Abstract
Importance
The value of routine pre-operative testing prior to most surgical procedures is widely considered to be low. To improve the quality of pre-operative care and reduce waste, two professional societies released guidance in 2002, but researchers and policymakers remain concerned about the health and cost burden of low-value care in the pre-operative setting.
Objective
To examine the long-term, national impact of 2002 professional guidance from the American College of Cardiology/American Heart Association (ACC/AHA) and American Society of Anesthesiologists (ASA), on physicians’ use of routine pre-operative testing.
Design, Setting, Participants
U.S. adults evaluated in pre-operative visits in the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) between 1997-2010. A quasi-experimental, differences-in-differences (DID) approach evaluated whether professional guidance in 2002 was associated with changes in pre-operative testing patterns, while adjusting for temporal trends in routine testing, as captured by testing patterns in general medical exams.
Main Measures
Physician orders for outpatient plain radiography, hematocrit, urinalysis, electrocardiogram (ECG), and cardiac stress testing.
Results
Over the 14-year period, the average annual number of pre-operative visits in the US increased from 6.8 million in 1997-1999, to 9.8 million in 2002-2004, to 14.3 million in 2008-2010. After accounting for temporal trends in routine testing, we found no statistically significant overall changes in the use of plain radiography (11.3% in 1997-2002 to 9.9% in 2003-2010, DID=−1.0-per-100-visits 95% CI-4.1,2.2), hematocrit (9.4% in 1997-2002 to 4.1% in 2003-2010, DID=+1.2-per-100-visits 95% CI-2.2,4.7), urinalysis (12.2% in 1997-2002 to 8.9% in 2003-2010, DID=+2.7-per-100-visits 95% CI-1.7,7.1), or cardiac stress testing (1.0% in 1997-2002 to 2.0% in 2003-2010, DID=+0.7-per-100-visits 95% CI-0.1,1.5) after release of professional guidance. However, the rate of ECG testing fell (19.4% in 1997-2002 to 14.3% in 2003-2010, DID=−6.7-per-100-visits, 95%CI-10.6,−2.7%) in the period after these guidelines.
Conclusions and Relevance
The release of 2002 guidance reduced the incidence of routine ECG but not of plain radiography, hematocrit, urinalysis, or cardiac stress testing. Because routine pre-operative testing is generally considered to provide low incremental value, more concerted efforts to understand physician behavior and remove barriers to guideline adherence may improve quality and reduce healthcare costs.
Background
The value of routine pre-operative testing prior to most elective surgical procedures is widely considered to be low.1-4 The national cost of this testing may be considerable, with 30 million Americans undergoing surgery annually and 60% of those patients undergoing ambulatory procedures.4 In recognition of these challenges and broader concerns about value, several major physician-education initiatives were undertaken to more appropriately guide medical decision-making, improve the quality of care that physicians delivered, and reduce the incidence of unnecessary testing. Three in particular—the American Board of Internal Medicine's (ABIM) “Medical Professionalism in the New Millennium: A Physician Charter”, which helped catalyze the Choosing Wisely campaign; the American College of Cardiology/American Heart Association (ACC/AHA) Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery; and the American Society of Anesthesiology's (ASA) Practice Advisory Guidelines for Preanesthesia Evaluation—were concurrently disseminated in 2002.5-8
While the ABIM charter focused on guiding principles important in the practice of medicine, such as improving the quality and cost-effectiveness of care, the ACC/AHA and ASA guidance made more specific recommendations about appropriate testing in the pre-operative setting.5 However, despite these efforts, many researchers and policymakers remain concerned that a substantial gap persists between practice guidelines and clinical care patterns.2,9 Evidence supporting their concerns includes the rise in cardiac stress testing among patients enrolled in Medicare prior to elective surgery,2 the wide use of laboratory blood testing in the pre-operative setting,1 and uncertainty among physicians about potential adverse consequences—such as delayed or canceled surgery—of performing fewer tests.10
While single site studies have reported poor rates of adherence to guidelines for pre-operative testing,2,9 and larger studies suggest that some pre-operative tests are overused and low-value for specific surgical procedures,1,2 the long-term, national impact of the 2002 initiatives informing pre-operative testing practices across diverse tests and surgery types is unknown. This is particularly important because routine pre-operative testing is common and costly,1,2 and ACC/AHA and ASA guidance—which were both updated in recent years—discourage this practice.11,12 In this study, we use nationally representative data from the National Ambulatory and Hospital Medical Care Surveys to examine pre-operative testing patterns between 1997 and 2010, before and after the release of 2002 guidance for pre-operative testing.
Methods
Study Design
We used a quasi-experimental, differences-in-differences (time by group interaction) approach to examine the long-term impact of professional guidance in 2002 on pre-operative testing patterns. The differences-in-differences approach measures changes in an outcome associated with a policy change, after accounting for secular trends in that outcome, as reflected in a control group that is not exposed to the policy change.13 In this study, we chose general medical exams, which primarily comprise routine annual exams or checkups, as our control group, as testing patterns in these visits likely capture temporal trends in the use of routine testing.14,15
Data and Study Population
We analyzed data collected in the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS) from 1997-2010.16 The National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention conduct the NAMCS and NHAMCS annually on a nationally representative sample of visits to office-based physicians, hospital-based outpatient clinics, and emergency departments in the United States. For the NAMCS, each physician is randomly assigned to a 1-week reporting period during which a systematic random sample of visits are surveyed. Data are collected on patients' symptoms and demographic characteristics, physicians' diagnoses, medications ordered or provided, and medical services provided. For the NHAMCS, a systematic random sample of patient visits in selected non-institutional general and short-stay hospitals are surveyed during a randomly assigned 4-week reporting period. The data collected on patient and provider characteristics are comparable to those collected in the NAMCS. From 1993 to 2010, the physician and hospital/outpatient clinic response rates in the NAMCS and NHAMCS ranged from 58% to 73% and 80% to 95%, respectively, and item nonresponse rates were generally 5% or less in both surveys.
The NAMCS and NHAMCS record up to three reasons for each visit (one “most important” and two “other” reasons) and three diagnoses related to the visit (one “primary” and two “other” diagnoses); these are all centrally coded using the NCHS Reason for Visit Classification (RVC) and the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9), respectively. Adult patients (≥18 years old) seeing physicians for pre-operative evaluations or general medical exams comprised our study population. Pre-operative visits were identified using RVC code 4200.0 or ICD-9 diagnosis codes V72.81-V72.84, and general medical exams were identified using RVC code 3100.0.
Primary Measures
The 2002 ACC/AHA guidelines, which aimed to “identify the most appropriate testing and treatment strategies... and avoid unnecessary testing,” in patients undergoing noncardiac surgery, primarily focused on the use of cardiac stress testing and invasive coronary angiography, though other pre-operative tests and management approaches were reviewed. In particular, these guidelines suggested that routine electrocardiogram (ECGs) generally be used only in patients with recent chest pain or ischemic equivalents, or undergoing higher risk surgery.6 The 2002 ASA advisory, which informs preanesthesia care in general and pre-operative testing in particular, addressed a broad range of pre-operative tests, emphasized the importance of assessing the comparative effectiveness of pre-operative testing strategies, and specifically recommended against routine testing, defined as “a test ordered in the absence of a specific clinical indication or purpose.”8 Therefore, their advisory is relevant to a broad range of physicians providing pre-operative care. Additional details about the context of the 2002 ACC/AHA and ASA guidelines, along with the 2007 ACC/AHA guideline update, are provided in the Appendix. Based on the tests reviewed by these professional societies and data availability in the NAMCS/NHAMCS, we considered orders for plain radiography, hematocrit, urinalysis, ECG, or cardiac stress testing as our primary outcomes. Data on hematocrit studies were only available from 1997-2004; these studies therefore were included only in subanalyses. We identified these tests using ICD-9 procedure codes or with survey fields that directly captured test referrals.17
Other Measures
To further account for patient and clinical characteristics that may be associated with testing, we extracted information on patient age, sex, race/ethnicity, insurance (private, Medicare, Medicaid, self-pay/no charge, and other/unknown), US census region (Northeast, Midwest, South, and West), urban or rural setting, smoker, and important comorbidities known to increase the risk of adverse events in the post-operative period (hypertension, coronary artery disease, diabetes, chronic kidney disease, and chronic obstructive pulmonary disease [COPD]).6 Race and ethnicity were determined, per NAMCS and NHAMCS guidance, according to the office or clinic's “usual practice, based on your knowledge of the patient, or from information in the medical record.” We categorized patients as non-Hispanic white, non-Hispanic black, Hispanic, other race, and unknown race/ethnicity, when information on ethnicity was missing. Comorbidities were identified using visit diagnoses and reasons for visit.18
Statistical Analysis
All analyses accounted for the complex sampling design of the NAMCS and NHAMCS.19 We performed descriptive data analysis and used simple logistic regressions, including ordinal and multinomial models, to compare the characteristics of patients in pre-operative and general medical exams. We also estimated simple and multivariate differences-in-differences logistic regressions and estimated the predicted probability of testing to examine the impact of 2002 professional society guidance on the use of each type of test in pre-operative visits. Our models were generally implemented as: Testing = β0 + β1 Pre-operative visit + β2 Post-guidelines + β3 Pre-operative visit X Post-guidelines, where Testing, Pre-operative visit, and Post-guidelines are indicator variables, and Post-guidelines specifically captures the period from 2003-2010.. The coefficient on the interaction term between Pre-operative visit and Post-guidelines estimates the impact of interest. Specifically, this coefficient captures the difference in routine testing rates between pre-operative visits and general medical exams in the time period before the release of professional guidance in 2002 to the difference after the release of this guidance; it therefore represents the independent relationship between 2002 guidance and pre-operative testing rates. We tested for a difference in pre-2002 testing trends between pre-operative visits and general medical exams by estimating simple logistic regressions limited to 1997-2002 and including an interaction variable between time and our indicator for pre-operative visits. The coefficient for this variable was not significant for any of the routine tests we examined.
Multivariable logistic regression models also adjusted for patients’ clinical risk factors and demographic characteristics, insurance, geographic region, and urban/rural setting. To account for secular trends in testing, we also included a continuous variable for year. In 2007, the ACC/AHA pre-operative testing guidelines were updated to further narrow recommendations for pre-operative ECG and cardiac stress testing,12 a development that could erroneously inflate any effects we attributed to the 2002 guidance alone. In light of this, we performed a sensitivity analysis, limiting the time horizon to 2007 instead of 2010. We also performed a sensitivity analysis in which we used ICD-9 procedure codes for cardiovascular and pulmonary procedures (see Appendix Section 4 for details) to identify patients that may have been referred for higher risk procedures. Analyses were performed using Stata version 12 (College Station, Texas).
Results
Patient characteristics
Over the 14-year period, the average annual number of pre-operative visits in the US increased from 6.8 million in 1997-1999, to 9.8 million in 2002-2004, to 14.3 million in 2008-2010. Compared to patients seen in general medical exams, patients seen in pre-operative visits were more likely to be female, less likely to be black versus white, more likely to have Medicare or be uninsured vs. having private insurance, and more likely to live in the South or West vs. living in the Northeast (Table 1). Patients in pre-operative visits were also less likely to have a visit diagnosis of hypertension, coronary artery disease, diabetes, or COPD, but more likely to have a visit diagnosis of chronic kidney disease.
Table 1.
Characteristics of patients seeing physicians for pre-operative visits or general medical exams in the U.S., 1997-2010
| Pre-operative visits | General medical exams | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Unweighted No. | Weighted Annual No. | Percent, % | Unweighted No. | Weighted Annual No. | Percent, % | P value |
| All visits | 8,845 | 10,266,817 | 100 | 29,287 | 49,496,063 | 100 | |
| Age, yrs | |||||||
| 18-44 | 2,859 | 3,073,666 | 29.9 | 10,108 | 14,876,146 | 30.1 | |
| 45-64 | 3,530 | 3,749,175 | 36.5 | 10,912 | 19,310,498 | 39.0 | |
| >=65 | 2,456 | 3,443,976 | 33.5 | 8,267 | 15,309,420 | 30.9 | 0.283 |
| Sex | |||||||
| Female | 5,469 | 6,657,816 | 64.8 | 17,798 | 28,661,083 | 57.9 | |
| Male | 3,376 | 3,609,001 | 35.2 | 11,489 | 20,834,980 | 42.1 | <0.001 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 4,520 | 5,750,637 | 56.0 | 14,684 | 28,279,841 | 57.1 | |
| Non-Hispanic black | 1,114 | 674,070 | 6.6 | 4,143 | 4,581,156 | 9.3 | 0.001 |
| Hispanic | 953 | 683,112 | 6.7 | 3,091 | 2,815,335 | 5.7 | 0.055 |
| Other/unknown | 2,258 | 3,158,998 | 30.8 | 7,369 | 13,819,732 | 27.9 | 0.136 |
| Insurance | |||||||
| Private | 3,592 | 4,986,042 | 48.6 | 11,870 | 27,646,706 | 55.9 | |
| Medicare | 2,316 | 2,878,154 | 28.0 | 7,563 | 13,345,849 | 27.0 | 0.002 |
| Medicaid | 1,292 | 605,670 | 5.9 | 4,665 | 2,929,045 | 5.9 | 0.152 |
| Other | 815 | 803,900 | 7.8 | 2,536 | 3,488,643 | 7.0 | 0.023 |
| Uninsured | 830 | 830 | 9.7 | 2,653 | 2,085,820 | 4.2 | <0.001 |
| Region | |||||||
| Northeast | 2,050 | 1,921,551 | 18.7 | 8,944 | 11,207,994 | 22.6 | |
| Midwest | 1,761 | 1,994,323 | 19.4 | 7,040 | 11,710,212 | 23.7 | 0.952 |
| South | 2,734 | 3,704,982 | 36.1 | 8,666 | 17,368,247 | 35.1 | 0.03 |
| West | 2,300 | 2,645,962 | 25.8 | 4,637 | 9,209,610 | 18.6 | <0.001 |
| Office/clinic setting | |||||||
| Urban | 8,070 | 9,021,071 | 87.9 | 25,583 | 41,957,506 | 84.8 | |
| Rural | 775 | 1,245,746 | 12.1 | 3,704 | 7,538,557 | 15.2 | 0.006 |
| Smoker | |||||||
| No | 3,487 | 4,587,678 | 44.7 | 10,069 | 19,805,561 | 40.0 | |
| Yes | 899 | 929,895 | 9.1 | 2,471 | 4,173,282 | 8.4 | 0.63 |
| Unknown | 4,459 | 4,749,243 | 46.3 | 16,747 | 25,517,220 | 51.6 | 0.001 |
| Visit diagnosis | |||||||
| Hypertension | 609 | 808,162 | 7.9 | 5,550 | 10,553,887 | 21.3 | <0.001 |
| Coronary artery disease | 300 | 340,893 | 3.3 | 1,356 | 2,406,372 | 4.9 | <0.001 |
| Diabetes | 318 | 378,773 | 3.7 | 2,843 | 4,568,556 | 9.2 | <0.001 |
| Chronic kidney disease | 187 | 118,588 | 1.2 | 257 | 330,871 | 0.7 | 0.039 |
| COPD | 73 | 117,875 | 1.1 | 519 | 971,150 | 2.0 | 0.005 |
Abbreviations: CI, confidence interval; Avg., average; No., number; ASA, American Society of Anesthesiologists; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; Thous., thousands; unadj., unadjusted; adj., adjusted
Notes: P values calculated with Wald chi-square test from simple ordinal (age) or binomial/multinomial logistic regression models comparing patients in pre-operative visits to patients in general medical exams.
Prevalence of Testing
We compared unadjusted rates of testing in pre-operative and general medical exams over the duration of our study period (Table 2). Patients presenting for pre-operative visits were more likely to receive plain radiographs (10.3% vs. 5.2%; OR 2.11, 95% CI 1.76, 2.54 P<0.001), ECG (16.0% vs. 10.5%; OR 1.63, 95% CI 1.38, 1.92 P<0.001) and cardiac stress testing (1.7% vs. 0.8%; OR 2.11, 95% CI 1.44, 3.10 P<0.001), compared to patients presenting for general medical exams, and less likely to undergo urinalysis (10.0% vs. 21.6%; OR 0.40, 95% CI 0.34, 0.48 P<0.001) and hematocrit testing (7.8% vs. 12.5%; OR 0.59, 95% CI 0.45, 0.77 P<0.001). Overall, patients in pre-operative visits were less likely to receive any test compared to patients in general medical exams (26.5% vs. 29.4%; OR 0.87, 95% CI 0.76, 0.99 P=0.035).
Table 2.
Prevalence of testing in patients seeing physicians for pre-operative visits or general medical exams in the U.S., 1997-2010
| Pre-operative visits | General medical exams | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-operative tests | Unweighted No. | Weighted Annual No. | Percent, % | Unweighted No. | Weighted Annual No. | Percent, % | OR for Difference | 95% CI | P value |
| Plain radiography | 953 | 1,060,083 | 10.3 | 1,492 | 2,556,729 | 5.2 | 2.11 | 1.76 to 2.54 | <0.001 |
| Hematocrit | 322 | 665,287 | 7.80 | 1,589 | 6,079,908 | 12.5 | 0.59 | 0.45 to 0.77 | <0.001 |
| Urinalysis | 798 | 1,027,702 | 10.0 | 5,714 | 10,686,372 | 21.6 | 0.40 | 0.34 to 0.48 | <0.001 |
| ECG | 1,300 | 1,638,962 | 16.0 | 2,126 | 5,178,623 | 10.5 | 1.63 | 1.38 to 1.92 | <0.001 |
| Cardiac stress test | 171 | 171,022 | 1.70 | 231 | 393,508 | 0.8 | 2.11 | 1.44 to 3.10 | <0.001 |
| Any test* | 2,370 | 2,723,526 | 26.5 | 7,840 | 14,552,887 | 29.4 | 0.87 | 0.76 to 0.99 | 0.035 |
| Any test† | 1,314 | 2,517,477 | 29.4 | 5,298 | 17,012,107 | 35.0 | 0.77 | 0.64 to 0.93 | 0.007 |
Abbreviations: CI, confidence interval; ECG, electrocardiography; No., number; OR, odds ratio
Does not include hematocrit blood tests, which were available from 1997-2004 only
Includes hematocrit; therefore time period limited to 1997-2004
Changes in Testing After Professional Guidance
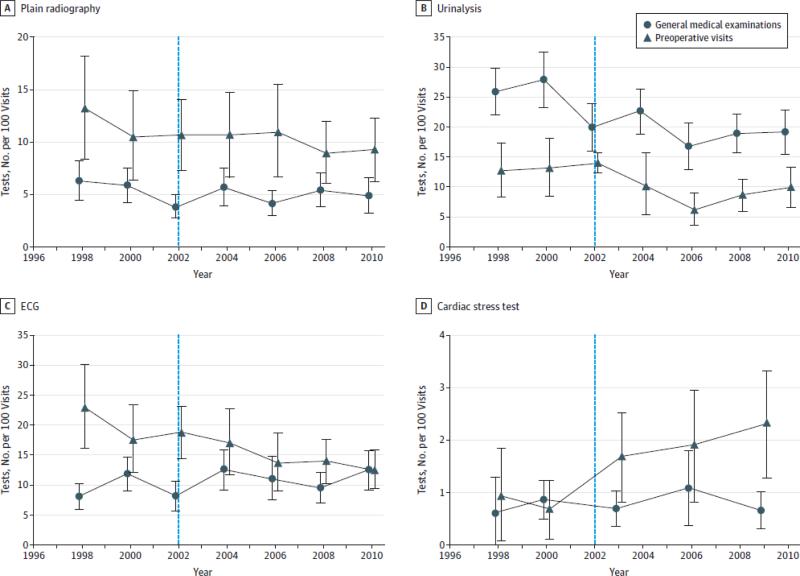
Trends in testing during pre-operative visits and general medical exams are shown in Figure 1. Overall rates of plain radiography and urinalysis fell over time across both visit types, as did rates of ECG in pre-operative visits. However, rates of cardiac stress testing trended upward in pre-operative visits.
Figure.
Rate of Testing in US Ambulatory Preoperative Visits Compared With General Medical Examinations, 1997-2010
The vertical line at year 2002 indicates the cutoff for the comparison of change in testing rates for preoperative visits to general medical examinations. Data points represent combined data for 2-year increments (eg, 1997-1998, 1999-2000), but cardiac stress testing rates were averaged for 3-year instead of 2-year intervals (except in 1997-1998) to improve statistical reliability. ECG indicates eletrocardiography.
Using our differences-in-differences regression models, we found that, in the years after release of professional guidance for pre-operative testing, there was no significant change in the use of plain radiography (11.3% in 1997-2002 to 9.9% in 2003-2010, DID=−1.0 per 100 visits, 95% CI −4.1, 2.2), hematocrit (9.4% in 1997-2002 to 4.1% in 2003-2010, DID=+1.2 per 100 visits, 95% CI −2.2, 4.7), urinalysis (12.2% in 1997-2002 to 8.9% in 2003-2010, DID=+2.7 per 100 visits, 95% CI −1.7, 7.1), or cardiac stress testing (1.0% in 1997-2002 to 2.0% in 2003-2010, DID=+0.7 per 100 visits, 95% CI −0.01, 1.5) (Table 3). Pre-operative guidance in 2002 was associated with a decrease in the use of ECG testing, however, which fell by −6.7 tests per 100 visits (95% CI −10.6, −2.7 P=0.001 in adjusted model). In a sensitivity analysis, we limited the follow-up period to 2007, the year that the ACC/AHA updated its pre-operative testing guidelines. This change did not significantly affect our results (adjusted change in ECG testing = −6.1 tests per 100 visits, P=0.006; no other testing changes statistically significant), which are presented in Appendix Table 8. In another sensitivity analysis, we adjusted for an indicator variable for patients who may have been referred for higher risk cardiovascular and pulmonary procedures. Our results, presented in Appendix Section 3, were not significantly affected.
Table 3.
Testing rate and net change (differences in differences estimate) in testing rates during pre-operative visits after 2002 AHA/ACC and ASA guidance in the U.S.
| Testing rate per 100 visits | Unadjusted impact | Adjusted impact | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-operative tests | Prior to prof. society guidance (1997-2002) | After prof. society guidance (2003-2010) | Net change after 2002 guidance, per 100 visits** | 95% CI | P value | Net change after 2002 guidance, per 100 visits** | 95% CI | P value |
| Plain radiography | 11.3 | 9.9 | −1.1 | −4.2 to 1.9 | 0.47 | −1.0 | −4.1 to 2.2 | 0.55 |
| Hematocrit | 9.4 | 4.1 | 0.4 | −3.5 to 4.3 | 0.85 | 1.2 | −2.2 to 4.7 | 0.48 |
| Urinalysis | 12.2 | 8.9 | 1.9 | −2.2 to 6.1 | 0.36 | 2.7 | −1.7 to 7.1 | 0.23 |
| ECG | 19.4 | 14.3 | −7.3 | −12 to −3.1 | <0.001 | −6.7 | −10.6 to −2.7 | 0.001 |
| Cardiac stress test | 1.0 | 2.0 | 0.8 | −0.1 to 1.7 | 0.073 | 0.7 | −0.1 to 1.5 | 0.082 |
| Any test* | 28.1 | 25.8 | 0.8 | −4.7 to 6.3 | 0.78 | 1.8 | −3.7 to 7.3 | 0.53 |
| Any test† | 30.5 | 27.0 | 0.2 | −8.4 to 8.8 | 0.97 | 1.9 | −5.8 to 9.6 | 0.63 |
Abbreviations: CI, confidence interval; ECG, electrocardiography; No., number; OR, odds ratio; Prof., professional
Net changes incorporate pre-2002 and post-2002 testing rates in both pre-operative visits and general medical exams and reflect differences-in-differences estimates.
Does not include hematocrit blood tests, which were available from 1997-2004 only
Includes hematocrit; therefore time period limited to 1997-2004
Discussion
We found that overall rates of routine testing in the US among patients evaluated in pre-operative visits declined across several—but not all—of the test categories we examined. However, after accounting for temporal trends in routine testing patterns, we found little evidence that the release of professional guidance in 2002 was associated with long-term changes in national pre-operative testing rates. In particular, with the exception of ECG testing, the rates of pre-operative testing with plain radiography, hemoglobin, urinalysis, and cardiac stress testing were not significantly affected by the release of ACC/AHA and ASA guidance in the years following 2002. Our findings are consistent with the conclusions of previous studies that report overuse of testing in the pre-operative setting, including studies of pre-operative cardiac stress test use among patients with Medicare,2 medical consultations for cataract surgery,3 and testing prior to hernia repair,1 among other studies.9,20 Though we found little evidence of guideline impact, it is noteworthy that rates of pre-operative testing have generally fallen over time. This likely reflects strong secular trends in testing, and may have been partially driven by temporal reductions in reimbursement.
National pre-operative testing guidance in 2002 may have had a limited long-term impact on physician behavior for several reasons. Physicians may not have been aware of these guidelines, or may have felt that the guidelines were not applicable to their patients.21 Prior research on physician behavior and practice guidelines also suggests that financial incentives that link physician compensation with test use may also increase utilization.22 The role of incentives might be most relevant for cardiac stress testing, a setting in which physicians are compensated for performing and interpreting study results. In a recent national study of cardiac stress testing, we found that rates of imaging stress tests—which are reimbursed by Medicare and private insurers at higher rates than non-imaging cardiac stress tests—increased substantially over the past two decades.18
More broadly, prior studies of physician behavior have reported limited effects of professional guidelines on physician behavior.21,23-29 Analyses of physician decision-making have proposed myriad reasons for poor adherence, including lack of awareness of guidelines, unfamiliarity among physicians with the content of guidance, uncertainty about whether a guideline will improve patient outcomes, uncertainty in the setting of conflicting guidelines, and inertia of existing practice patterns.21 These factors may explain the absence of a robust response to 2002 guidance on pre-operative testing. In addition, some evidence suggests that physicians are more likely to follow guidelines that add, rather than eliminate, a test or procedure 23,27 These behavioral patterns among physicians have broad implications for clinical quality and healthcare costs, as professional societies routinely aim to improve quality of care and reduce inappropriate variation in practice patterns.21
In 2012, the American Society of Anesthesiologists updated the 2002 practice advisory with new evidence but concluded that no change in their recommendations was necessary.11 More recently, the ASA, as part of the Choosing Wisely Campaign, surveyed practicing anesthesiologists to develop a “top-five” list of low-value pre-operative practices. Consistent with earlier ASA guidance, their final list included routine laboratory studies and cardiac stress testing, with the latter a major focus of ACC/AHA guidelines. Because cardiac stress tests, in particular, are costly and frequently result in downstream tests and procedures, more concerted efforts to understand physician behavior and remove barriers to guideline adherence may improve quality and reduce healthcare costs.18
Our study has several limitations. The NAMCS and NHAMCS provide only a limited amount of clinical information on each patient visit, and we were therefore unable to adjust for surgery type or identify patients referred for high-risk surgery. Because high-risk surgeries may be associated with higher rates of pre-operative testing, we examined published national rates of cardiac and pulmonary procedures in using the National Hospital Discharge Surveys, to determine whether the distribution of these procedures changed during our study period. Our analysis demonstrated little difference in the distribution of these procedures between 2003 to 2010 (14.5% to 15.7% for cardiac procedures, 2.3% to 2.7% for pulmonary procedures).30-32 Another important limitation is that we were unable to separate differential effects of the ACC/AHA and ASA guidance. In particular, the ASA guidelines may have been less frequently accessed by internists, cardiologists, and other physicians who routinely perform pre-operative evaluations. Another limitation is the relatively low incidence of some tests, such as cardiac stress tests, which may have limited our ability to detect overall effects of 2002 professional pre-operative guidance. However, our overall estimates of test use meet statistical reliability requirements issued by the NCHS. Because our study is cross-sectional, we also do not have information on patient outcomes. Another limitation of our analysis is that general medical exams may not be an adequate control group for pre-operative evaluations, though these visits are frequently also characterized by the routine use of tests that may be low in value. Finally, temporal trends in testing, reflected by the use of routine testing in general medical exams, may have been influenced by other factors that differentially affected physician behavior in general medical visits, compared to pre-operative testing visits.33,34
Physicians play a critical role in improving the quality of care patients receive and reducing the use of low-value care. In the context of pre-operative care, our findings suggest that professional guidance aimed at improving quality and reducing waste had little impact on physician practice. In so doing, we add to the body of evidence that underscores the urgency of employing evidence-based and novel approaches to remove barriers to adherence and influence physician behavior in the provision of high-quality care.
Supplementary Material
Acknowledgements
Alana E. Sigmund, MD, NYU School of Medicine, Division of General Internal Medicine- conceived and designed study, contributed to analyses and writing. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Elizabeth R. Stevens, MPH NYU Department of Population Health- contributed to analyses and writing
Jeanna Blitz, MD, NYU School of Medicine, Department of Anesthesiology- contributed to interpretation of the data and writing
Joseph A. Ladapo, MD, PhD, NYU School of Medicine, Department of Population Health, Division of General Internal Medicine – supervised and designed study, contributed to analyses, interpretation of the data, and writing. Dr. Ladapo's work is supported by the National Heart, Lung, and Blood Institute (K23 HL116787) and he serves as a consultant to CardioDx, Inc. The preceding sponsors did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Annals of surgery. 2012;256(3):518–528. doi: 10.1097/SLA.0b013e318265bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheffield KM, McAdams PS, Benarroch-Gampel J, et al. Overuse of preoperative cardiac stress testing in medicare patients undergoing elective noncardiac surgery. Annals of surgery. 2013;257(1):73–80. doi: 10.1097/SLA.0b013e31826bc2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleisher LA. Preoperative consultation before cataract surgery: are we choosing wisely or is this simply low-value care? JAMA internal medicine. 2014;174(3):389–390. doi: 10.1001/jamainternmed.2013.12298. [DOI] [PubMed] [Google Scholar]
- 4.Onuoha OC, Arkoosh VA, Fleisher LA. Choosing Wisely in Anesthesiology: The Gap Between Evidence and Practice. JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2014.2309. [DOI] [PubMed] [Google Scholar]
- 5.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- 6.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Journal of the American College of Cardiology. 2002;39(3):542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 7.Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Journal of the American College of Cardiology. 1996;27(4):910–948. doi: 10.1016/0735-1097(95)99999-x. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists Task Force on Preanesthesia E Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96(2):485–496. doi: 10.1097/00000542-200202000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Benarroch-Gampel J, Riall TS. What laboratory tests are required for ambulatory surgery? Advances in surgery. 2013;47:81–98. doi: 10.1016/j.yasu.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Patey AM, Islam R, Francis JJ, Bryson GL, Grimshaw JM, Canada PPT. Anesthesiologists' and surgeons' perceptions about routine pre-operative testing in low-risk patients: application of the Theoretical Domains Framework (TDF) to identify factors that influence physicians' decisions to order pre-operative tests. Implementation science : IS. 2012;7:52. doi: 10.1186/1748-5908-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee on S. Practice P, Apfelbaum JL, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522–538. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 12.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Journal of the American College of Cardiology. 2007;50(17):e159–241. doi: 10.1016/j.jacc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Ladapo JA, Rodwin BA, Ryan AM, Trasande L, Blustein J. Scientific publications on firearms in youth before and after Congressional action prohibiting federal research funding. JAMA. 2013;310(5):532–534. doi: 10.1001/jama.2013.119355. [DOI] [PubMed] [Google Scholar]
- 14.Qaseem A, Alguire P, Dallas P, et al. Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med. 2012;156(2):147–149. doi: 10.7326/0003-4819-156-2-201201170-00011. [DOI] [PubMed] [Google Scholar]
- 15.Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142–148. doi: 10.1001/2013.jamainternmed.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics (U.S.) [June 4, 2013];Ambulatory Health Care Data: NAMCS and NHAMCS description. 2013 http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm.
- 17.National Center for Health Statistics (U.S.) 2009 NAMCS Public-use Data File Documentation. 2009 2009. ftp://ftp.cdc.gov/pub/health_statistics/NCHS/Dataset_Documentation/NAMCS/doc09.pdf.
- 18.Ladapo JA, Blecker S, Douglas PS. Physician Decision Making and Trends in the Use of Cardiac Stress Testing in the United States: An Analysis of Repeated Cross-sectional Data. Ann Intern Med. 2014;161(7):482–490. doi: 10.7326/M14-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics (U.S.) [June 4, 2013];Ambulatory Health Care Data: NAMCS and NHAMCS, Reliability of Estimates. 2013 http://www.cdc.gov/nchs/ahcd/ahcd_estimation_reliability.htm.
- 20.Siriussawakul A, Nimmannit A, Rattana-arpa S, Chatrattanakulchai S, Saengtawan P, Wangdee A. Evaluating compliance with institutional preoperative testing guidelines for minimal-risk patients undergoing elective surgery. BioMed research international. 2013;2013:835426. doi: 10.1155/2013/835426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA : the journal of the American Medical Association. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Andersen R, Brook R, Kominski G, Albert PS, Wenger N. The Effects of Payment Method on Clinical Decision-Making: Physician Responses to Clinical Scenarios. Medical care. 2004;42(3):297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 23.McCormick B, Ottesen RA, Hughes ME, et al. Impact of guideline changes on use or omission of radiation in the elderly with early breast cancer: practice patterns at national comprehensive cancer network institutions. Journal of the American College of Surgeons. 2014;219(4):796–802. doi: 10.1016/j.jamcollsurg.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Grise EM, Adeoye O, Lindsell C, et al. Emergency department adherence to American Heart Association guidelines for blood pressure management in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2012;43(2):557–559. doi: 10.1161/STROKEAHA.111.637983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson BL, Pearlman M, Griffin J, Schulkin J. Conflicting and changing breast cancer screening recommendations: survey study of a national sample of ob-gyns after the release of the 2009 USPSTF guidelines. Journal for healthcare quality : official publication of the National Association for Healthcare Quality. 2013;35(4):25–35. doi: 10.1111/jhq.12009. [DOI] [PubMed] [Google Scholar]
- 26.McCulloh RJ, Smitherman SE, Koehn KL, Alverson BK. Assessing the impact of national guidelines on the management of children hospitalized for acute bronchiolitis. Pediatric pulmonology. 2014;49(7):688–694. doi: 10.1002/ppul.22835. [DOI] [PubMed] [Google Scholar]
- 27.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(14):1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudiver F, Guibert R, Haggerty J, et al. What influences family physicians' cancer screening decisions when practice guidelines are unclear or conflicting? The Journal of family practice. 2002;51(9):760. [PubMed] [Google Scholar]
- 29.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Medical care. 2001;39(8 Suppl 2):II2–45. [PubMed] [Google Scholar]
- 30.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital and health statistics. Series 13, Data from the National Health Survey. 1998;(139):1–119. [PubMed] [Google Scholar]
- 31.Cullen Karen A., Ph.D. MPHMJH, Ph.D., Golosinsky Aleksandr. Division of Health Care Statistics. Ambulatory Surgery in the United States, 2006. National Health Statistics Reports. 2009:11. [PubMed] [Google Scholar]
- 32.Buie Verita C., Dr.pH, Owings Maria, Ph.D CJD, Ph.D., Golosinsky Alexander., M.S. Division of Care Statistics National Hospital Discharge Survey. 2006 Annual Summary Division of Health Care Statistics; 2006. p. 79. [PubMed] [Google Scholar]
- 33.Summaries for patients. Screening for coronary heart disease: recommendations from the United States Preventive Services Task Force. Annals of internal medicine. 2004;140(7):I95. doi: 10.7326/0003-4819-140-7-200404060-00049. [DOI] [PubMed] [Google Scholar]
- 34.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Journal of the American College of Cardiology. 2002;40(8):1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.