high level of oxidative stress. DM and its complications are related to high levels of oxidative stress. However, the level of oxidative stress in diabetic DE and DM groups were at low levels, implying that there may be special physiological mechanisms for a DM-activated compensatory reaction against oxidative stress. In the 4th week, FOXO and MnSOD expression in the diabetic DE group and DM group maintained up-regulation. In the 8th week, FOXO and MnSOD expression in the diabetic DE and DM groups significantly decreased; thus, the level of oxidative stress was significantly up-regulated. At every time point, FOXO and MnSOD in DE mice kept low expression and the corneas of DE mice exhibited significant oxidative stress.
The expression of SIRT1, FOXO3, and MnSOD showed that there may be a link between them. SIRT1, FOXO3, and MnSOD play positive roles in the regulation of oxidative stress. During oxidative stress, SIRT1 can regulate the expression of FOXO3 while FOXO3 can increase MnSOD. We believe that a pathway exists for SIRT1-FOXO3-MnSOD. With the progression of diabetic DE, SIRT1 expression rises in the first stage and then decreases. These interesting changes suggest a role for SIRT1 against oxidative stress. Our further research will concentrate on these issues and attempt to find the role that SIRT1 plays in the pathogenesis of diabetic dry eye.
In conclusion, significant oxidative stress was detected in diabetic DE, DE, and DM model mice. Our work shows a link between SIRT1 and the changes in the cornea of diabetic DE, DE, and DM mice models.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81400373) and Shanghai Municipal Commission of Health Youth Talents Training Plan of Tongji University and Family Planning (2013SY041). The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure of conflict of interest
None.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard JD. Guidelines for the treatment of chronic dry eye disease. Manag Care. 2003;12:20–25. [PubMed] [Google Scholar]
- 3.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 4.Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45(Suppl 2):S199–202. doi: 10.1016/s0039-6257(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 5.Augustin AJ, Spitznas M, Kaviani N, Meller D, Koch FH, Grus F, Göbbels MJ. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:694–698. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura S, Shibuya M, Nakashima H, Hisamura R, Masuda N, Imagawa T, Uehara M, Tsubota K. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Invest Ophthalmol Vis Sci. 2007;48:1552–1558. doi: 10.1167/iovs.06-1027. [DOI] [PubMed] [Google Scholar]
- 7.Uchino Y, Kawakita T, Ishii T, Ishii N, Tsubota K. A new mouse model of dry eye disease: oxidative stress affects functional decline in the lacrimal gland. Cornea. 2012;31(Suppl 1):S63–67. doi: 10.1097/ICO.0b013e31826a5de1. [DOI] [PubMed] [Google Scholar]
- 8.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 10.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:19–21. doi: 10.1136/bjo.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifart U, Strempel I. [The dry eye and diabetes mellitus] . Ophthalmologe. 1994;91:235–239. [PubMed] [Google Scholar]
- 13.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 14.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 15.Kubota S, Kurihara T, Ebinuma M, Kubota M, Yuki K, Sasaki M, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. Am J Pathol. 2010;177:1725–1731. doi: 10.2353/ajpath.2010.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman S, Islam R. Mammalian Sirt1: insights on its biological functions. Cell Commun Signal. 2011;9:11. doi: 10.1186/1478-811X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 18.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 19.Mimura T, Kaji Y, Noma H, Funatsu H, Okamoto S. The role of SIRT1 in ocular aging. Exp Eye Res. 2013;116:17–26. doi: 10.1016/j.exer.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Jaliffa C, Ameqrane I, Dansault A, Leemput J, Vieira V, Lacassagne E, Provost A, Bigot K, Masson C, Menasche M, Abitbol M. Sirt1 involvement in rd10 mouse retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:3562–3572. doi: 10.1167/iovs.08-2817. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 22.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 23.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 24.Kresge N, Simoni RD, Hill RL. Forty years of superoxide dismutase research: the work of Irwin Fridovich. Journal of Biological Chemistry. 2006;281:e17–e17. [Google Scholar]
- 25.Park CY, Zhuang W, Lekhanont K, Zhang C, Cano M, Lee WS, Gehlbach PL, Chuck RS. Lacrimal gland inflammatory cytokine gene expression in the botulinum toxin B-induced murine dry eye model. Mol Vis. 2007;13:2222–2232. [PubMed] [Google Scholar]
- 26.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, Pflugfelder SC. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 27.Ninichuk V, Kulkarni O, Clauss S, Anders H. Tubular atrophy, interstitial fibrosis, and inflammation in type 2 diabetic db/db mice. An accelerated model of advanced diabetic nephropathy. Eur J Med Res. 2007;12:351–355. [PubMed] [Google Scholar]
- 28.Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Dry eye reversal and corneal sensation restoration with topical naltrexone in diabetes mellitus. Arch Ophthalmol. 2009;127:1468–1473. doi: 10.1001/archophthalmol.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 30.Brennan LA, McGreal RS, Kantorow M. Oxidative stress defense and repair systems of the ocular lens. Front Biosci (Elite Ed) 2012;4:141–155. doi: 10.2741/365. [DOI] [PubMed] [Google Scholar]
- 31.Wojcik KA, Kaminska A, Blasiak J, Szaflik J, Szaflik JP. Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy. Int J Mol Sci. 2013;14:19294–19308. doi: 10.3390/ijms140919294. [DOI] [PMC free article] [PubMed] [Google Scholar]


