Abstract
Human diploid cell strains (HDCSs), possessing identical chromosome sets known to be free of all known adventitious agents, are of great use in developing human vaccines. However it is extremely difficult to obtain qualified HDCSs that can satisfy the requirements for the mass production of vaccines. We have developed a new HDCS, Walvax-2, which we derived from the lung tissue of a 3-month-old fetus. We established primary, master and working cell banks successfully from reconstituted frozen cells. Observations during the concurrent propagation of Walvax-2 and MRC-5 cells revealed differences in terms of growth rate, cell viability and viral sensitivities. Specifically, Walvax-2 cells replicated more rapidly than MRC-5 cells, with Walvax-2 cells attaining the same degree of confluence in 48 hours as was reached by MRC-5 cells in 72 hours. Moreover, Walvax-2 cells attained 58 passages of cell doublings whereas MRC-5 reached 48 passages during this period. We also assessed the susceptibility of these cells to rabies, hepatitis A, and Varicella viruses. Analysis of virus titers showed the Walvax-2 cells to be equal or superior to MRC-5 cells for cultivating these viruses. Furthermore, in order to characterize the Walvax-2 cell banks, a series of tests including cell identification, chromosomal characterization, tumorigenicity, as well as tests for the presence of microbial agents, exogenous viruses, and retroviruses, were conducted according to standard international protocols. In conclusion, results from this study show that Walvax-2 cell banks are a promising cell substrate and could potentially be used for the manufacturing of HDCVs.
Keywords: biological characteristics, cell substrate, human diploid cell strain (HDCSs), human diploid cell vaccines (HDCVs), viral sensitivities
Abbreviations
- ATCC
American Type Culture Collection
- CCID50
50% cell culture infectious dose
- CCTCC
China Center for Type Culture Collection
- CPE
cytopathogenic effect
- ELISA
enzyme-linked immuno sorbent Assay
- FFU
fluorescent focus units
- G6PD
glucose 6 phosphate dehydrogenase
- GM
growth medium
- HAV
hepatitis A virus
- HDCSs
human diploid cell strains
- HDCV
human diploid cell vaccine
- LD
lactate dehydrogenase
- MCB
master cell bank
- MDCK
Madin–Darby canine kidney
- MOI
multiplicity of infection
- NIFDC
National Institute for Food and Drug Control
- PAGE
polyacrylamide gelelectrophoresis
- PCB
primary cell bank
- PFU
plaque forming units
- PPLO
pleuropneumonia-Like organisms
- STR
Short tandem repeats
- VZV
varicella zoster virus
- WCB
Working cell bank
Introduction
The replication of viruses occurs only when the virus enters into host cells, often resulting in diseases that are difficult to treat. Currently, there are no widely accepted therapeutics available to treat such diseases, therefore prophylactic vaccines play an imperative role in the fight against viral diseases. Antibodies produced for most kinds of viral diseases when the immune system is stimulated by intact viral particles,.1,2 Owing to this property, the vast majority of viral vaccines still adopt the traditional cell substrate culture method. Three cell substrates, human diploid cells, continuous cell lines and primary cell lines, are always used for developing vaccines.3 However, continuous and primary cell lines used for vaccine production suffer from the limitation of being potentially strongly tumorigenic. Four Additionally the primary cell lines, which are obtained from animals, introduce potentially risky exogenous agents.4 In contrast, human diploid cell strains (HDCSs), acquired from embryos or other tissue cells of human origin, possess identical chromosome sets that are free of all known adventitious agents.5 These unique properties explain the value of such materials and the current interest in their use in the development of human viral vaccines.6,7,8 Human diploid cell vaccines (HDCVs) have been licensed all over the world. Many studies have demonstrated superior immunogenicity and safety of HDCVs relative to those using any other tissue culture, such as hamster kidney cells or vero cell vaccines.9 The WHO recommends HDCS as the safest cell culture substrate for the production of viral vaccines10 and consequently they have become the preferred cell substrate for vaccine production worldwide.
Hayflick in 19618 and Jacobs in 19677 developed the 2 most well known HDCSs, Wistar Institute (WI)-38 and Medical Research Council (MRC)-5, respectively, that currently serve as international standardized cell strains. Since then, there has been continuing interest in the development of HDCSs. Eleven,12 However, it is extremely hard to obtain human fetal tissue from which to derive qualified human diploid cell strains. This is due to issues that include the requirement for strict ethical review, the possibility of environmental degradation, and food safety hazards, all of which may lead to chromosomal aberrations such as the presence of aneuploidy and polyploidy for the karyotype.13 Most importantly, strict requirements regarding the methods for obtaining suitable tissues from which to derive HDCS via abortion render the acquisition of appropriate material difficulty. Even if a new HDCS is derived successfully, it might not satisfy requirements for industrial production due to its inability to sustain multiple passages, the IMR-9 cell line being an example.14,15 Due to the diminishing supply of WI-3810 cells, the MRC-5 line has become the most widely used cell strain in the production of HDCS-derived human vaccines. China consequently confronts 2 key challenges for the production of viral vaccines from MRC-5 cells (which are mainly obtained from abroad): concerns about influences of limited passages, and the policies of the countries from which the cells are imported. More specifically, the numbers of passages of the imported MRC-5 cells are generally higher, generally later than the 20th passage, resulting in restricted mass production due to decreased growth vitality. Additionally, according to the standard for the Pharmacopoeia of the People's Republic of China (2010), Volume III, the use of the HDCSs is limited to generations within 2-thirds of the primary cell lifespan for the manufacture of vaccines. Due to the scarce HDCSs resources, the research and production of viral HDCVs in China are substantially restricted. For example, human diploid cell rabies vaccine, which is considered to be the gold standard for rabies vaccines, is not currently available in China.16 Furthermore, the productive cell generations for the OKA-HDC on the Chinese market from 3 manufactures are MRC-5 cells in the 32nd and 33rd passages,17 which have therefore already reached the limit required as described above in Chinese Pharmacopeia (the 33rd passage is the last cell doubling that could be used in the production for the MRC-5). Relying on imported HDCSs, may lead to unstable supply as well as unpredictable costs. Therefore the intention of this study is to develop a completely new HDCS of Chinese origin that could be used in manufacturing viral vaccines.
This study, therefore, aims to (i) establish and characterize Walvax-2, a totally new HDCS; and (ii) evaluate the susceptibility to 3 kinds of viral vaccine strains, namely the CTN-V/PV strain of rabies, the YN-5 strain of hepatitis A, and the Oka strain of Varicella virus in Walvax-2, thereby preparing for the industrial development of HDCVs in China.
Results
Source tissue material
We obtained 9 fetuses through rigorous screening based on carefully specified inclusion criteria (see Methods section). The Walvax-2 strain of cells met all of these criteria and proved to be the best cell line following careful evaluation. Therefore it was used for establishing a human diploid cell strain. Walvax-2 was derived from a fetal lung tissue, similar to WI-38 and MRC-5, and was obtained from a 3-month old female fetus aborted because of the presence of a uterine scar from a previous caesarean birth by a 27-year old healthy woman.
Primary cell stock and cell bank system
After incubation for 48 h, a confluent cell monolayer formed and then increased in density over the following 48 hours. After a series of successful cultures, these cells were specified as the primary cell seeds of the Walvax-2 human diploid cell line. Thereafter, a 3-tiered cell bank consisting of pre-master cell bank (PCB,P6), master cell bank (MCB, P14), and working cell bank (WCB, P20) was established.
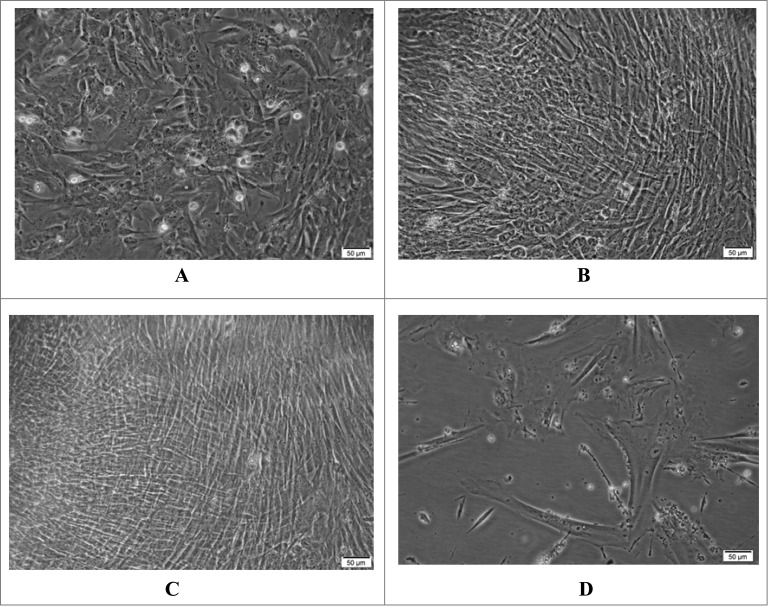
Figure 1 shows a gradual growing procedure for cells after propagation. Initially, round cells with clear and relatively dark edges were observed; as time went by, the cells elongated to become spindle shaped and translucent fibroblasts (Fig. 1A). Over a period of 24 hours during which the cells divided and proliferated, the cells grew into flame shaped, typical plump diploid fibroblasts with good refractive properties, and rearranged into highly polarized areas with curling patterns (Fig. 1B). Finally, the cells formed a dense confluent monolayer after 48 hours (Fig. 1C).
Figure 1.
Morphology of the Walvax-2 cells. The cells were cultured and incubated at 37 °C. The photos were taken at 4 h (A), 24 h (B) and 48 h (C) and at 72 h post-subculture for the 58th passage (D).
Walvax-2 cells maintained excellent capabilities for growth and proliferation until the 50th passage, after which these abilities degraded. At passage 58, cells exhibited blurred edges and could not form a confluent monolayer after being cultured for 72 h. Also noted were increasing black spots in cells, as well as dead cells in the media. (Fig. 1D). Cell death was eventually observed after 20 d
Cryopreservation stability and recovery viability
The Walvax-2 3-tiered cell bank, composed of PCB (P6), MCB (P14), and WCB (P20), exhibited a homogeneous growth pattern and attained identical population doublings (58) when compared with the primary cell seed. All the cells restored from frozen stock reached adherent growth in 2–8 h and formed a confluent monolayer in 24 h, with the percentage of viable cells in the range of 80–90% (Fig. 2). Each of the curves in Figure 2 demonstrates the growth features for the Walvax-2 primary cell seed as well as the cell banks. Generally speaking, the typical diploid cell with limited replicative lifespan follows a “slow-logarithmic-slow” model. However, Walvax-2 cells show strong cell proliferation, with the missing “slow” pattern at the beginning for each curve, until the 50th passage, after which the viability of the cells decreases dramatically. Furthermore, comparative cell doubling times are summarized in Table 1. The results confirm that the Walvax-2 cells reconstituted from the frozen state do not alter their stability and viability, and could potentially be used as a cell substrate due to these crucial properties.
Figure 2.
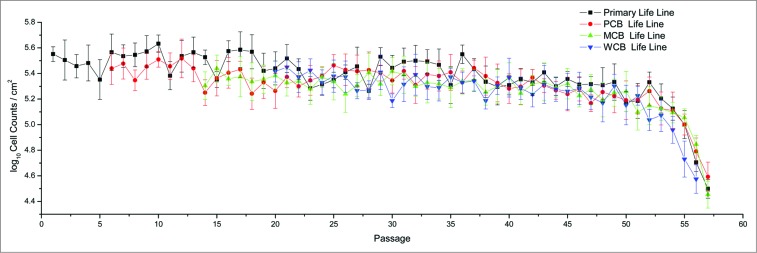
The growth patterns of Walvax-2 cell banks. Primary cells were isolated from fetal lung tissue, frozen at the 6th, 14th and 20th passages, and then recovered and subcultured continuously until cell senescence occurred.
Table 1.
Population doubling times of the Walvax-2 cells with and without being subjected to freezing
| Passage number | Without being subjected to freezing | Reconstituted from the frozen state | |
|---|---|---|---|
| Population doubling time(h) | Cell origin | Population doubling time(h) | |
| P 10 | 18–20 | PCB,P6 | 18–20 |
| P 20 | 29–31 | MCB, P14 | 30–32 |
| P25 | 30–32 | WCB, P20 | 30–32 |
| P32 | 38–40 | The 28th passage from the WCB | 39–41 |
| P43 | 39–41 | The 38th passage from the WCB | 40–42 |
| P55 | 55–60 | The 48th passage from the WCB | 57–62 |
Cell identification
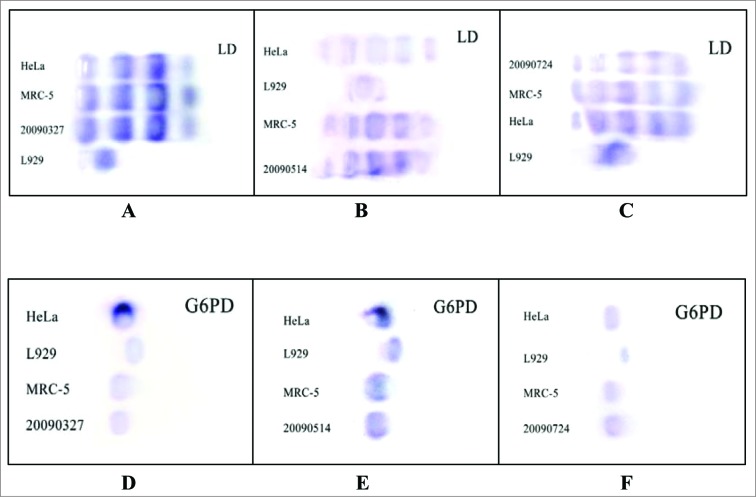
As shown in Figure 3, the isozyme patterns of the Walvax-2 cells, using LD and G6PD as indicators, are identical to those of human diploid cells (MRC-5) and the human cervical cancer cell line (Hela) preserved in China Center for Type Culture Collection (CCTCC), whereas the mouse fibroblast cell line (L929) exhibits entirely different results. These findings confirm the fact that the Walvax-2 cell banks behave in a manner similar to other human-derived cell lines.
Figure 3.
Isoenzyme tests for the Walvax-2 cells. Firstly, LD and G6PD, 2 isoenzymes used as indicators, were isolated from HeLa, L929, MRC-5 and Walvax-2 cells, and then subjected to PAGE and stained. The numbers of 20090327, 20090514 and 20090724 illustrated in the pictures represent Walvax-2 cells for the 18th, 30th and 50th passages.
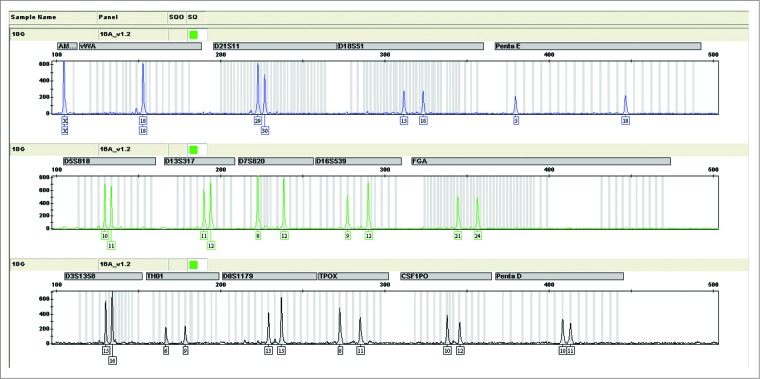
STR profiles of 16 DNA fragments of gene locus for Walvax-2 cells are shown in Figure 4, from which we see that they match the targeted alleles as expected. In Table 2 the data from this study are compared with those of STR databases in ATCC of USA and DSMZ of Germany. Three qualified laboratories, CCTCC, NIFDC (National Institutes for Food and Drug Control) and Law School of Kunming Medical University, draw the same conclusions that the Walvax-2 cell line displays its own specific DNA profile of human individual origin distinct from the MRC-5 and the Hela cell lines.
Figure 4.
The Short Tandem Repeat (STR) map of Walvax-2 cells for the 18th passage. According to the instructions supplied with the Goldeneye 16A identification kit (people spot), the DNA of Walvax-2 cells at the 18th passage were isolated and amplified by multiplex PCR with primers of 16 STR sites. Then the STR map was obtained by analyzing the samples of PCR by capillary electrophoresis (CE). The STR maps of Walvax-2 cells at the 30th and 50th passages (not shown) were the same as shown.
Table 2.
The STR mapping of the Walvax-2 cells
| gene locus | Walvax-2 | MRC-5* | HeLa* | gene locus | Walvax-2 | MRC-5* | HeLa* |
|---|---|---|---|---|---|---|---|
| Amelogenin | X | X,Y | X | D16S539 | 09,12 | 9,11 | 9,10 |
| vWA | 18 | 15 | 16,18 | FGA | 21,24 | - | - |
| D21S11 | 29,30 | - | - | D3S1358 | 15,16 | - | - |
| D18S51 | 15,18 | - | - | THO1 | 06,09 | 8 | 7 |
| PentaE | 05,18 | - | - | D8S1179 | 13,15 | - | - |
| D5S818 | 10,11 | 11,12 | 11,12 | TPOX | 08,11 | 8 | 8,12 |
| D13S317 | 11,12 | 11,14 | 12.13.3 | CSF1PO | 10,12 | 11,12 | 9,10 |
| D7S820 | 08,12 | 10,11 | 8,12 | PentaD | 10,11 | - | - |
*Data from ATCC and DSMZ
Chromosomal characterization
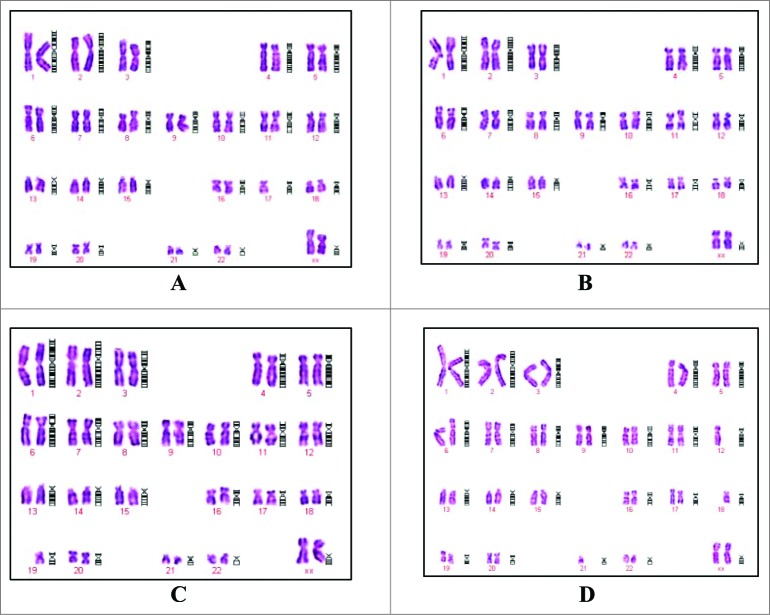
The chromosomal characterization for PCB (P6), MCB (P14), WCB (P20) from the 38th passage, which is the last passage that could be used for producing viral vaccines according to the requirements of Chinese Pharmacopeia, are illustrated in Figure 5. They show clearly that the Walvax-2 cells are 46/XX, typical diploid type of human origin. The chromosomal analysis of the Walvax-2 cells as summarized in Table 3 demonstrate that the karyological properties of Walvax-2 cells satisfy the requirements of diploid cells of human origin to be used for producing viral vaccines, with the frequencies of abnormalities being considerably lower than the corresponding national standards.
Figure 5.
Chromosomes from Walvax-2 cell banks. Walvax-2 cells were incubated 1 day post-subculture, after which the colcemid and then Giemsa banded karyotype analyses were carried out. Pictures were the karyotype of Walvax-2 cells at the 6th (A), 14th (B), 20th (C) and 38th (D) passages
Table 3.
The accumulated results of chromosomal analysis of Walvax-2 cells
| Passage | Structural abnormalities | Aneuploidy | Polyploidy | Hyperdiploidy | Breaks or gaps |
|---|---|---|---|---|---|
| Standard* | ≤2 % | ≤18 % | ≤4 % | ≤2 % | ≤8 % |
| 10-19 | 0/3500 | 265/3500 (7.57%) | 1/3500 (0.03%) | 22/3500 (0.63%) | 0/3500 |
| 20-29 | 0/6000 | 538/6000 (8.97%) | 3/6000 (0.05%) | 49/6000 (0.82%) | 1/6000 (0.17%) |
| 30-39 | 0/4500 | 423/4500 (9.4%) | 1/4500 (0.02%) | 40/4500 (0.89%) | 1/4500 (0.02%) |
| 40-50 | 0/9000 | 945/9000 (10.5%) | 7/9000 (0.08%) | 113/9000 (1.26%) | 7/9000 (0.08%) |
*Chinese pharmacopeia, volume III, 2010 edition
Microbial agent tests
No cultivable bacteria or fungi were found in broth and agar cultures. Mycoplasma tests using both the culture method and DNA staining technique, also met the corresponding requirements.
Exogenous virus agent tests
Results for the testing of general (non-specific) as well as specific adventitious viral agents were negative for all tested viruses as described in detail in the “materials and methods” section.
Retrovirus test
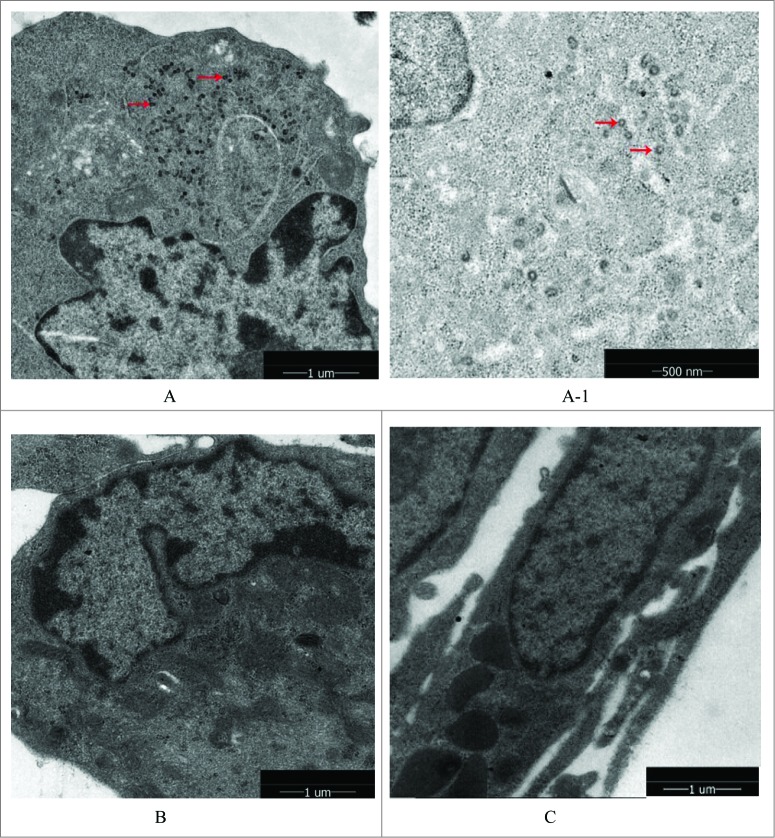
The results in Figure 6 make it clear that no retroviruses were found in the Walvax-2 cells, as well as the system control cells of MRC-5. However obvious retroviruses were found in the Sp2/0-Ag14 cells of the positive control group, seen as tiny black dots in the figure.
Figure 6.
Retrovirus tests of Walvax-2. The results were observed by mirror electron microscopy(200Kv 5000x/160Kv 7800x). The arrows point to virus particles detected as shown in the picture. (A) and (A-1) were represented positive controls (Sp2/0-Ag14), (A-1) was partial enlarged detail of (A). (B) was represented negative control (MRC-5). (C) was represented the cells of Walvax-2 of the 24th passage.
Tumorigenicity test
Tumorigenicity tests were conducted at 2 points following the inoculation of cells into the nude mice, 21 and 84 d All mice survived in all study groups. During the animal tests, no pathological abnormalities of nodule growths were found in the experimental as well as the parallel negative control group (MRC-5), and for both groups there was no pathological heterogeneous cell growth observed at the inoculating site or other sites including heart, liver, spleen, lung, kidney, brain and mesenteric lymph nodes after autopsy. In contrast, nodule and heterogeneous cell growth were easily found in the inoculating site in the positive control group (Hela). These results show that the Walvax-2 cells can be used for the production of vaccines with little risk of potential carcinogenesis.
Susceptibility to viruse tests
Infectivity titers of the CTN-1V strain for the rabies virus are presented in Table 4. CTN-1V virus was well adapted in Walvax-2 relative to MRC-5. Maximum infectivity titers of CTN-1V virus for Walvax-2 and MRC-5 were 8.14 and 7.41 FFU/ml, respectively. During the period for virus propagation, the titers in Walvax-2 cells were consistently higher than those of MRC-5 cells, although the differences were not always statistically significant. However, analysis of the overall situation of the adaptation of the CTN-1V in Walvax-2 cells relative to MRC-5 cells yielded a significant difference (P﹤0.001) by a 2-tailed t-test. Similarly, the results for the PV strain adaptation in both human diploid cells demonstrated a consistent trend, which exhibited distinct differences for the titers.
Table 4.
Propagation of CTN-1V or PM virus in the Walvax-2 or MRC-5 cells
| Virus Passage NO. | CTN-1V virus ( log FFU/ml)a | PVvirus ( log FFU/ml)a | ||||
|---|---|---|---|---|---|---|
| Walvax-2 cells | MRC-5 cells | Pb | Walvax-2 cells | MRC-5 cells | Pb | |
| original | 7.50 | 7.50 | / | 7.50 | 7.50 | / |
| 1 | 4.84± 0.62 | 4.58± 0.40 | >0.05 | 4.40± 0.27 | 3.62± 0.23 | >0.05 |
| 2 | 5.40± 0.21 | 5.02±0.34 | <0.05 | 5.04±0.18 | 4.75± 0.24 | <0.05 |
| 3 | 6.10± 0.37 | 5.41± 0.24 | <0.05 | 5.30± 0.33 | 4.83± 0.25 | <0.05 |
| 4 | 6.530.31 | 6.09± 0.17 | <0.05 | 5.86± 0.10 | 5.02± 0.13 | <0.05 |
| 5 | 6.78± 0.40 | 6.14± 0.16 | <0.05 | 6.21± 0.21 | 5.63± 0.05 | <0.05 |
| 6 | 7.08± 0.15 | 6.57± 0.42 | >0.05 | 6.57± 0.53 | 6.02± 0.18 | >0.05 |
| 7 | 7.34± 0.22 | 6.89± 0.21 | <0.05 | 7.01± 0.70 | 6.00± 0.23 | >0.05 |
| 8 | 7.51± 0.21 | 7.16± 0.08 | >0.05 | 6.93± 0.19 | 6.28± 0.25 | <0.05 |
| 9 | 7.67± 0.18 | 7.09± 0.10 | <0.05 | 7.23± 0.23 | 6.59± 0.26 | <0.05 |
| 10 | 8.14± 0.31 | 7.41± 0.35 | <0.05 | 8.02± 0.19 | 7.11± 0.38 | <0.05 |
Passages the 1th to 4th, subculture; Passages the 5th to 8th, cell-mixing; Passages the 9th to 10th, cell-free medium;
a±SD.
b Significance of difference (P value) determined by 2-tailed t-test
Data for the VZV virus propagating in Walvax-2 and MRC-5 cells is given in Table 5. In Walvax-2 cells the virus titer grew rapidly to 6.28 log PFU/ml, and reached a peak of 6.59 log PFU/ml at passage 41. In contrast, the virus titers in MRC-5 were much lower, with the overall numbers less than 6.0 log PFU/ml. All comparisons except that of the earliest generation were all statistically significant, indicating strong adaptation of the VZV strain for Walvax-2 cells relative to MRC-5 cells.
Table 5.
Propagation of VZV strain in the Walvax-2 or MRC-5 cells
| Virus Passage No. | Virus Titer in Walvax-2 cell (log PFU/ml)a | Virus Titer in MRC-5 cell(log PFU/ml) a | Pb |
|---|---|---|---|
| 31(original) | 5.0 | 5.0 | |
| 33 | 6.28± 0.28 | 5.42± 0.19 | >0.05 |
| 35 | 6.13± 0.12 | 5.56± 0.11 | <0.05 |
| 37 | 6.31± 0.28 | 5.52± 0.08 | <0.05 |
| 39 | 6.27± 0.14 | 5.58± 0.12 | <0.05 |
| 41 | 6.59± 0.06 | 5.74± 0.13 | <0.05 |
a±SD.
b Significance of difference (P value) determined by 2-tailed t-test
The comparative results for YN5 adaptability are listed in Table 6. The titer after one generation in Walvax-2 reached 7.32 log CCID50/ml, even higher than the value in the original cells (passage 23). During the course of 8 passages propagated continuously in the Walvax-2 cells, the infectious virus titers increased from 7.32 to 7.65 log CCID50/ml, which was marginally higher than those of MRC-5 cells (7.0 to 7.36 log CCID50/ml).
Table 6.
The titers of HAV (YN5) adapted in human diploid cells
| Virus Passage NO. | Infectivity titer in Walvax-2 cells(log CCID50/ml)a | Infectivity titer in MRC-5 cells(log CCID50/ml) a | Pb |
|---|---|---|---|
| 23(original) | 7.0 | 7.0 | |
| 24 | 7.32± 0.28 | 6.27± 0.27 | <0.05 |
| 25 | 7.47± 0.09 | 7.01± 0.23 | >0.05 |
| 26 | 7.50±0.17 | 7.35± 0.14 | >0.05 |
| 27 | 7.62± 0.06 | 7.18± 0.38 | >0.05 |
| 28 | 7.97± 0.09 | 7.50± 0.23 | >0.05 |
| 29 | 8.21± 0.29 | 7.54± 0.24 | <0.05 |
| 30 | 7.81± 0.17 | 7.35± 0.14 | <0.05 |
| 31 | 7.65± 0.14 | 7.36± 0.34 | >0.05 |
a±SD.
b Significance of difference (P value) determined by 2-tailed t-test
Discussion
HDCS, deemed as the safest cell substrate, play a vital role in the production of viral human vaccines. However, it is extremely hard to obtain qualified HDCSs that meet the requirements for mass production. It took us 4 y to successfully establish Walvax-2 cell lines and a 3-tiered cell bank, namely PCB, MCB and WCB. Complete records for the cell bank establishment, cell culture conditions, and tests are available. The criteria used for characterizing the Walvax-2 cell banks are those recommended internationally18,19 and concurrent titrations were set up using MRC-5 cells (the most widely used human diploid cell substrate as a parallel control. Walvax-2 cells have received qualification test reports from the NIFDC and CCTCC, an important step in their use for the production of human viral vaccines in China. Given that the availability of HDCSs, and therefore the production of HDCVs, is currently subject to external forces, the development of an HDCS of Chinese origin has great implications for improving the stability of the supply of HDCVs in China.
Walvax-2 cells displayed a fibroblastic morphology similar to that of MRC-5 cells. However, observations during the concurrent propagation of Walvax-2 and MRC-5 cells revealed differences in terms of growth rates and cell viability. The Walvax-2 cells replicated more rapidly than MRC-5 –they attained the same degree of confluence in 48 hours as was reached by MRC-5 in 72 hours, and the results are in line with measured cell doubling times as listed in Table 1. After freezing and recovering, the growth characteristics and patterns of the 3 life lines (PCB, MCB, and WCB) are similar to those of the primary life line, and attained 58 passages of cell doublings whereas MRC-5 reached 48 passages, with the difference decreasing gradually with increasing hours of freezing.8 In conclusion, these results may indicate that Walvax-2 is a cell line with superior characteristic of high growth ability, as well as strong viability compared to MRC-5. It could be used as a host for the cultivation and inoculation of viruses, although different schedules for inoculation and propagation should be further studied based on the growth characteristics of particular viruses. Furthermore, the stability of the karyotype is another crucial issue when using the HDCS in the manufacture of vaccines. The results for karyological data on Walvax-2 cells, as summarized in Table 3, demonstrate increases of aneuploidy and hyperdiploidy with age. However, this is not a concern on the grounds that the 2 “middle groups," which are directly related to those to be used in the manufacture of vaccines according to the requirements of Chinese Pharmacopeia, have frequencies of aneuploidy and hyperdiploidy of 9.4% and 0.89% respectively, which are substantially lower than the national standards of 18% and 2%, respectively.
The susceptibility of the human fetal cell strain MRC-5 to viruses infectious in man has been well demonstrated over the past 10 years, indicating the value of such material for the isolation of viruses and the development of vaccines. In this study, the Walvax-2 cell line served as a host for the cultivation of the CTN-V/PV strain of rabies, the YN-5 strain of hepatitis A, the Oka strain of Varicella virus, with results that demonstrate good sensitivity to these viruses. Compared to the MRC-5 cells, titers for viruses in the Walvax-2 cells are higher, with the overall numbers achieving statistical significance. These discrepancies elucidate that Walvax-2, as a new human diploid cell line, is equal or superior to MRC-5 for the propagation of viruses. Generally speaking, as the cell passage number increases the viral titers will experience an initial decrease, and then increase gradually as the cell substrate adapts to the virus. This trend is observed for the propagation of rabies virus in our study. However, the results are not the same for the propagation of VZV and HAV strains in HDCSs, which exhibit increased titers after only one generation. To the best of our knowledge, this may be attributed to the fact that these 2 virus strains are quite sensitive to HDCSs, and particularly to the Walvax-2 cells. Alternatively, the higher titers for Walvax-2 may relate to the characteristics of high growth ability as well as strong viability compared with MRC-5, as described in the “Results” section. Nevertheless, more research needs to be done to investigate the susceptibility of Walvax-2 cells to a greater variety of viruses, and to develop fully the potential of Walvax-2 cells as a cell substrate platform for producing viral vaccines for human use in China.
The sensitivity to rabies virus of Walvax-2 has important implications for China. Human diploid cell rabies vaccine, which is free of complications but is highly immunogenic,20 is considered to be the gold standard for rabies vaccine.16 According to the report by the WHO, there are roughly 55000 human deaths caused by rabies annually.21 Following India, China ranks in second place for the highest number of human cases in the world.22 However, there is no such gold standard rabies vaccine on the Chinese market, where the disease burden is remarkably high. Possible reasons are as follows: the vaccine, regarded as liquid gold by the general public, represents a financial burden and hence has lower usage in developing countries. To minimize costs as well as make it affordable for Indians, the Serum Institute of India indigenously developed Ravivax ( Pitman-Moore strain, MRC-5), decreasing the cost for the vaccine dramatically (from US $40 dropped to $7).20 This is also one of the motivations for this study, to develop a totally new HDCS that could be used as a culture medium in manufacturing viral vaccines in China. Recently, a document from the Chinese pharmacopeia commission indicates that the current 2 kinds of cell substrate rabies vaccine presently on the international market, PVRV and PHK, may not be included in the updated Chinese pharmacopeia (2015).23 The explanations for the removed vaccines are that they will no longer be manufactured or will be replaced by others. Human diploid cell rabies vaccine is gaining increased national attention in China. We tested the susceptibility of 2 rabies strains concurrently in our study, CTN-V and PV. We found that the titers of CTN-1V strain are higher than those of PV strain, independent of the effects of adaptation by the cell substrates. Both strains have been used for production in China over the years, and the safety and immunogenicity of the vaccines have been verified.24 Consequently, considering the impact on future production, CTN-1V will be the preferred rabies strain for research and production in the future. Although we have reported results of the susceptibility of 3 viruses in this study, we prepared rabies vaccines using the preferred CTN-1V-HDC (Walvax-2) viral strain (15th passage) and determined the potency to be higher than 6.0IU/dose, which was significantly greater than the WHO-recommended standard of 2.5 IU/ dose16 (described in detail in another study25). The efficacies of the diploid rabies vaccines on animal tests would further confirm the use of the Walvax-2 cells in human viral vaccine production.
There are several limitations to this study. More work is required regarding the adaptation of a greater variety of viruses on the Walvax-2 cells and the possibility for the industrial development of appropriate vaccines. In recent years, a large number of research papers have reported the application of Gene chip technology and high-throughput sequencing PCR technology for detecting potential contaminations of viruses. Thus, further screening of human-derived viruses needs to be conducted, especially for tumorigenic DNA viruses, retroviruses et al. Currently, we have been conducting tests for the human herpes simplex virus 6 and 7, and further screening will be carried out soon.
In conclusion, we have successfully established and characterized a new human diploid cell line designated Walvax-2, and evaluated its susceptibility to 3 kinds of viral vaccine strains. The Walvax-2 cells are equally susceptible, and in some cases superior to, the MRC-5 line for the cultivation of viruses. Results from this study suggest that the Walvax-2 cell banks are a promising cell substrate and could potentially be used for the manufacturing of HDCVs.
Materials and Methods
Cells and viruses
HeLa, MRC-5, L929, MDCK, VeroandSp2/0-Ag14 cells were obtained from the America Center for Type Culture Collection (CCL-2, CCL-171, CCL-1, CCL-34, CCL-81, andCRL-158). Rabies fixed virus CTN-1Vstrain26,27 and Pasteur strain were provided by the National Institute for Food and Drug Control (NIFDC, P.R. China) and Jiangsu Simcere Vaxtec Bio-Pharmaceutical Co., Ltd, respectively. The Varicella zoster virus Oka strain28 was provided by American Type Culture Collection (ATCC). The hepatitis A virus (HAV) YN5 strain was isolated in 2003 from a hepatitis A patient in Kunming, China.29
Laboratory animals
Kunming mice, guinea pigs and rabbits were supplied by Guangdong Medical Laboratory Animal Center (Guangdong Province, P.R. China). Specific-pathogen-free (SPF) eggs were purchased from Beijing Merial Vital Laboratory Animal Technology Co., Ltd. Nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd.
The care and use of laboratory animals were approved by the Animal Care and Use Committee of Yunnan Walvax Biotechnology Co., Ltd. All animals were treated humanely and euthanized by cervical dislocation at the end of the experimental period.
Culture medium and other reagents
The growth medium (GM) for all cells was Eagle's minimum essential medium (M0769; Sigma) supplemented with 10 percent calf serum, 2 percent 2 M Glutamine (G8540; Sigma) and 2 percent 0.83 M NaHCO3. The cryopreservation solution was GM added with 10 percent DMSO (D8418, sigma). Inorganic salts were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, P.R. China).
Source tissue material
The fetal material was provided by the Department of Obstetrics and Gynecology of Yunnan Hospital, with legal and ethical agreements from the donator. Before the study, we made strict and comprehensive inclusion criteria in order to guarantee a high quality cell strain: 1) gestational age 2 to 4 months; 2) induction of labor with the water bag method; 3) the parents career should not involve contact with chemicals and radiation; 4) both parents are in good health without neoplastic and genetic diseases, and with no history of human tissue or organ transplantation in the families traced for 3 generations; and 5) no infectious diseases. The tissues from the freshly aborted fetuses were immediately sent to the laboratory for the preparation of the cells.
Preparation of primary cell stock and cell banks
The preparations of the primary cell stock and serial propagation of cells were carried out according to the methods of Jacobs in 19708 and Hayflick in 1961.30 The selected primary Walvax-2 cell seed was used for passaging, with the inoculation concentration of 5×105 cells /ml. Subsequent subculture was conducted at a 1:2 split ratio immediately subsequent to the formation of a dense cell monolayer. When the cells reached the 6th, 14th, and 29th cell doublings, cultures were harvested and frozen to form a pre-master cell bank (PCB), master cell bank (MCB) and working cell bank (WCB).
Cryopreservation stability and recovery viability
Cryopreservation was performed 6 times for Walvax-2 cells with the cell lines designated as P6, P14, P20, P28, P38 and P48. These particular cell lines were chosen on the grounds that they represented the corresponding cell banks of PCB, MCB, WCB, the major working passages, and the entire lifecycle of the Walvax-2 cells. The cells were centrifuged and re-suspended in cryopreservation solution, and the cell concentration was adjusted to 6∼10×106 cells /ml. The suspension was dispensed in 1.0 ml to 2.0 ml Cryogenic Vials (#430659,Corning). Following the manufacturer's instructions for the use of programmed cooling boxes (Nalgene Mr. Frosty,Thermo Fisher), the cryogenic vials were then sealed and put into the boxes at −70C overnight. Then cryogenic vials were placed directly into liquid nitrogen for long term cryopreservation. The frozen cells were recovered according to the procedures given by Jacobs in 19708 and Hayflick in 1961.30
Cells reconstituted from the frozen state were taken immediately for the calculation of population doubling times by cell counting. The three-tiered banks were propagated serially for doubling time assessments. The experiments were repeated 8 times, and the doubling times were compared with that of cells that had not been frozen.
Cell identification
The cell identification was evaluated by a 2-step procedure: Firstly isoenzyme analysis was performed using lactate dehydrogenase (LD) and Glucose 6 phosphate dehydrogenase (G6PD) as indicators to confirm Walvax-2 cell banks were human-derived cells. Then, Short Tandem Repeat (STR) analysis was conducted using MRC-5 as a parallel control by 3 qualified laboratories: China Center for Type Culture Collection (CCTCC), National Institutes for Food and Drug Control (NIFDC), and Law School of Kunming Medical University to assure that the cells were derived from the tissue of a specific human individual and different from any other established human diploid cell lines.
Chromosomal characterization
Chromosome examinations were conducted for every 10 passages by counting percentages for 5 types of chromosomal aberrations, including structural abnormalities, aneuploidy, polyploidy, hyperploidy and breaks or gaps. Chromosome specimen slides were obtained using the method of Coburn and Leykauf,31 and then stained with Giemsa. Giemsa-banded karyotypes were recorded by Applied Imaging Software-Karyotyping 3.0 (England).
Microbial agents tests
The presence of bacterium, fungus and mycoplasmas for Walvax-2 cells were tested according to the requirements of ATCC and WHO.18,19 Bacillus subtilis (CMCC(B)63501), Clostridium sporogenes (CMCC(B)64941) and Candida albicans (CMCC(F)98001) were used as positive controls for the tests of bacteria and fungi. A total of 19 cell passages were tested for sterility. The cell samples were tested under different temperatures for 2 weeks to confirm that no bacterial and fungal contamination was present. The mycoplasma test was conducted as per requirement in Volume III of Chinese Pharmacopeia, using the culture method and DNA staining technique, and B6yh4 cells were used as a positive control. All positive controls were provided by the National Institute for Food and Drug Control (NIFDC, P.R. China).
Exogenous virus agents tests
Tests for adventitious viral agents of Walvax-2 cells were conducted as per requirements for Preparation and Control of Animal Cell Substrates Used for Production and Testing of Biologics in Volume III of Chinese Pharmacopeia, including testing for general adventitious viral agents (non-specific virus) and specific adventitious viral agents.
General adventitious agents included embryonated egg inoculation by the yolk sac, allantoic cavity; i.c. and i.p. inoculation of adult and suckling mice, i.p. inoculation of guinea pigs; monolayer cell culture using MRC-5, and vero cells for detection of various human viruses.
Tests for specific adventitious agents consisted of human derived virus, bovine derived virus and porcine virus. For the human derived virus test, 6 viruses including HBV, HCV, HIV, Human cytomegalovirus, human nasopharyngeal virus and human parvovirus B19, were carried out based on per testing kit, using ELISA and PCR methods, respectively. For the bovine derived virus test, 3 methods were used: (i) the microscopic CPE observation method; (ii) different cell culture conditions for hemadsorption activity, and (iii) fluorescence quantitative RT-PCR method (bovine adenovirus, bovine parvovirus, bovine diarrhea virus, bovine influenza virus, bovine parainfluenza virus, rabies virus and retrovirus). The possible swine viral contamination was examined using RT-PCR and PCR methods for classical swine fever virus, Japanese encephalitis virus and Pseudorabies virus.
Retrovirus test
The retrovirus test was performed according to procedures described in “ Reverse transcriptase activity assay in attenuated live vaccine”(Yan Kong et al)32 and “Development of an improved product enhanced reverse transcriptase assay” (Audrey Chang, et al).33 More specifically, the testing methods included product-enhanced reverse transcriptase (PERT) assay, infection test and direct observation by transmission electron microscopy. The mouse bone marrow cell line Sp2/0-Ag14 served as a positive control while MRC-5 cells were used for the system control.
Tumorigenicity test
To ascertain whether the cells had any neoplastic properties, P10, P20, P28, P38 and P48 Walvax-2 cells were implanted into 10 nude mice aged 4–6 weeks, in the thigh of the right hind leg of each mouse according to the requirements of Chinese Pharmacopeia. MRC-5 cells served as the negative control, and Hela cells served as the positive control. All animals were examined after 21 and 84 d following the inoculation of the cells. Animals not surviving the full period were examined post mortem, and observations for neoplastic growth were conducted for all tested animals.
Susceptibility to viruses test
Particular cell generations that would potentially be used for producing viral vaccines, were used to determine susceptibility to viruses after 25 to 30 cell doublings. Three kinds of viral vaccine strains (rabies, Varicella zoster and Hepatitis A) were used for the assays. To determine the susceptibility of Walvax-2 cells relative to MRC-5 cells, concurrent titrations were compared for the same cell doublings.
Rabies Virus
Virus propagation
The CTN-1V and Pasteur strains were propagated in Walvax-2 and MRC-5 cells by the method of Wiktor et al.34 The virus maintenance medium was consistent with GM with the addition of 2% (v/v) fetal calf serum. A multiplicity of infection (MOI) of 0.01 was used. The viruses were incubated at 34–35ºC.
Virus titration
The rabies virus was titrated using a modified test as described by Smith et al.35 Virus titer was expressed in fluorescent focus units (FFU)/ml. Briefly, a monolayer of BSR cells in 96-well plates was incubated with serial fold5- virus dilutions at 37°C in a 5% CO2 humidified incubator for 24 h. The cells were then fixed with 80% cold acetone at -20°C for 30 minutes, and then stained with the Rabies DFA Reagent (5100; Millipore). The plates were examined by fluorescence microscopy (Olympus Corp., Tokyo, Japan), and the numbers of fluorescent foci presented in the wells were recorded. The highest dilutions with fluorescent foci less than 30 were defined as endpoints, and virus titers were calculated by the following formula: virus titer (FFU/ml) = (the mean foci number in the endpoint wells × 5 + the mean foci number in the wells with lower dilutions next to the endpoint well) ÷2 × the dilution factor of the lower dilutions ÷ the volume of virus dilution inoculated into each well.
Varicella Zoster Virus (VZV)
Virus propagation
The Oka strain at passage 31 was inoculated into Walvax-2 and MRC-5 cells and grown into a confluent monolayer at an MOI of 0.01.36 Infected cells were incubated at 36 °C for 48–52 hrs till the cytopathogenic effect (CPE) was estimated to be approximately 75% to 100%. The cells were then trypsinized and resuspended in cryopreservation solution and stored at -196°C. The virus was serially propagated 8 times as described above for Walvax-2 and MRC-5 cells.
Virus titration
A plaque assay37,38 was used and virus titer was expressed in plaque forming units (PFU)/ml. When Walvax-2 and MRC-5 cells in 6-well plates grew to a near confluent monolayer, the old medium was poured off and the monolayer was infected with cell-associated virus in fresh medium (with 2% fetal calf serum and 1% penicillin-streptomycin). Infections were allowed to proceed for 8–9 days, at which point the first signs of CPE was visible, the cells were stained and plaques counted.
Hepatitis A Virus
Virus propagation
According to the method of Wang et al,39 the HAV YN5 strain was propagated in Walvax-2 cells and MRC-5. Briefly, Walvax-2 cells were trypsinized and inoculated with HAV at a MOI of 0.01 and stirred gently with a magnetic stirrer for 2 h at 37°C. The cells were then seeded in T225 flasks filled with GM at 37°C for 3–4 d until a confluent monolayer was formed. The GM was replaced by virus maintenance medium, consisting of MEM supplemented with 2% (v/v) fetal calf serum, 0.35% (m/v) NaHCO3, 2% (v/v) and 2 M Glutamine; Cells were incubated at 35°C for 25 d Afterwards the cells were harvested and stored at −80°C.
Virus titration
An enzyme-linked immunosorbent assay (ELISA) was used to determine the virus infectivity titer of HAV.40 The monolayers of Walvax-2 and MRC-5 were inoculated with serial fold5- cell-associated virus dilutions and incubated at 37°C for 1 h. Each dilution was assayed in quadruplicate. Then the inoculums were removed and replaced with a 1 ml nutrient MEM overlay containing 2 % fetal calf serum and incubated at 35°C for 25 d. The infected cells were harvested and sonicated. The presence of HAV-Ag was tested by ELISA. The CCID50 value was calculated by a modified Reed-Muench's method.41
Acknowledgments
We are grateful to Dr Shen Chao (College of Life Sciences at Wuhan University) for generous technical assistance and advice. Many thanks to all those who were involved in this work.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by National High Technology Research and Development Program of China (2014AA021002), the National Key Technology Support Program (2008BAI54B04–09), National Science and Technology Major Project (2013ZX10004003) and Major Project of Yunnan Provence Programs for Fundamental Research (2013FC005).
References
- 1. Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3 12:2374-95; PMID: 22355444; http://dx.doi.org/ 10.3390/v3122374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciupe SM, Ribeiro RM, Perelson AS. Antibody responses during hepatitis B viral infection. PLoS Comput Biol 2014; 10 7:e1003730; PMID: 25078553; http://dx.doi.org/ 10.1371/journal.pcbi.1003730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO study group on cell substrates for production of biologicals. WHO Headquarters, 2007; 11-12, Geneva, Switzerland: [DOI] [PubMed] [Google Scholar]
- 4. Zhang DL, Ji L, Li LJ, Huang GS. Systematically experimental investigation on carcinogenesis or tumorigenicity of VERO cell lines of different karyotypes in nude mice in vivo used for viral vaccine manufacture. Yi Chuan Xue Bao 2004; 31 7:647-60; PMID: 15473315 [PubMed] [Google Scholar]
- 5. Pagano JS, Bottiger M, Bonnevier JO, Gard S. The response and the lack of spread in swedish school children given an attenuated poliovirus vaccine prepared in a human diploid cell strain. Am J Hyg 1964; 79:74-85; PMID: 14114357 [DOI] [PubMed] [Google Scholar]
- 6. Fletcher MA, Hessel L, Plotkin SA. Human diploid cell strains (HDCS) viral vaccines. Dev Biol Stand 1998; 93 97-107; PMID: 9737384 [PubMed] [Google Scholar]
- 7. Hayflick L. History of cell substrates used for human biological. Dev Biol Stand 1989; 70:11-26; PMID: 2668070 [PubMed] [Google Scholar]
- 8. Jacobs JP, Jones CM, Baille JP. Characteristics of a human diploid cell designated MRC-5. Nature 1970; 227 5254:168-70; PMID: 4316953; http://dx.doi.org/ 10.1038/227168a0 [DOI] [PubMed] [Google Scholar]
- 9. Guo SH, Tao H, Ying ZF. Study on safety and immunogenicity of oral poliomyelitis attenuated live vaccine (human diploid cell). Zhongguo Yi Miao He Mian Yi 2010; 16 3:193-6; PMID:20726255 [PubMed] [Google Scholar]
- 10. WHO Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks. WHO Expert Committee on Biological Standardization Geneva, World Health Organization, In press (Adopted, 2010). 2010 [Google Scholar]
- 11. Zhang K, Na T, Wang L, Gao Q, Yin W, Wang J, Yuan BZ. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014;32(50):6820-6827; PMID: 25086263 [DOI] [PubMed] [Google Scholar]
- 12. Yang E, Cheng C, Zhang Y, Wang J, Che Y, Pu J, Dong C, Liu L, He Z, Lu S, et al. Comparative study of the immunogenicity in mice and monkeys of an inactivated CA16 vaccine made from a human diploid cell line. Hum Vaccin Immunother 2014; 10(5):1266-1273; PMID:24583556; http://dx.doi.org/ 10.4161/hv.28083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo YZ, Zhou Y. The effects of environmental pollution on human chromosome instability. Proc Clinical Med J 2003; 12 12:912-3 [Google Scholar]
- 14. von Seefried A, Chun JH. Serially subcultivated cells as substrates for poliovirus production for vaccine. Dev Biol Stand 1981; 47:25-33; PMID: 6262151 [PubMed] [Google Scholar]
- 15. Leiva R. A brief history of human diploid cell strains. Natl Cathol Bioeth Q 2006; 6 3:443-51; PMID: 17091551; http://dx.doi.org/ 10.5840/ncbq20066328 [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization Rabies Vaccines. WHO Position Paper, Weekly Epidemiological Record, No. 85, 6th August 2010, Geneva, Switzerland [Google Scholar]
- 17. Fang HH. Current activities of varicella vaccine in china. Conference About Quality Control of Live Attenuated Viral Vaccine. 2006. 18-19, Shanghai, China [Google Scholar]
- 18. WHO Recommendation for the evaluation of animal cell culture as substrates for manufacture of biological medicine products and for the characterization of cell banksIn: WHO expert committee on biological standardization. Sixty-First Report. 2013 Annex 3. In WHO Technical Report Series No. 978 World Health Organization, Geneva [Google Scholar]
- 19. FDA. Guidance for Industry:“Characterization and Qualification of cell Substrates and other biological materials used in the production of viral vaccines for infectious disease Indications”. 2010 [Google Scholar]
- 20. Sudarshan MK, Bhardwaj S, Mahendra BJ, Sharma H, Sanjay TV, Ashwathnarayana DH, Bilagumba G. An immunogenicity, safety and post-marketing surveillance of a novel adsorbed human diploid cell rabies vaccine (rabivax) in indian subjects. Hum Vaccin 2008; 4 4:275-9; PMID: 18682695; http://dx.doi.org/ 10.4161/hv.4.4.5588 [DOI] [PubMed] [Google Scholar]
- 21. WHO WHO Expert Consultation on Rabies, First Report, Technical Report Series, 931, 2005, Geneva, Switzerland: [PubMed] [Google Scholar]
- 22. Tang Q, Li H. Epidemic situation and related factors analysis of rabies in China. Chin J Epidemiol 2005; 26: 223-224 [Google Scholar]
- 23. http://www.chp.org.cn/cms/business/biological/000064.html [Google Scholar]
- 24. Qian H, Yu W, Liu Y, Dou ZY, Du XL, Wang Q. Studies on side effect and immunological effect of CTN-1V stain. China Public Health 2002; 18:946-7 [Google Scholar]
- 25. He LF, Chen M, Zhang YL, Reng ZY, Hu C, Gao XZ, Zhao ZX, Ma W, Li B, Bai M. Adaption and passage rabies virus strain CTN-1V in human diploid cell strain walvax-2. Prog in Microbiol Immunol 2014;42(5):1-4 [Google Scholar]
- 26. Yan ZL, Han XJ, Ji YJ, Song ZZ, Yu YX. Studies on the passage and adaptation of a rabies street virus in mice brain and human diploid cell strain. Chin J Microbiol Immunol 1983, 3 5:305-307 [Google Scholar]
- 27. Dong GM, Liu ZS, Yu YX, Yan ZL, Liu JH. Studies of adaptation and passage on rabies virus (CTN-1) strain in vero cell culture. Prog in Microbiol Immunol 1995; 23 2:82-4 [Google Scholar]
- 28. Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974; 2 7892:1288-90; PMID: 4139526; http://dx.doi.org/ 10.1016/S0140-6736(74)90144-5 [DOI] [PubMed] [Google Scholar]
- 29. Chen EJ, Ma B, Wei SZ. The study on adaptation of hepatitis a virus YN5 strain in vero cell. Prog in Microbiol Immunol 2003; 31:1-4 [Google Scholar]
- 30. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25 585-621; PMID: 13905658; http://dx.doi.org/ 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- 31. Coburn SP, Leykauf RB. Human Chromosome Analysis Biokit. Burlington, NC: Carolina Biological Supply Co, 1976 [Google Scholar]
- 32. Kong Y, LI J, An Q, Yang LH, Liu WX, Dong GM. Reverse transcriptase assay of biological products. J Microbiol 2003; 2: 005 [Google Scholar]
- 33. Chang A, Ostrove JM, Bird RE. Development of an improved product enhanced reverse transcriptase assay. J Virol Methods 1997; 65 1:45-54; PMID: 9128861; http://dx.doi.org/ 10.1016/S0166-0934(96)02168-4 [DOI] [PubMed] [Google Scholar]
- 34. Wiktor TJ, Fernandes MV, Koprowski H. Cultivation of rabies virus in human diploid cell strain WI-38, J Immunol 1964; 93:353-66; PMID: 14218592 [PubMed] [Google Scholar]
- 35. Smith JS, Yager PA, Baer GM: A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ 1973; 48:535-41; PMID: 4544144 [PMC free article] [PubMed] [Google Scholar]
- 36. Grose C, Brunel PA. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32°C. Infect Immun 1978; 19 1:199-203; PMID: 203532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rager-Zisman B, Merigan TC. A useful quantitative semi-micromethod for viral plaque assay. Proc Soc Exp Biol Med 1973; 142:1174-9; PMID: 4121080; http://dx.doi.org/ 10.3181/00379727-142-37202 [DOI] [PubMed] [Google Scholar]
- 38. Grose C, Perrotta DM, Brunell PA, Smith GC. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol 1979; 43 1:15-27; PMID: 225414; http://dx.doi.org/ 10.1099/0022-1317-43-1-15 [DOI] [PubMed] [Google Scholar]
- 39. Wang ZQ, Ma L, Gao H, Dong XG. Propagation of hepatitis a virus in human diploid fibroblast cells. acta virol 1986; 30 6:463-7; PMID: 2881456 [PubMed] [Google Scholar]
- 40. Wang KQ, Nielsen CM, Vestergaard BF. Isolation and adaptation characteristics of hepatitis a virus in primary african green monkey kidney cells: production of antigen useful for ELISA serology. Intervirology 1985; 24 2:99-107; PMID: 2997078; http://dx.doi.org/ 10.1159/000149625 [DOI] [PubMed] [Google Scholar]
- 41. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938; 27:493-7 [Google Scholar]